Beyond Nerve Entrapment: A Narrative Review of Muscle–Tendon Pathologies in Deep Gluteal Syndrome
Abstract
1. Introduction
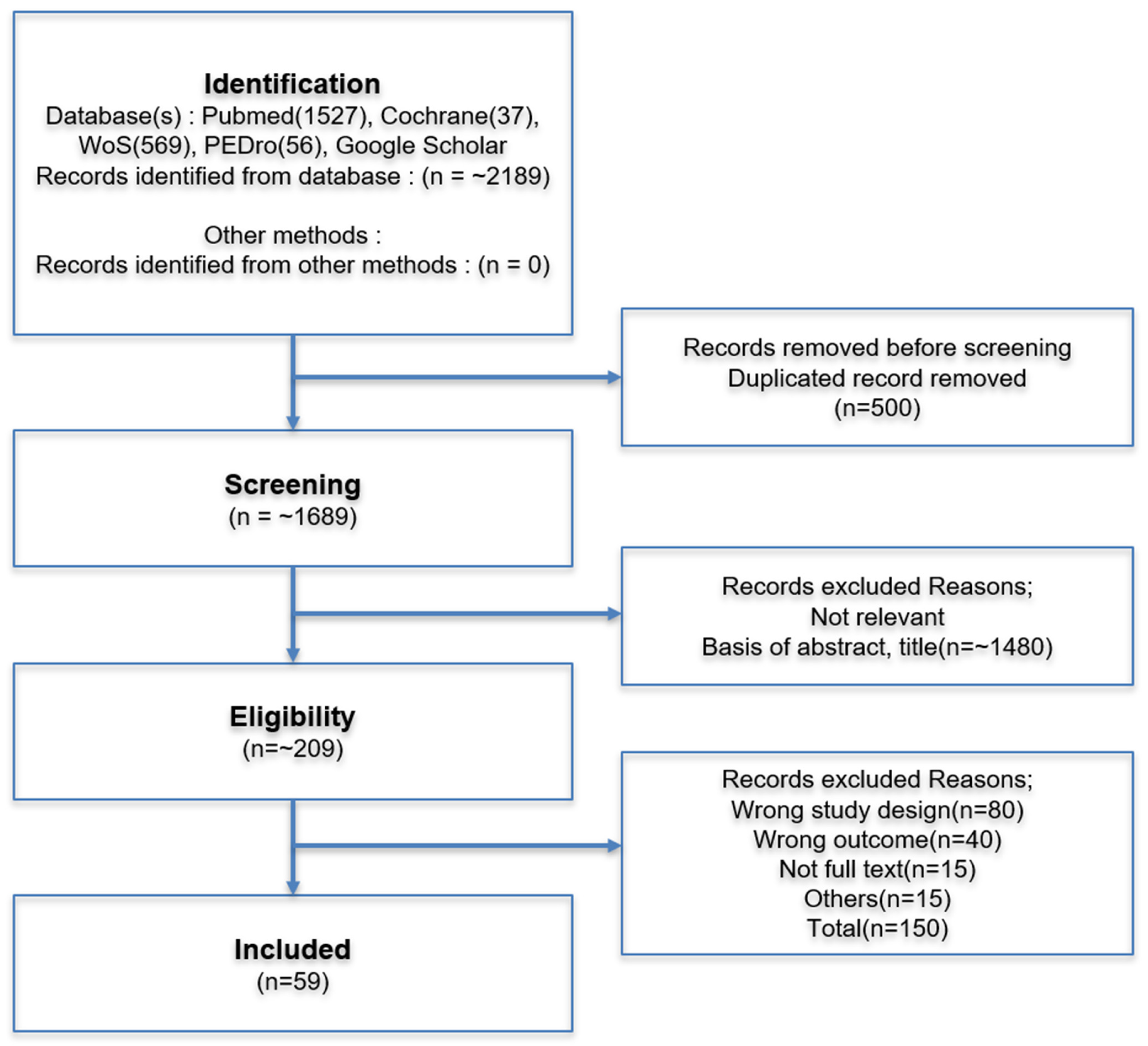
2. Method
2.1. Literature Search Strategy
2.2. Study Selection and Inclusion Criteria
- The anatomy of the deep gluteal space,
- Diagnostic approaches (e.g., history, physical examination, imaging), or
- Therapeutic interventions for DGS beyond classic nerve entrapment, particularly those involving muscular or tendinous pathologies.
2.3. Data Synthesis and Limitations
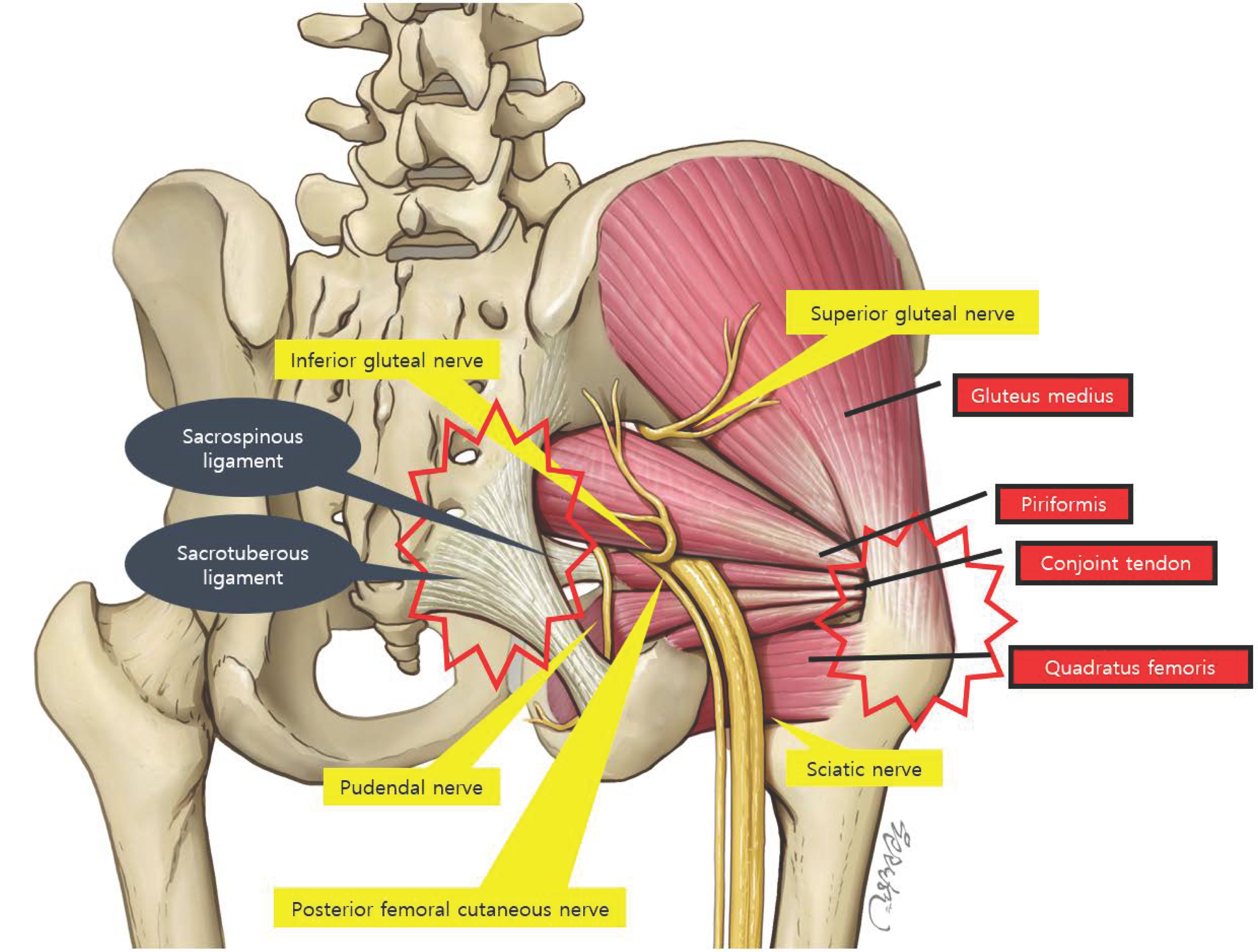
3. Anatomy and Pathogenesis
3.1. Traditional View: Sciatic Nerve Compression
3.2. Expanding View: Muscle-Origin Pain and Enthesopathy
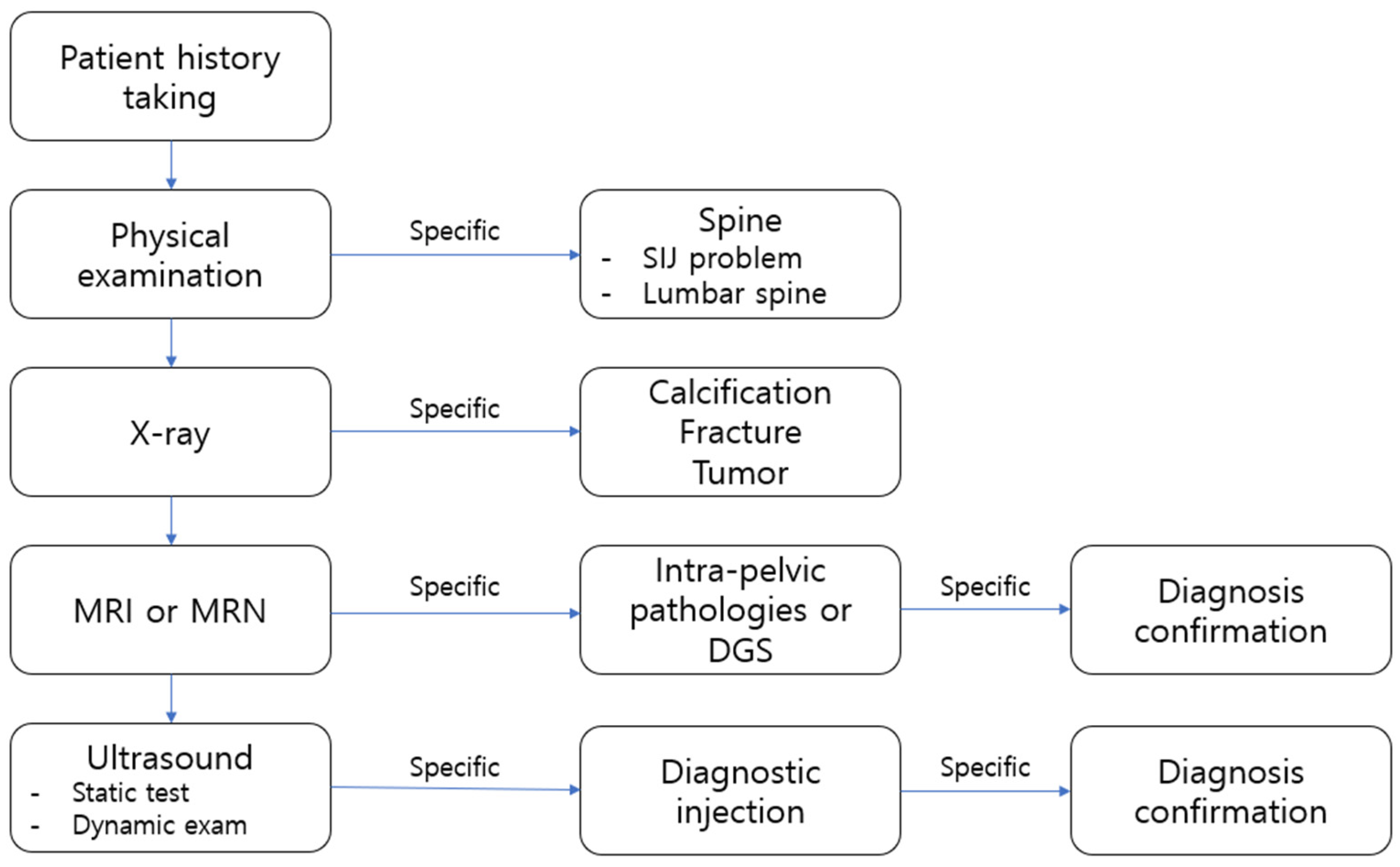
4. Diagnosis
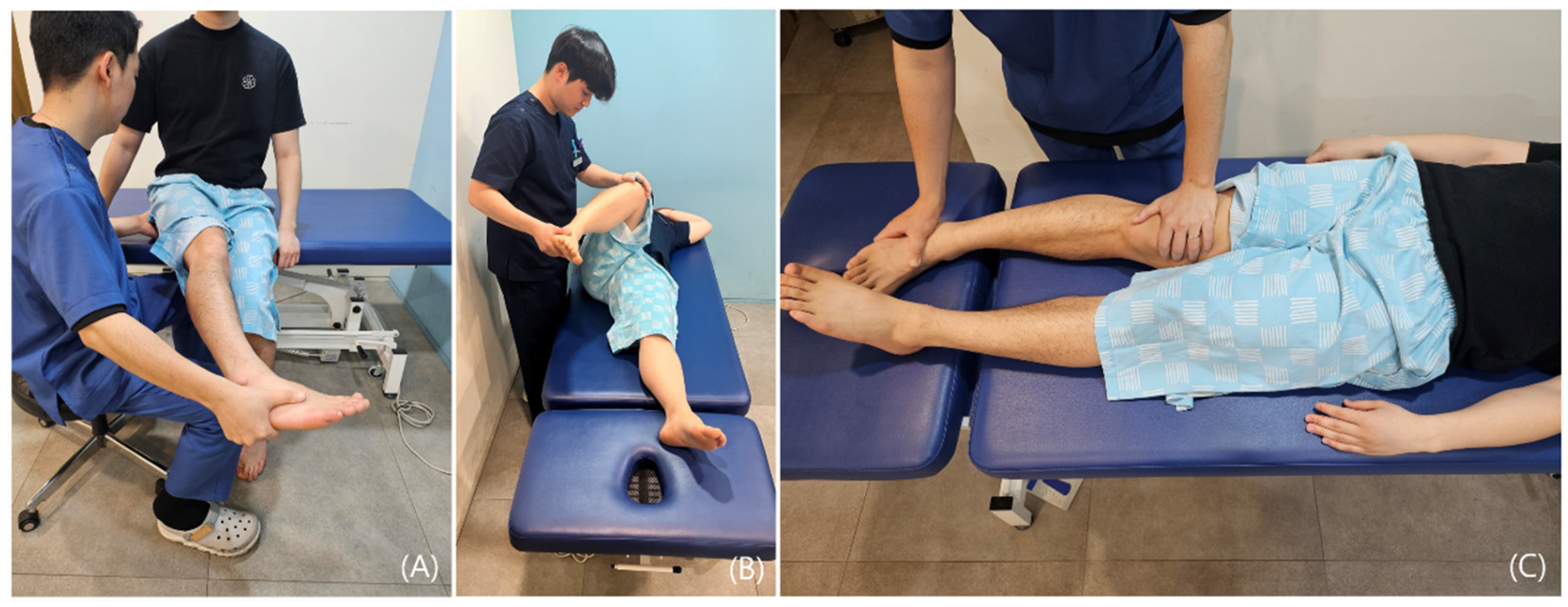
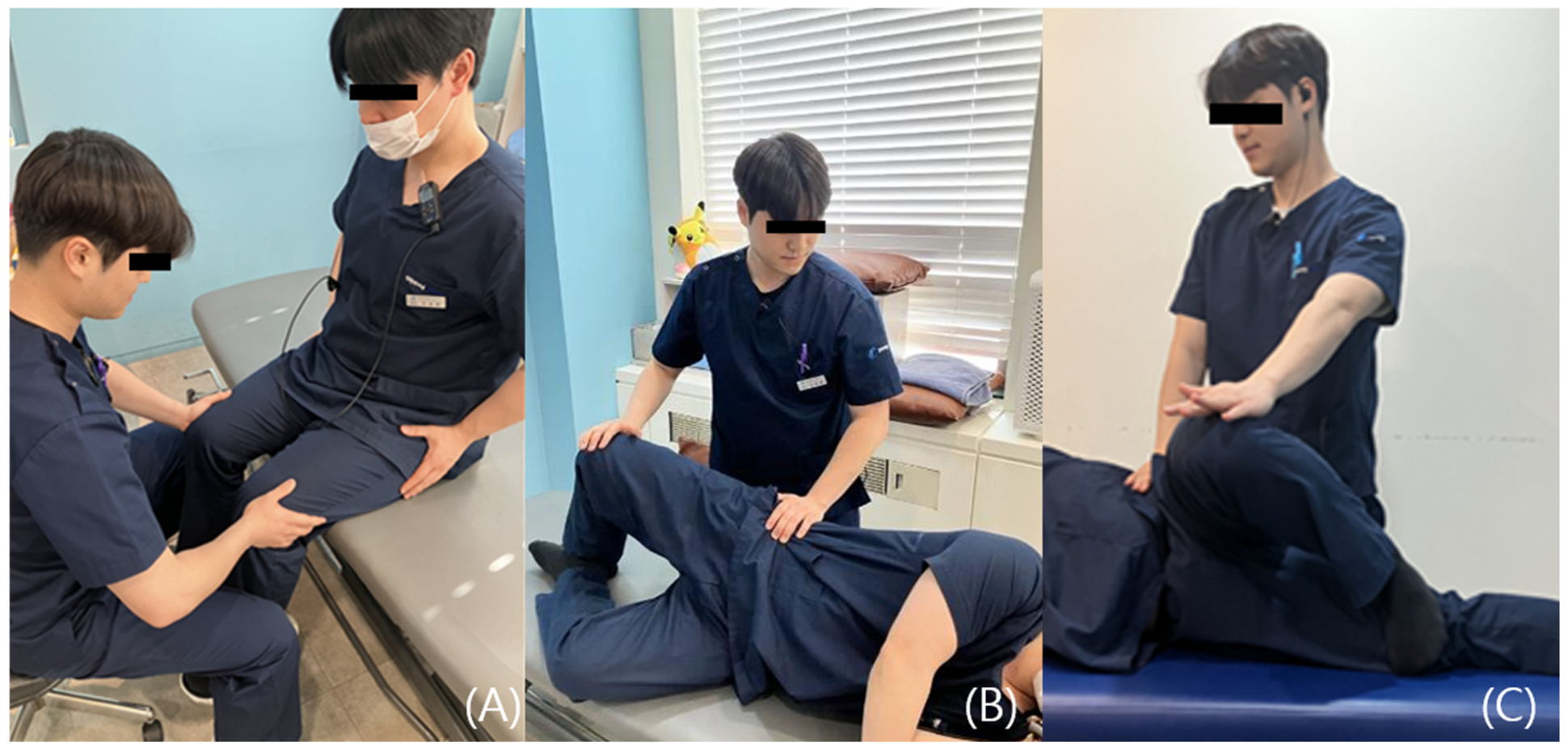
4.1. History Taking
4.2. Clinical Differentiation Between Nerve and Tendon Causes
- Pain and weakness suggest a partial tear of the muscle or tendon, wherein pain-induced inhibition contributes to decreased strength.
- Painless weakness may indicate either a complete rupture of the muscle or tendon or a neurologic deficit.
- Full range of motion with pain typically indicates tendinous pathology, commonly associated with enthesopathy.
- Restricted range of motion with pain during muscle stretch is suggestive of intrinsic muscular pathology such as a muscle tear or may reflect movement limitation due to protective spasm.
4.3. Imaging
4.3.1. Plain Radiographs
4.3.2. CT
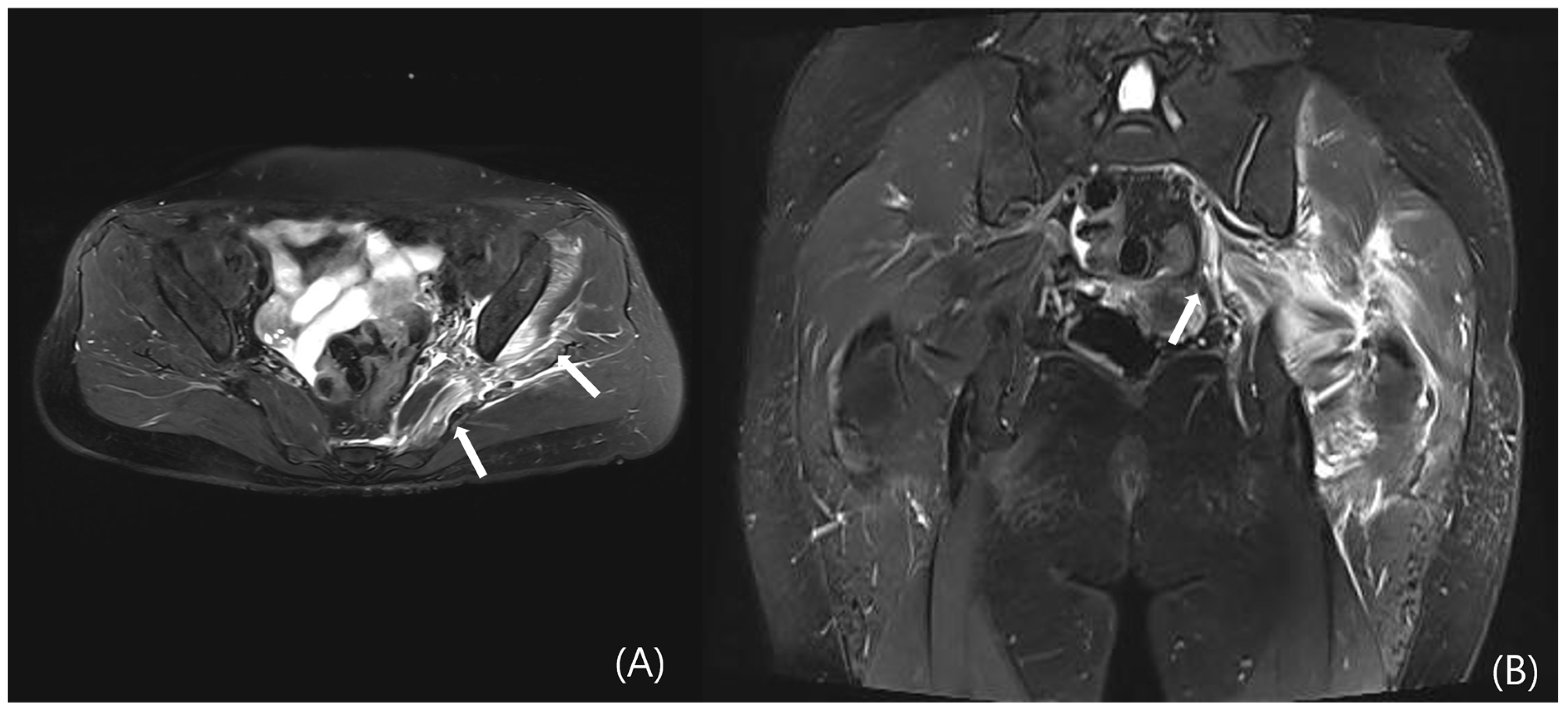
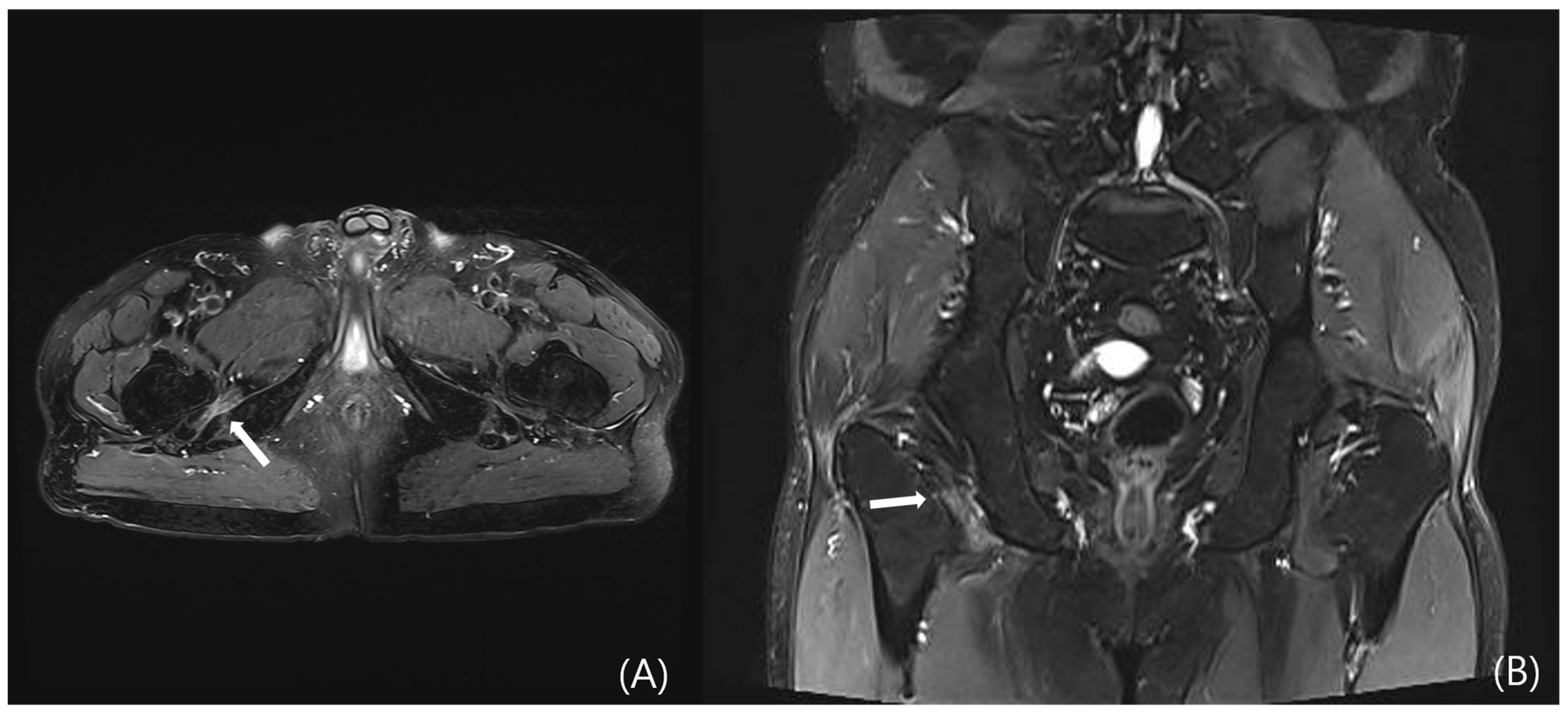
4.3.3. MRI
4.3.4. Ultrasound
5. Therapeutic Consideration
5.1. Conservative Treatment
5.2. Injection Therapy
- Steroid or local anesthetic injection: These agents are injected either intramuscularly or perineurally to reduce inflammation.
- Botulinum toxin injection: Particularly effective in piriformis syndrome, botulinum toxin serves to relax hypertonic muscles and alleviate pain. However, a critical appraisal of the literature reveals that evidence is limited to small, unblinded studies and case series (LoE: 4) with a high risk of performance and detection bias [53]. Systematic reviews have highlighted the inconsistency in results across studies and the lack of long-term follow-up data. The therapeutic effects are also temporary, necessitating repeated injections. Consequently, it should be considered an option only after more conventional therapies have failed, with patients counseled about the limited and temporary nature of the evidence supporting its use.
- Ultrasound-Guided Hydrodissection: Recently, ultrasound-guided hydrodissection and prolotherapy have gained attention as novel non-surgical treatment options for Deep Gluteal Syndrome (DGS). Hydrodissection involves the mechanical separation of perineural adhesions using an injected solution composed of 5% dextrose, lidocaine, and betamethasone, thereby restoring nerve mobility. In a study by Yen et al. (LoE: 4), 53 patients with DGS underwent ultrasound-guided SN hydrodissection; among them, 73.6% reported ≥50% pain reduction at 1 week post-injection, and 62.3% maintained this improvement at final follow-up [54]. While these results are promising, it is critical to note the limitations inherent to this level of evidence. As a single-arm case series, the study lacks a control group for comparison, making it difficult to attribute the improvements solely to hydrodissection versus placebo effect or natural history of the condition. Additionally, the mid-term follow-up period and relatively small sample size limit the generalizability and strength of these findings. Confirmation through larger, randomized controlled trials with sham-procedure control groups is necessary to firmly establish efficacy.
5.3. Role of Surgical Options
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guedes, F.; Brown, R.S.; Lourenço Torrão-Júnior, F.J.; Siquara-de-Sousa, A.C.; Pires Amorim, R.M. Nondiscogenic Sciatica: What Clinical Examination and Imaging Can Tell Us? World Neurosurg. 2020, 134, e1053–e1061. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.B.; Nagle, D. Piriform syndrome. West J. Med. 1976, 124, 435–439. [Google Scholar]
- Sun, G.; Fu, W.; Li, Q.; Yin, Y. Arthroscopic treatment of deep gluteal syndrome and the application value of high-frequency ultrasound. BMC Musculoskelet. Disord. 2023, 24, 742. [Google Scholar] [CrossRef]
- McCrory, P.; Bell, S. Nerve entrapment syndromes as a cause of pain in the hip, groin and buttock. Sports Med. 1999, 27, 261–274. [Google Scholar] [CrossRef]
- Koh, E. Imaging of peripheral nerve causes of chronic buttock pain and sciatica. Clin. Radiol. 2021, 76, 626.e1–626.e11. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P. The Oxford 2011 Levels of Evidence. [Background Document] 2011. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 6 September 2025).
- Martin, H.D.; Reddy, M.; Gómez-Hoyos, J. Deep gluteal syndrome. J. Hip Preserv. Surg. 2015, 2, 99–107. [Google Scholar] [CrossRef]
- Miller, S.L.; Webb, G.R. The proximal origin of the hamstrings and surrounding anatomy encountered during repair. Surgical technique. J. Bone Joint Surg. Am. 2008, 90 Pt 1 (Suppl. 2), 108–116. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Alshami, A.M.; Babri, A.S.; Souvlis, T.; Kippers, V.; Hodges, P.W. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J. Orthop. Res. 2006, 24, 1883–1889. [Google Scholar] [CrossRef]
- Martin, H.D.; Khoury, A.N.; Schroder, R.; Gomez-Hoyos, J.; Yeramaneni, S.; Reddy, M.; James Palmer, I. The effects of hip abduction on sciatic nerve biomechanics during terminal hip flexion. J. Hip Preserv. Surg. 2017, 4, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kivlan, B.R.; Martin, R.L.; Martin, H.D. Defining the greater trochanter-ischial space: A potential source of extra-articular impingement in the posterior hip region. J. Hip Preserv. Surg. 2016, 3, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Smoll, N.R. Variations of the piriformis and sciatic nerve with clinical consequence: A review. Clin. Anat. 2010, 23, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Torriani, M.; Souto, S.C.; Thomas, B.J.; Ouellette, H.; Bredella, M.A. Ischiofemoral impingement syndrome: An entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am. J. Roentgenol. 2009, 193, 186–190. [Google Scholar] [CrossRef]
- Goom, T.S.; Malliaras, P.; Reiman, M.P.; Purdam, C.R. Proximal Hamstring Tendinopathy: Clinical Aspects of Assessment and Management. J. Orthop. Sports Phys. Ther. 2016, 46, 483–493. [Google Scholar] [CrossRef]
- Leite, M.J.; Pinho, A.R.; Silva, M.R.; Lixa, J.C.; Madeira, M.D.; Pereira, P.G. Deep gluteal space anatomy and its relationship with deep gluteal pain syndromes. Hip Int. 2022, 32, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.E.; Ho, G.W.K.; Tortland, P.D. Deep Gluteal Syndrome: A Pain in the Buttock. Curr. Sports Med. Rep. 2021, 20, 279–285. [Google Scholar] [CrossRef]
- Geler Külcü, D. Deep Gluteal syndrome: An underestimated cause of posterior hip pain. Turk. J. Phys. Med. Rehabil. 2024, 70, 4–16. [Google Scholar] [CrossRef]
- De Lorenzis, E.; Natalello, G.; Simon, D.; Schett, G.; D’Agostino, M.A. Concepts of Entheseal Pain. Arthritis Rheumatol. 2023, 75, 493–498. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, Y.-K.; Lee, Y.J.; Shin, S.; Kang, Y.; Koo, K.-H. Deep gluteal syndrome as a cause of posterior hip pain and sciatica-like pain. Bone Jt. J. 2020, 102, 556–567. [Google Scholar] [CrossRef]
- Kulaklı, S.; Atakan, N.; Çağlayan, G.; Akıncı, A.; Özçakar, L. From a dermatologist point of view, enthesopathy and peripheral neuropathy in psoriasis patients. Arch. Curr. Med. Res. 2022, 3, 193–198. [Google Scholar] [CrossRef]
- Battaglia, P.J.; Mattox, R.; Haun, D.W.; Welk, A.B.; Kettner, N.W. Dynamic Ultrasonography of the Deep External Rotator Musculature of the Hip: A Descriptive Study. PM&R 2016, 8, 640–650. [Google Scholar] [CrossRef]
- Koulouris, G.; Connell, D. Hamstring muscle complex: An imaging review. Radiographics 2005, 25, 571–586. [Google Scholar] [CrossRef]
- Lempainen, L.; Johansson, K.; Banke, I.J.; Ranne, J.; Mäkelä, K.; Sarimo, J.; Niemi, P.; Orava, S. Expert opinion: Diagnosis and treatment of proximal hamstring tendinopathy. Muscles Ligaments Tendons J. 2015, 5, 23–28. [Google Scholar] [CrossRef]
- Kulcu, D.G.; Naderi, S. Differential diagnosis of intraspinal and extraspinal non-discogenic sciatica. J. Clin. Neurosci. 2008, 15, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, K.; Uchida, S.; Shanmugaraj, A.; Aquino, C.C.; Duong, A.; Simunovic, N.; Martin, H.D.; Ayeni, O.R. Deep gluteal syndrome is defined as a non-discogenic sciatic nerve disorder with entrapment in the deep gluteal space: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Ombregt, L. A System of Orthopaedic Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ropper, A.H.; Zafonte, R.D. Sciatica. N. Engl. J. Med. 2015, 372, 1240–1248. [Google Scholar] [CrossRef]
- Kobayashi, S.; Shizu, N.; Suzuki, Y.; Asai, T.; Yoshizawa, H. Changes in nerve root motion and intraradicular blood flow during an intraoperative straight-leg-raising test. Spine 2003, 28, 1427–1434. [Google Scholar] [CrossRef]
- Hermosura, J.; Lohman, E., 3rd; Bartnik-Olson, B.; Venezia, J.; Daher, N. The usage of a modified straight-leg raise neurodynamic test and hamstring flexibility for diagnosis of non-specific low back pain: A cross-sectional study. PLoS ONE 2024, 19, e0298257. [Google Scholar] [CrossRef]
- Robinson, D.R. Pyriformis syndrome in relation to sciatic pain. Am. J. Surg. 1947, 73, 355–358. [Google Scholar] [CrossRef]
- Martin, H.D.; Khoury, A.; Schröder, R.; Palmer, I.J. Ischiofemoral Impingement and Hamstring Syndrome as Causes of Posterior Hip Pain: Where Do We Go Next? Clin. Sports Med. 2016, 35, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.W.; Petersen, C.; Falconer, J. An examination of Cyriax’s passive motion tests with patients having osteoarthritis of the knee. Phys. Ther. 1994, 74, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.D.; Kivlan, B.R.; Palmer, I.J.; Martin, R.L. Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 882–888. [Google Scholar] [CrossRef]
- Meknas, M.K. Retro-Trochanteric Sciatica-like Pain: In-Depth Analyses of Clinical Symptoms, Treatment Options, Histological and Ultra Structural Findings in Tendon Biopsies. Ph.D. Thesis, University of Tromsø, Tromsø, Norway, 2010. [Google Scholar]
- Filler, A.G.; Haynes, J.; Jordan, S.E.; Prager, J.; Villablanca, J.P.; Farahani, K.; McBride, D.Q.; Tsuruda, J.S.; Morisoli, B.; Batzdorf, U.; et al. Sciatica of nondisc origin and piriformis syndrome: Diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J. Neurosurg. Spine 2005, 2, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Kean Chen, C.; Nizar, A.J. Prevalence of piriformis syndrome in chronic low back pain patients. A clinical diagnosis with modified FAIR test. Pain Pract. 2013, 13, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Cyriax, J. Textbook of Orthopaedic Medicine; Baillière, Tindall & Cassell: London, UK, 1975; 756p. [Google Scholar]
- Gonzalez-Lomas, G. Deep Gluteal Pain in Orthopaedics: A Challenging Diagnosis. J. Am. Acad. Orthop. Surg. 2021, 29, e1282–e1290. [Google Scholar] [CrossRef]
- Bârzu, M. Diagnostic Methods in Piriformis Syndrome. Timis. Phys. Educ. Rehabil. J. 2013, 6, 22–28. [Google Scholar] [CrossRef]
- van der Windt, D.A.; Simons, E.; Riphagen, I.I.; Ammendolia, C.; Verhagen, A.P.; Laslett, M.; Devillé, W.; Deyo, R.A.; Bouter, L.M.; de Vet, H.C.; et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst. Rev. 2010, 17, Cd007431. [Google Scholar] [CrossRef]
- Homayouni, K.; Jafari, S.H.; Yari, H. Sensitivity and Specificity of Modified Bragard Test in Patients With Lumbosacral Radiculopathy Using Electrodiagnosis as a Reference Standard. J. Chiropr. Med. 2018, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R. Hip Pain in Adults: Evaluation and Differential Diagnosis. Am. Fam. Physician 2021, 103, 81–89. [Google Scholar]
- Fishman, L.M.; Dombi, G.W.; Michaelsen, C.; Ringel, S.; Rozbruch, J.; Rosner, B.; Weber, C. Piriformis syndrome: Diagnosis, treatment, and outcome—A 10-year study. Arch. Phys. Med. Rehabil. 2002, 83, 295–301. [Google Scholar] [CrossRef]
- Kirschner, J.S.; Foye, P.M.; Cole, J.L. Piriformis syndrome, diagnosis and treatment. Muscle Nerve 2009, 40, 10–18. [Google Scholar] [CrossRef]
- Wilson, J.J.; Furukawa, M. Evaluation of the patient with hip pain. Am. Fam. Physician 2014, 89, 27–34. [Google Scholar] [PubMed]
- Hernando, M.F.; Cerezal, L.; Pérez-Carro, L.; Abascal, F.; Canga, A. Deep gluteal syndrome: Anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal. Radiol. 2015, 44, 919–934. [Google Scholar] [CrossRef]
- Filler, A.G.; Howe, F.A.; Hayes, C.E.; Kliot, M.; Winn, H.R.; Bell, B.A.; Griffiths, J.R.; Tsuruda, J.S. Magnetic resonance neurography. Lancet 1993, 341, 659–661. [Google Scholar] [CrossRef]
- Filippucci, E.; Aydin, S.Z.; Karadag, O.; Salaffi, F.; Gutierrez, M.; Direskeneli, H.; Grassi, W. Reliability of high-resolution ultrasonography in the assessment of Achilles tendon enthesopathy in seronegative spondyloarthropathies. Ann. Rheum. Dis. 2009, 68, 1850–1855. [Google Scholar] [CrossRef]
- Gandjbakhch, F.; Terslev, L.; Joshua, F.; Wakefield, R.J.; Naredo, E.; D’Agostino, M.A. Ultrasound in the evaluation of enthesitis: Status and perspectives. Arthritis Res. Ther. 2011, 13, R188. [Google Scholar] [CrossRef]
- Martin, H.D.; Gómez-Hoyos, J. Deep gluteal syndrome. In Posterior Hip Disorders: Clinical Evaluation and Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 167–187. [Google Scholar]
- Ellis, R.F.; Hing, W.A.; McNair, P.J. Comparison of longitudinal sciatic nerve movement with different mobilization exercises: An in vivo study utilizing ultrasound imaging. J. Orthop. Sports Phys. Ther. 2012, 42, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Tsutsumi, M.; Kawanishi, K.; Wada, M.; Kudo, S. Effects of Radial Extracorporeal Shockwave Therapy on Piriformis Syndrome: A Single-Case Experimental Design. Cureus 2024, 16, e61873. [Google Scholar] [CrossRef] [PubMed]
- Hopayian, K.; Mirzaei, M.; Shamsi, M.; Arab-Zozani, M. A systematic review of conservative and surgical treatments for deep gluteal syndrome. J. Bodyw. Mov. Ther. 2023, 36, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.S.; Lin, C.H.; Chiang, C.H.; Wu, C.Y. Ultrasound-Guided Sciatic Nerve Hydrodissection Can Improve the Clinical Outcomes of Patients with Deep Gluteal Syndrome: A Case-Series Study. Diagnostics 2024, 14, 757. [Google Scholar] [CrossRef]
- Trescot, A. Everything old is new again: New developments in prolotherapy. Tech. Reg. Anesth. Pain Manag. 2015, 19, 14–18. [Google Scholar] [CrossRef]
- Hauser, R.A.; Lackner, J.B.; Steilen-Matias, D.; Harris, D.K. A Systematic Review of Dextrose Prolotherapy for Chronic Musculoskeletal Pain. Clin. Med. Insights Arthritis Musculoskelet Disord. 2016, 9, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Shin, H.Y.; Koo, G.H.; Park, H.G.; Ha, Y.C.; Park, Y.H. Ultrasound-guided Prolotherapy with Polydeoxyribonucleotide Sodium in Ischiofemoral Impingement Syndrome. Pain Pract. 2014, 14, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Metikala, S.; Sharma, V. Endoscopic Sciatic Neurolysis for Deep Gluteal Syndrome: A Systematic Review. Cureus 2022, 14, e23153. [Google Scholar] [CrossRef]
- Martin, H.D.; Shears, S.A.; Johnson, J.C.; Smathers, A.M.; Palmer, I.J. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy 2011, 27, 172–181. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Banke, I.J.; Schauwecker, J.; Gerdesmeyer, L.; Suren, C. How to address ischiofemoral impingement? Treatment algorithm and review of the literature. J. Hip Preserv. Surg. 2017, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]


| No. | Clinical Tests | Clinical Subtype | Sensitivity | Specificity | LoE | Title | Reference | Year | Journal |
|---|---|---|---|---|---|---|---|---|---|
| 1 | straight leg test (SLRT) | discogenic radiculopathy | 28% (95% CI: 22 to 35) | 90% (95% CI: 85 to 94) | 1a | Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain | [40] | 2010 | Cochrane Database Syst. Rev. |
| 2 | straight leg test (SLRT) | discogenic radiculopathy | 0.15 (0.05 to 0.33) | 0.95 (0.68 to 1.00) | 4 | Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region | [33] | 2014 | Knee Surg. Sports Traumatol. Arthrosc. |
| 3 | straight leg test (SLRT) | discogenic radiculopathy | 62.24% (56.75–67.72) | 45.91% (38.66–53.20) | 4 | Sensitivity and Specificity of Modified Bragard Test in Patients with Lumbosacral Radiculopathy Using Electrodiagnosis as a Reference Standard | [41] | 2018 | J. Chiropr. Med. |
| 4 | Modified Straight Leg Raise Test (mSLRT) | discogenic radiculopathy | Not reported | Not reported | 5 | Frequently described in clinical practice, but no validated diagnostic accuracy studies are available; primarily supported by expert opinion. | |||
| 5 | Bowstring Test | discogenic radiculopathy | Not reported | Not reported | 5 | Historically reported in the orthopedic literature; however, no standardized sensitivity or specificity data exist, and evidence is limited to descriptive accounts. | |||
| 6 | Seated piriformis stretching | piriformis | 0.52 (0.33 to 0.71) | 0.90 (0.60 to 0.98) | 4 | Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region | [33] | 2014 | Knee Surg. Sports Traumatol. Arthrosc. |
| 7 | Seated piriformis stretching | piriformis | 91% | 80% | 5 | Hip Pain in Adults: Evaluation and Differential Diagnosis | [42] | 2021 | Am. Fam. Physician |
| 8 | Seated piriformis stretching | piriformis | 52% | 90% | 5 | Deep Gluteal Syndrome: A Pain in the Buttock | [16] | 2021 | Curr. Sports Med. Rep. |
| 9 | Freiberg Sign | piriformis | Not reported | Not reported | 5 | -Commonly used to assess piriformis-related pain yet lacks quantitative validation; diagnostic accuracy remains based on anecdotal and expert reports. (LoE: 5) | |||
| 10 | Flexion Adduction Internal Rotation (FAIR) | piriformis, Gemelli-Obturator Internus Complex | 69.6% (372) | 59.9% (147) | 4 | Piriformis syndrome: diagnosis, treatment, and outcome—a 10-year study | [43] | 2002 | Arch. Phys. Med. Rehabil. |
| 11 | Flexion Adduction Internal Rotation (FAIR) | piriformis, Gemelli-Obturator Internus Complex | 59% to 100% | 4% to 75% | 5 | Hip Pain in Adults: Evaluation and Differential Diagnosis | [42] | 2021 | Am. Fam. Physician |
| 12 | Flexion Adduction Internal Rotation (FAIR) | piriformis, Gemelli-Obturator Internus Complex | 88.10% | 83.20% | 5 | Deep Gluteal Syndrome: A Pain in the Buttock | [16] | 2021 | Curr. Sports Med. Rep. |
| 13 | Flexion Adduction Internal Rotation (FAIR) | piriformis, Gemelli-Obturator Internus Complex | 85.00% | 82.00% | 5 | Piriformis syndrome, diagnosis and treatment | [44] | 2009 | Muscle Nerve |
| 14 | Beatty sign | piriformis | Not reported | Not reported | 5 | -Widely cited in textbooks and case reports, but no formal studies evaluating sensitivity or specificity; current use relies on clinical tradition rather than validated evidence. (LoE: 5) | |||
| 15 | Pace’s test | piriformis | Not reported | Not reported | 5 | Frequently performed in clinical settings to evaluate piriformis involvement; nonetheless, diagnostic performance has not been systematically validated and rests on expert opinion. (LoE: 5) | |||
| 16 | Modified Beatty Sign | piriformis | Not reported | Not reported | 5 | Proposed as a variant of the Beatty test; described in limited clinical reports without supporting accuracy studies; evidence remains anecdotal. (LoE: 5) | |||
| 17 | Active piriformis test | piriformis | 0.78 (0.58 to 0.90) | 0.80 (0.49 to 0.94) | 4 | Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region | [33] | 2014 | Knee Surg. Sports Traumatol. Arthrosc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.H.; Hwang, J.H.; Lee, H.w.; Lee, M.; Park, C.; Lee, J.; Kim, S.; Lee, J.; de Castro, J.C.; Lam, K.H.S.; et al. Beyond Nerve Entrapment: A Narrative Review of Muscle–Tendon Pathologies in Deep Gluteal Syndrome. Diagnostics 2025, 15, 2531. https://doi.org/10.3390/diagnostics15192531
Yoon YH, Hwang JH, Lee Hw, Lee M, Park C, Lee J, Kim S, Lee J, de Castro JC, Lam KHS, et al. Beyond Nerve Entrapment: A Narrative Review of Muscle–Tendon Pathologies in Deep Gluteal Syndrome. Diagnostics. 2025; 15(19):2531. https://doi.org/10.3390/diagnostics15192531
Chicago/Turabian StyleYoon, Yong Hyun, Ji Hyo Hwang, Ho won Lee, MinJae Lee, Chanwool Park, Jonghyeok Lee, Seungbeom Kim, JaeYoung Lee, Jeimylo C. de Castro, King Hei Stanley Lam, and et al. 2025. "Beyond Nerve Entrapment: A Narrative Review of Muscle–Tendon Pathologies in Deep Gluteal Syndrome" Diagnostics 15, no. 19: 2531. https://doi.org/10.3390/diagnostics15192531
APA StyleYoon, Y. H., Hwang, J. H., Lee, H. w., Lee, M., Park, C., Lee, J., Kim, S., Lee, J., de Castro, J. C., Lam, K. H. S., Suryadi, T., & Youn, K. H. (2025). Beyond Nerve Entrapment: A Narrative Review of Muscle–Tendon Pathologies in Deep Gluteal Syndrome. Diagnostics, 15(19), 2531. https://doi.org/10.3390/diagnostics15192531








