Assessing REM Sleep as a Biomarker for Depression Using Consumer Wearables
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
- A binary MLP (Multi-Layer Perceptron) classifier first detects sleep versus wake epochs (wake/sleep).
- Epochs classified as sleep are then processed by a multi-class classifier (also MLP) to identify REM versus NREM sleep stages (REM/NREM).
3. Results
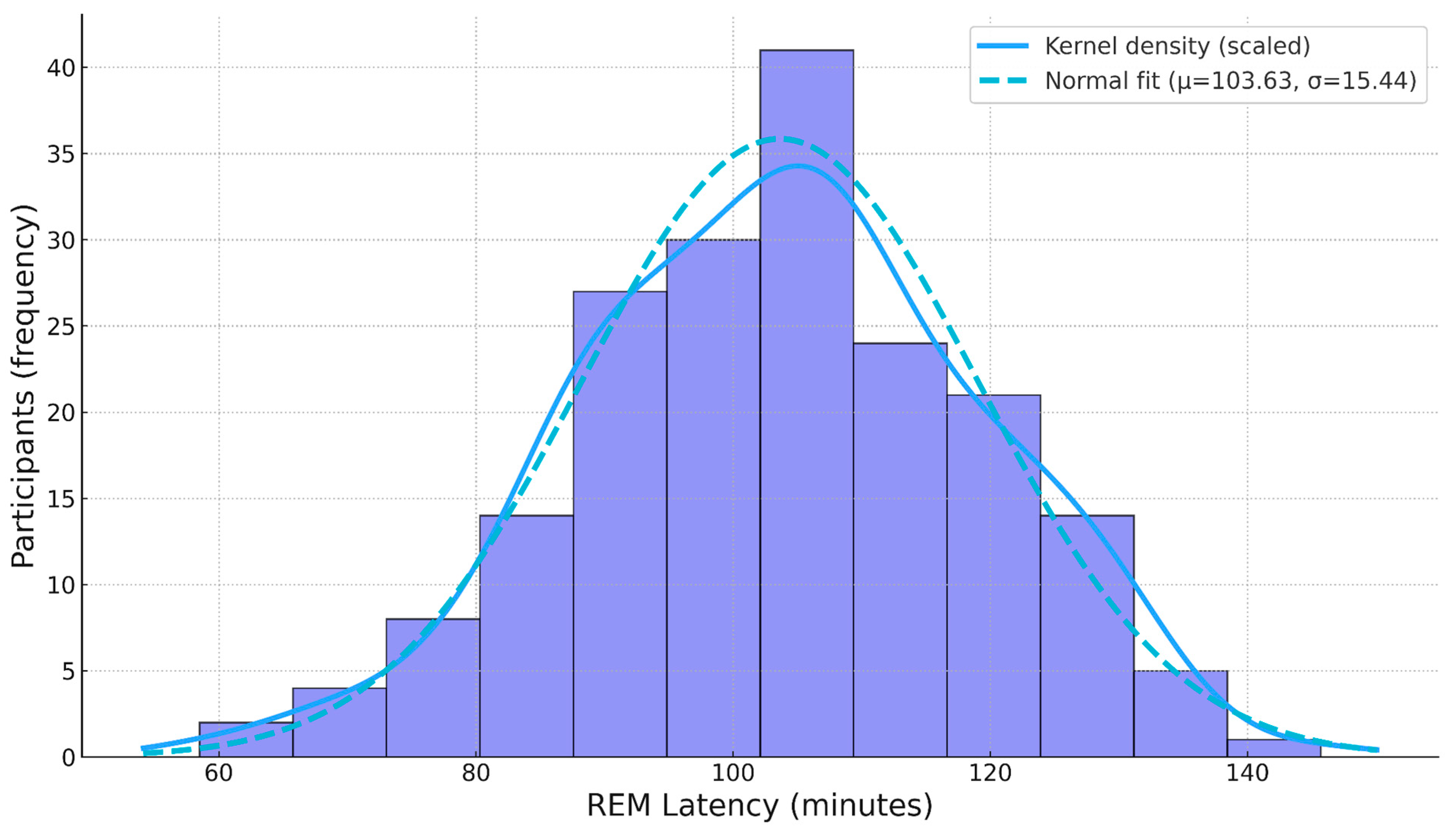
3.1. Descriptive Statistics
3.2. Correlation Analysis
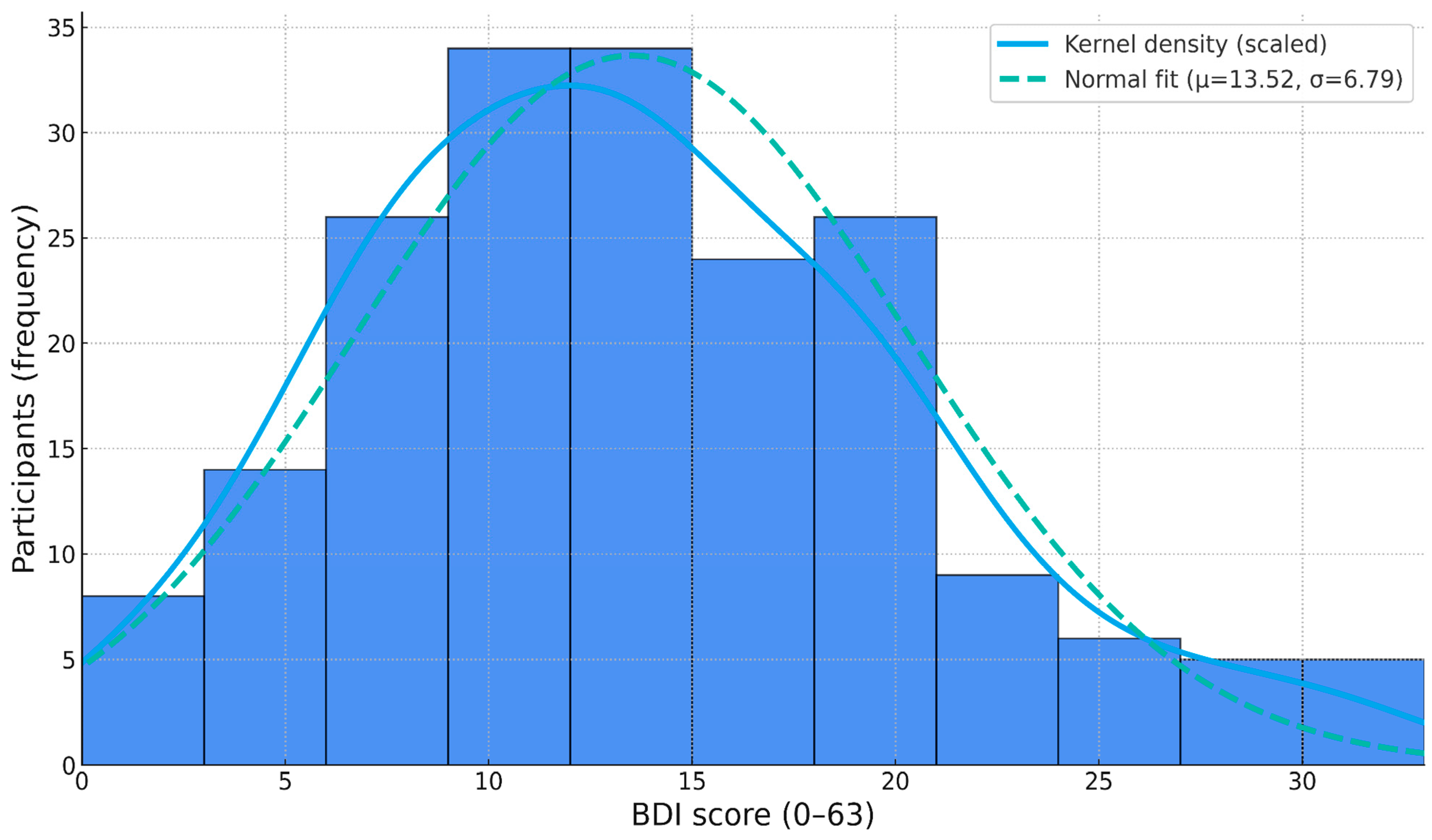
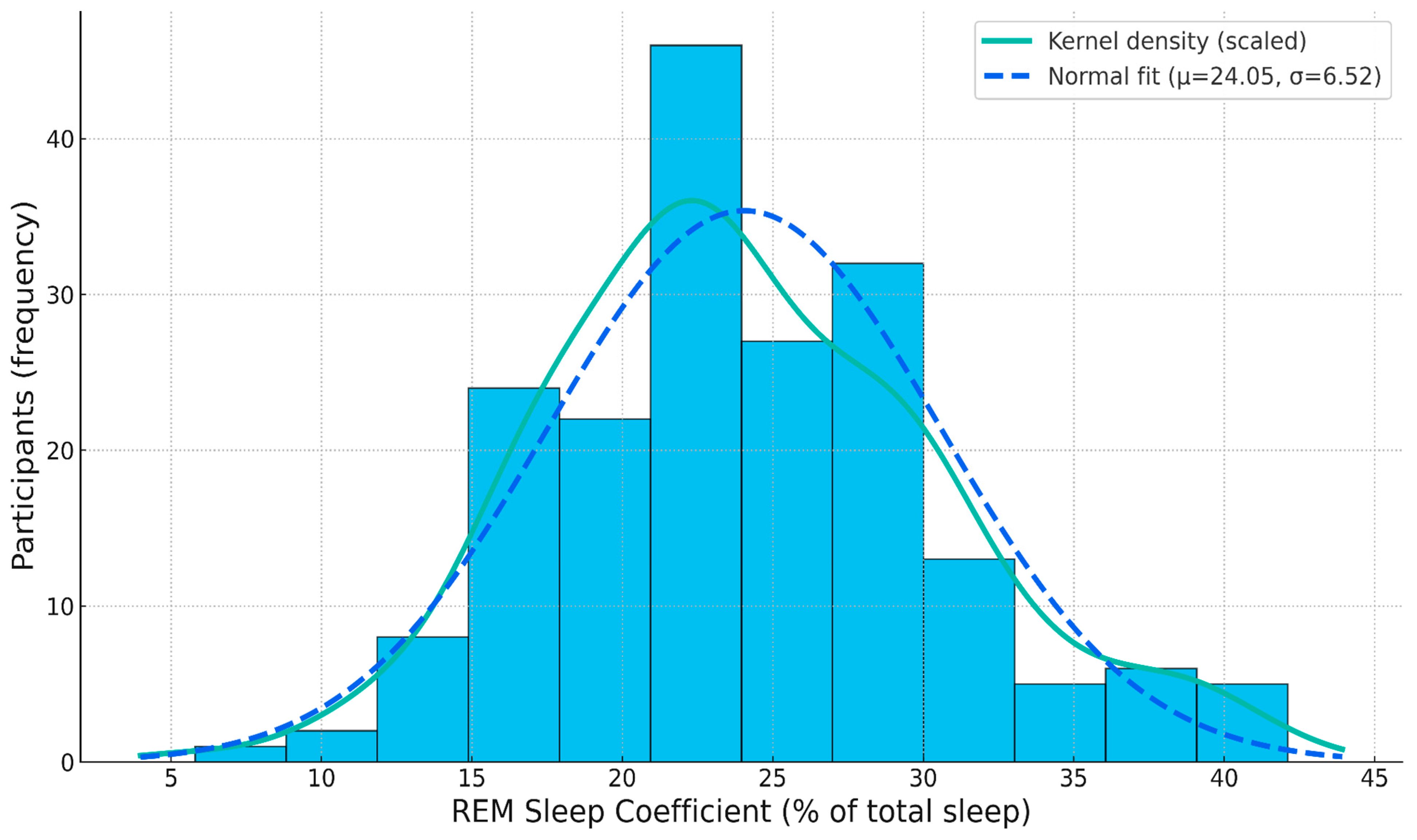
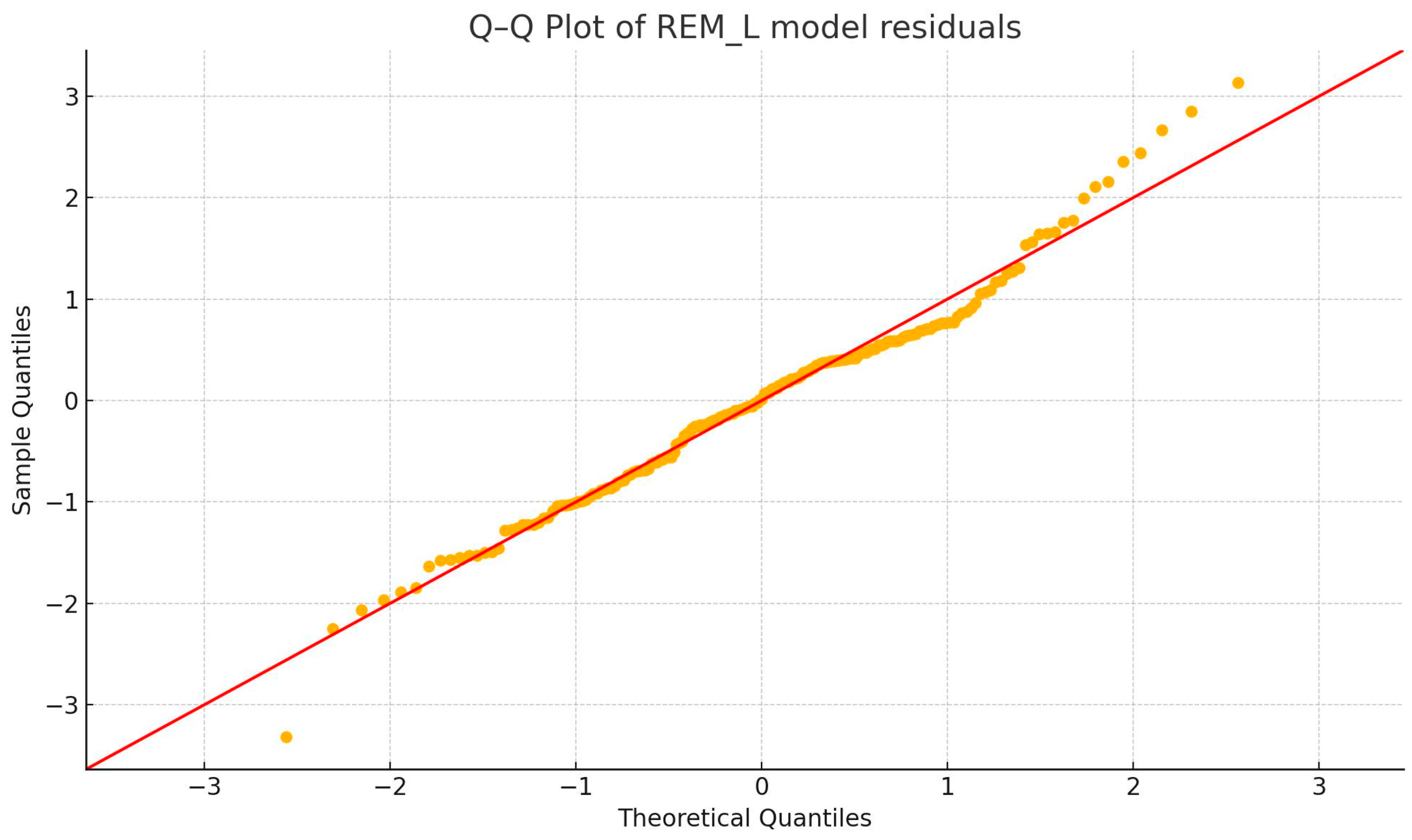
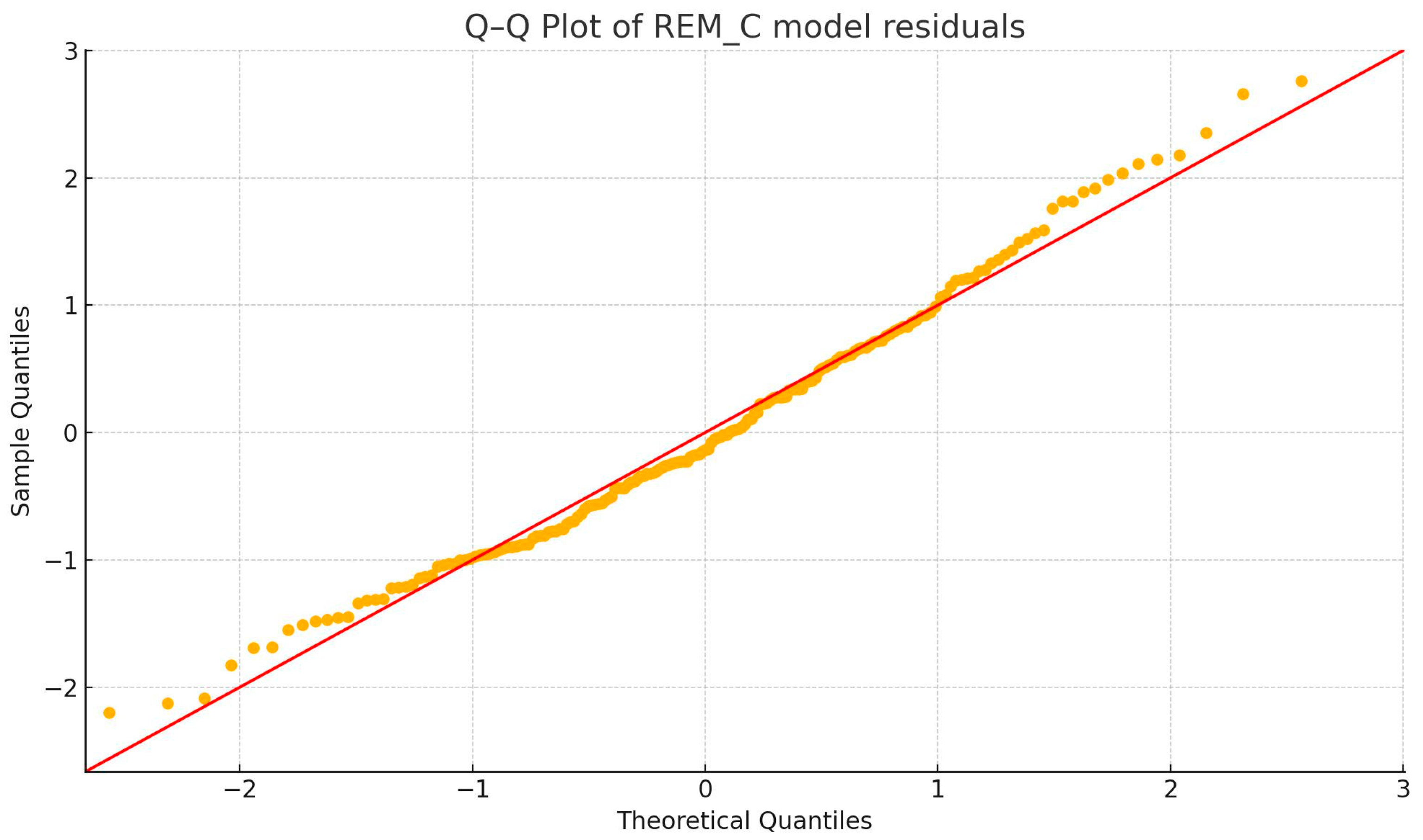
3.2.1. Outlier and Normality Tests
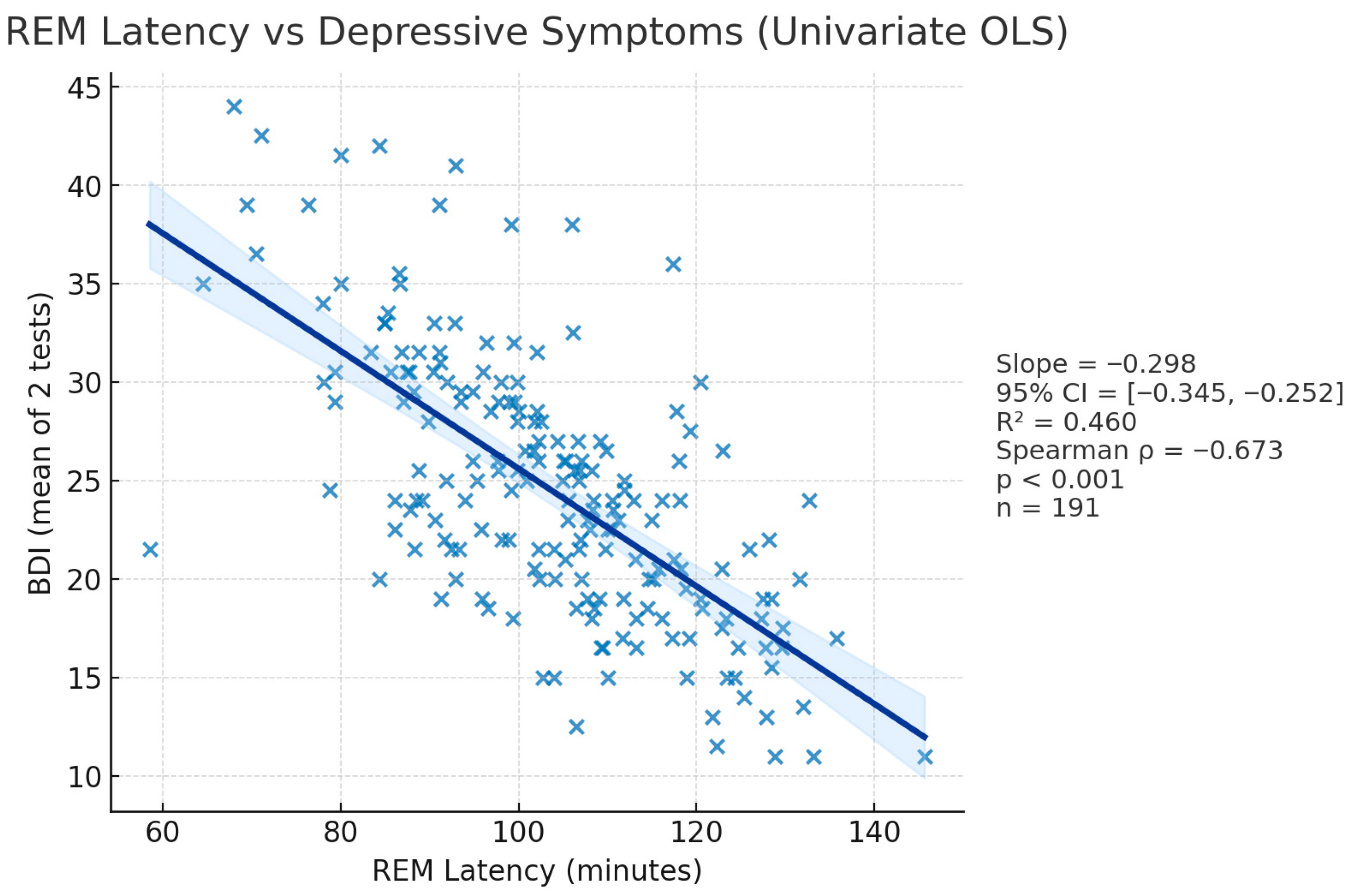
3.2.2. BDI—REM_L Correlation (Figure 4)
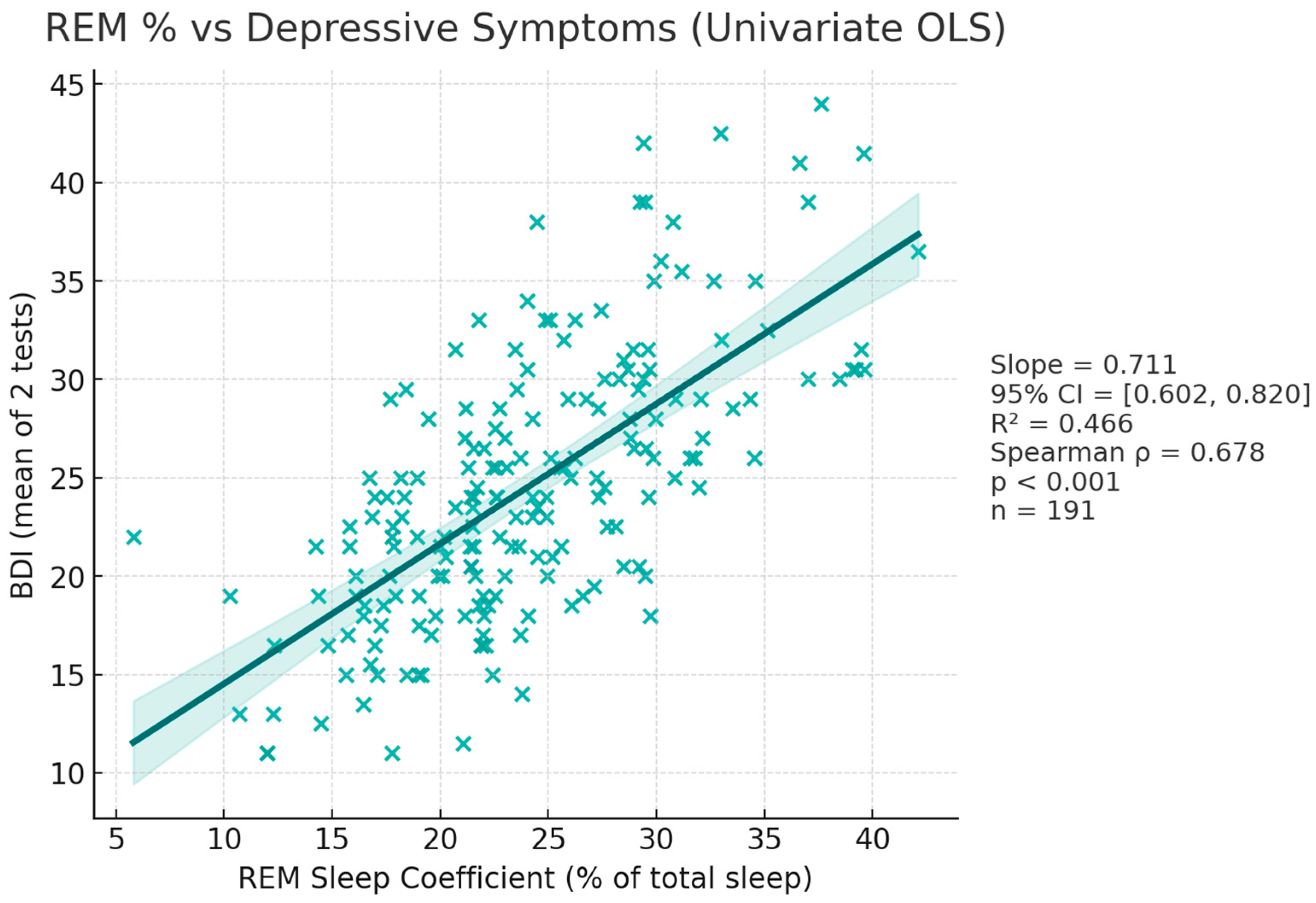
3.2.3. BDI—REM_C Correlation (Figure 5)
3.3. Linear Regression
3.4. Covariate Analysis
3.5. Multiple Testing Correction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falup-Pecurariu, C.; Diaconu Ștefania Țînț, D.; Falup-Pecurariu, O. Neurobiology of sleep (Review). Exp. Ther. Med. 2021, 21, 272. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7851648/ (accessed on 29 December 2023). [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist 2014, 20, 203–219. [Google Scholar] [CrossRef]
- Rasch, B.; Born, J. About Sleep’s Role in Memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef]
- Scammell, T.E.; Arrigoni, E.; Lipton, J. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- Hobson, J.A. Sleep and dreaming: Induction and mediation of REM sleep by cholinergic mechanisms. Curr. Opin. Neurobiol. 1992, 2, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Mendoza Alvarez, M.; Balthasar, Y.; Verbraecken, J.; Claes, L.; van Someren, E.; van Marle, H.J.F.; Vandekerckhove, M.; De Picker, L. Systematic review: REM sleep, dysphoric dreams and nightmares as transdiagnostic features of psychiatric disorders with emotion dysregulation—Clinical implications. Sleep Med. 2025, 127, 1–15. [Google Scholar] [CrossRef]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; 947p. [Google Scholar]
- Belzung, C.; Willner, P.; Philippot, P. Depression: From psychopathology to pathophysiology. Curr. Opin. Neurobiol. 2015, 30, 24–30. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 20 September 2024).
- Bains, N.; Abdijadid, S. Major Depressive Disorder. In StatPearls; StatPearls Publishing: Treasure Islands, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559078/ (accessed on 27 August 2025).
- Booij, S.H.; Bos, E.H.; Bouwmans, M.E.J.; van Faassen, M.; Kema, I.P.; Oldehinkel, A.J.; de Jonge, P. Cortisol and α-Amylase Secretion Patterns Between and Within Depressed and Non-Depressed Individuals. PLoS ONE 2015, 10, e0131002. [Google Scholar] [CrossRef] [PubMed]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Carniel, B.P.; da Rocha, N.S. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110151. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef]
- Palagini, L.; Baglioni, C.; Ciapparelli, A.; Gemignani, A.; Riemann, D. REM sleep dysregulation in depression: State of the art. Sleep Med. Rev. 2013, 17, 377–390. [Google Scholar] [CrossRef]
- Crișan, C.A.; Stretea, R.; Bonea, M.; Fîntînari, V.; Țața, I.M.; Stan, A.; Micluția, I.V.; Cherecheș, R.M.; Milhem, Z. Deciphering the Link: Correlating REM Sleep Patterns with Depressive Symptoms via Consumer Wearable Technology. J. Pers. Med. 2024, 14, 519. [Google Scholar] [CrossRef]
- Rykov, Y.; Thach, T.Q.; Bojic, I.; Christopoulos, G.; Car, J. Digital Biomarkers for Depression Screening with Wearable Devices: Cross-sectional Study with Machine Learning Modeling. JMIR Mhealth Uhealth 2021, 9, e24872. [Google Scholar] [CrossRef]
- APA. Beck Depression Inventory (BDI). Available online: https://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/beck-depression (accessed on 7 January 2024).
- Arıkan, M.K.; Uysal, Ö.; Gıca, Ş.; Orhan, Ö.; İlhan, R.; Esmeray, M.T.; Bakay, H.; Metin, B.; Pogarell, O.; Turan, Ş. REM parameters in drug-free major depressive disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 73, 101876. [Google Scholar] [CrossRef] [PubMed]
- Ricciardiello, A.; Teh, J.Z.; Lam, A.K.F.; Marshall, N.S.; Naismith, S.L.; D’Rozario, A.L. Objective measures of sleep in adults and older adults with and without depression: A systematic review and meta-analysis. Sleep Med. 2024, 124, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Leitner, C.; Dalle Piagge, F.; Tomic, T.; Nozza, F.; Fasiello, E.; Castronovo, V.; De Gennaro, L.; Baglioni, C.; Ferini-Strambi, L.; Galbiati, A. Sleep alterations in major depressive disorder and insomnia disorder: A network meta-analysis of polysomnographic studies. Sleep Med. Rev. 2025, 80, 102048. [Google Scholar] [CrossRef]
- Goldstein, A.N.; Walker, M.P. The Role of Sleep in Emotional Brain Function. Ann. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef]
- van der Helm, E.; Yao, J.; Dutt, S.; Rao, V.; Saletin, J.M.; Walker, M.P. REM sleep de-potentiates amygdala activity to previous emotional experiences. Curr. Biol. 2011, 21, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Vogel, G.W.; Vogel, F.; McAbee, R.S.; Thurmond, A.J. Improvement of depression by REM sleep deprivation. New findings and a theory. Arch. Gen. Psychiatry 1980, 37, 247–253. [Google Scholar] [CrossRef]
- Cartwright, R.; Baehr, E.; Kirkby, J.; Pandi-Perumal, S.R.; Kabat, J. REM sleep reduction, mood regulation and remission in untreated depression. Psychiatry Res. 2003, 121, 159–167. [Google Scholar] [CrossRef]
- Crișan, C.A.; Milhem, Z.; Stretea, R.; Țața, I.M.; Cherecheș, R.M.; Micluția, I.V. A Narrative Review on REM Sleep Deprivation: A Promising Non-Pharmaceutical Alternative for Treating Endogenous Depression. J. Pers. Med. 2023, 13, 306. [Google Scholar] [CrossRef]
- Ioannou, M.; Wartenberg, C.; Greenbrook, J.T.V.; Larson, T.; Magnusson, K.; Schmitz, L.; Sjögren, P.; Stadig, I.; Szabó, Z.; Steingrimsson, S. Sleep deprivation as treatment for depression: Systematic review and meta-analysis. Acta Psychiatr. Scand. 2021, 143, 22–35. [Google Scholar] [CrossRef]
- Hu, B.; Liu, C.; Mou, T.; Luo, F.; Lv, T.; Qian, C.; Zhang, J.; Ye, M.; Liu, Z. Meta-Analysis of Sleep Deprivation Effects on Patients with Depression. Front. Psychiatry 2021, 12, 783091. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.K.; Kelly, S.J.; Adams, C.E.; Glazebrook, C. A systematic review of studies of depression prevalence in university students. J. Psychiatr. Res. 2013, 47, 391–400. [Google Scholar] [CrossRef]
- Chouraki, A.; Tournant, J.; Arnal, P.; Pépin, J.L.; Bailly, S. Objective multi-night sleep monitoring at home: Variability of sleep parameters between nights and implications for the reliability of sleep assessment in clinical trials. Sleep 2023, 46, zsac319. [Google Scholar] [CrossRef] [PubMed]
- Apple Inc. Estimating Sleep Stages from Apple Watch; Apple Inc.: Cupertino, CA, USA, 2023. [Google Scholar]
- Birrer, V.; Elgendi, M.; Lambercy, O.; Menon, C. Evaluating reliability in wearable devices for sleep staging. NPJ Digit. Med. 2024, 7, 74. [Google Scholar] [CrossRef]
- Walch, O.; Huang, Y.; Forger, D.; Goldstein, C. Sleep stage prediction with raw acceleration and photoplethysmography heart rate data derived from a consumer wearable device. Sleep 2019, 42, zsz180. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Cui, L.; Mueller, R.; Tao, S.; Kim, M.; Rueschman, M.; Mariani, S.; Mobley, D.; Redline, S. The National Sleep Research Resource: Towards a sleep data commons. J. Am. Med. Inf. Assoc. 2018, 25, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Deak, M.C.; Gault, D.; Goldstein, C.A.; Hwang, D.; Kwon, Y.; O’HEarn, D.; Schutte-Rodin, S.; Yurcheshen, M.; Rosen, I.M.; et al. Consumer Sleep Technology: An American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2018, 14, 877–880. [Google Scholar] [CrossRef]
- Baglioni, C.; Regen, W.; Teghen, A.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Riemann, D. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Med. Rev. 2014, 18, 195–213. [Google Scholar] [CrossRef]
- Xu, J.; Liu, N.; Polemiti, E.; Garcia-Mondragon, L.; Tang, J.; Liu, X.; Lett, T.; Yu, L.; Nöthen, M.M.; Feng, J.; et al. Effects of urban living environments on mental health in adults. Nat. Med. 2023, 29, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.M.; Drewnowski, A.; Andersen, M.R.; Rose, C.; Buszkiewicz, J.; Mou, J.; Ko, L.K. Investigating the effects of rurality on stress, subjective well-being, and weight-related outcomes. Wellbeing Space Soc. 2023, 5, 100171. [Google Scholar] [CrossRef]
- Svendsen, M.T.; Bak, C.K.; Sørensen, K.; Pelikan, J.; Riddersholm, S.J.; Skals, R.K.; Mortensen, R.N.; Maindal, H.T.; Bøggild, H.; Nielsen, G.; et al. Associations of health literacy with socioeconomic position, health risk behavior, and health status: A large national population-based survey among Danish adults. BMC Public Health 2020, 20, 565. [Google Scholar] [CrossRef]
- Nagappan, A.; Krasniansky, A.; Knowles, M. Patterns of Ownership and Usage of Wearable Devices in the United States, 2020-2022: Survey Study. J. Med. Internet Res. 2024, 26, e56504. [Google Scholar] [CrossRef]
- Floyd, J.A.; Janisse, J.J.; Jenuwine, E.S.; Ager, J.W. Changes in REM-Sleep Percentage Over the Adult Lifespan. Sleep 2007, 30, 829–836. [Google Scholar] [CrossRef]
- Van Cauter, E.; Leproult, R.; Plat, L. Age-Related Changes in Slow Wave Sleep and REM Sleep and Relationship with Growth Hormone and Cortisol Levels in Healthy Men. JAMA 2000, 284, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.B.; Kim, H.C.; Jeon, Y.J.; Jung, S.J. Association between socioeconomic status and longitudinal sleep quality patterns mediated by depressive symptoms. Sleep 2021, 44, zsab044. [Google Scholar] [CrossRef]
- Rao, U.; Hammen, C.L.; Poland, R.E. Risk Markers for Depression in Adolescents: Sleep and HPA Measures. Neuropsychopharmacology 2009, 34, 1936–1945. [Google Scholar] [CrossRef]
- Giles, D.E.; Jarrett, R.B.; Roffwarg, H.P.; Rush, A.J. Reduced rapid eye movement latency. A predictor of recurrence in depression. Neuropsychopharmacology 1987, 1, 33–39. [Google Scholar] [CrossRef]
- Adil, M.; Atiq, I.; Younus, S. Effectiveness of the Apple Watch as a mental health tracker. J. Glob. Health 2024, 14, 03010. [Google Scholar] [CrossRef]
- Olinic, M.-S.; Stretea, R.; Cherecheș, C. Wearables in ADHD: Monitoring and Intervention—Where Are We Now? Diagnostics 2025, 15, 2359. [Google Scholar] [CrossRef] [PubMed]
- Crișan, C.A.; Milhem, Z.; Stretea, R.; Hossu, R.M.; Florean, I.S.; Cherecheș, R.M. Coping Mechanisms during the War in Ukraine: A Cross-Sectional Assessment among Romanian Population. Healthcare 2023, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Crișan, C.A.; Pop, R.; Stretea, R.; Milhem, Z.; Forray, A.I. Coping strategies, resilience and quality of life: Reaction to the COVID-19 pandemic among Romanian physicians. Hum. Resour. Health 2024, 22, 28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stretea, R.; Milhem, Z.; Fîntînari, V.; Crișan, C.A.; Stan, A.; Petreuș, D.; Micluția, I.V. Assessing REM Sleep as a Biomarker for Depression Using Consumer Wearables. Diagnostics 2025, 15, 2498. https://doi.org/10.3390/diagnostics15192498
Stretea R, Milhem Z, Fîntînari V, Crișan CA, Stan A, Petreuș D, Micluția IV. Assessing REM Sleep as a Biomarker for Depression Using Consumer Wearables. Diagnostics. 2025; 15(19):2498. https://doi.org/10.3390/diagnostics15192498
Chicago/Turabian StyleStretea, Roland, Zaki Milhem, Vadim Fîntînari, Cătălina Angela Crișan, Alexandru Stan, Dumitru Petreuș, and Ioana Valentina Micluția. 2025. "Assessing REM Sleep as a Biomarker for Depression Using Consumer Wearables" Diagnostics 15, no. 19: 2498. https://doi.org/10.3390/diagnostics15192498
APA StyleStretea, R., Milhem, Z., Fîntînari, V., Crișan, C. A., Stan, A., Petreuș, D., & Micluția, I. V. (2025). Assessing REM Sleep as a Biomarker for Depression Using Consumer Wearables. Diagnostics, 15(19), 2498. https://doi.org/10.3390/diagnostics15192498





