Associations Between Regulatory Immune Cells, Thymus Cellular Remodeling, and Vascular Aging in Advanced Coronary Atherosclerosis: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Thymus Biopsy
2.3. Blood Processing
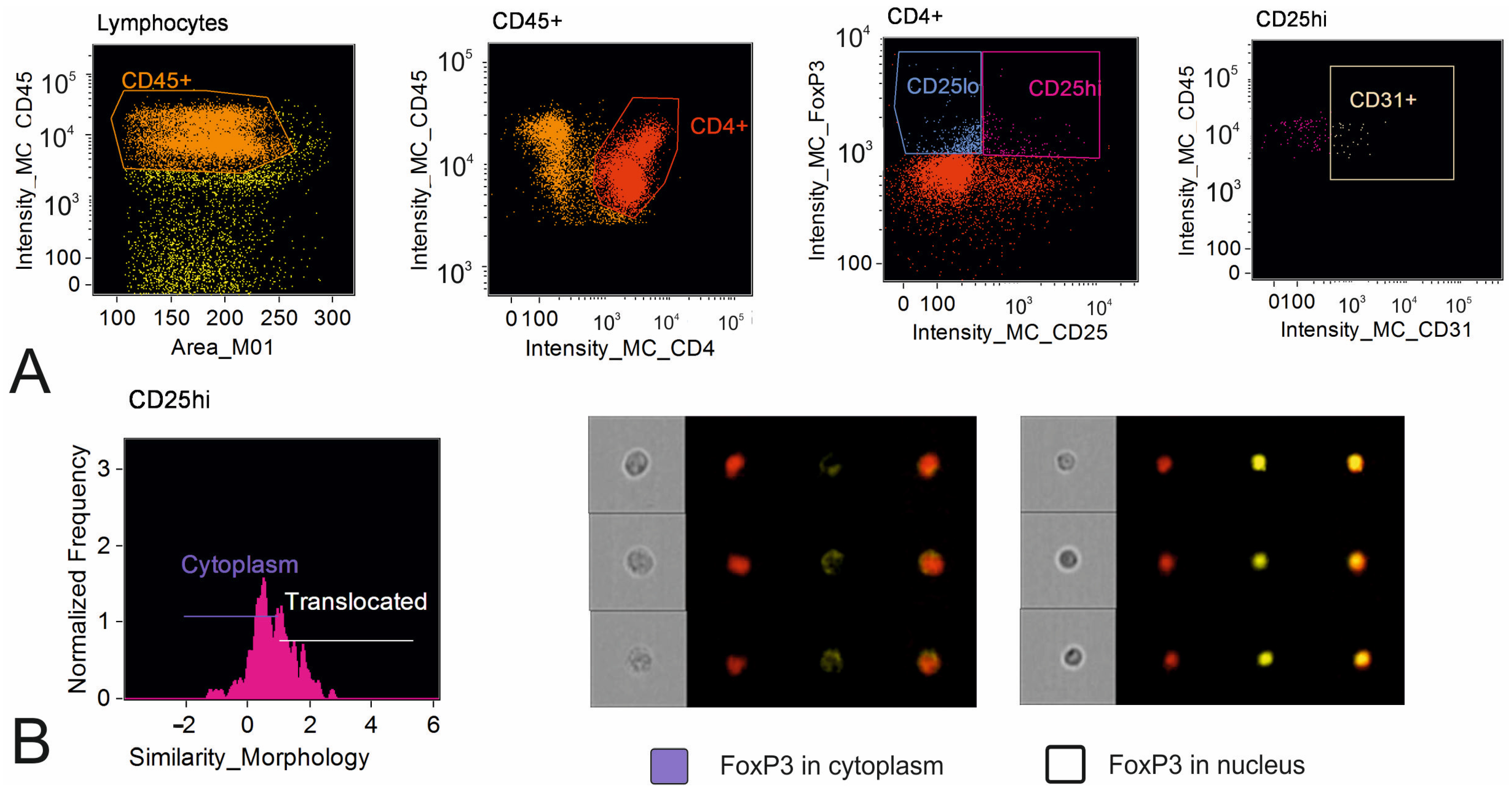
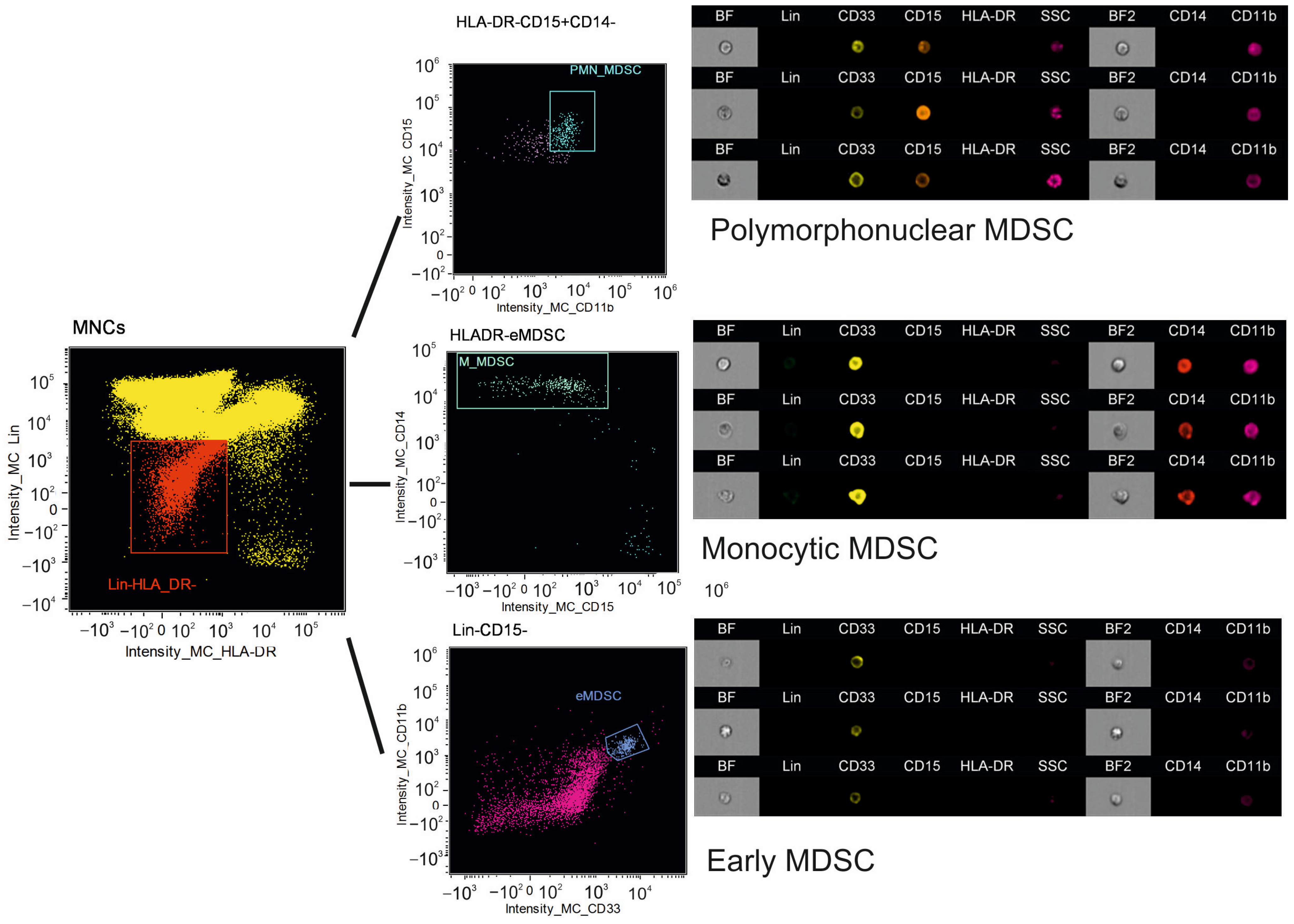
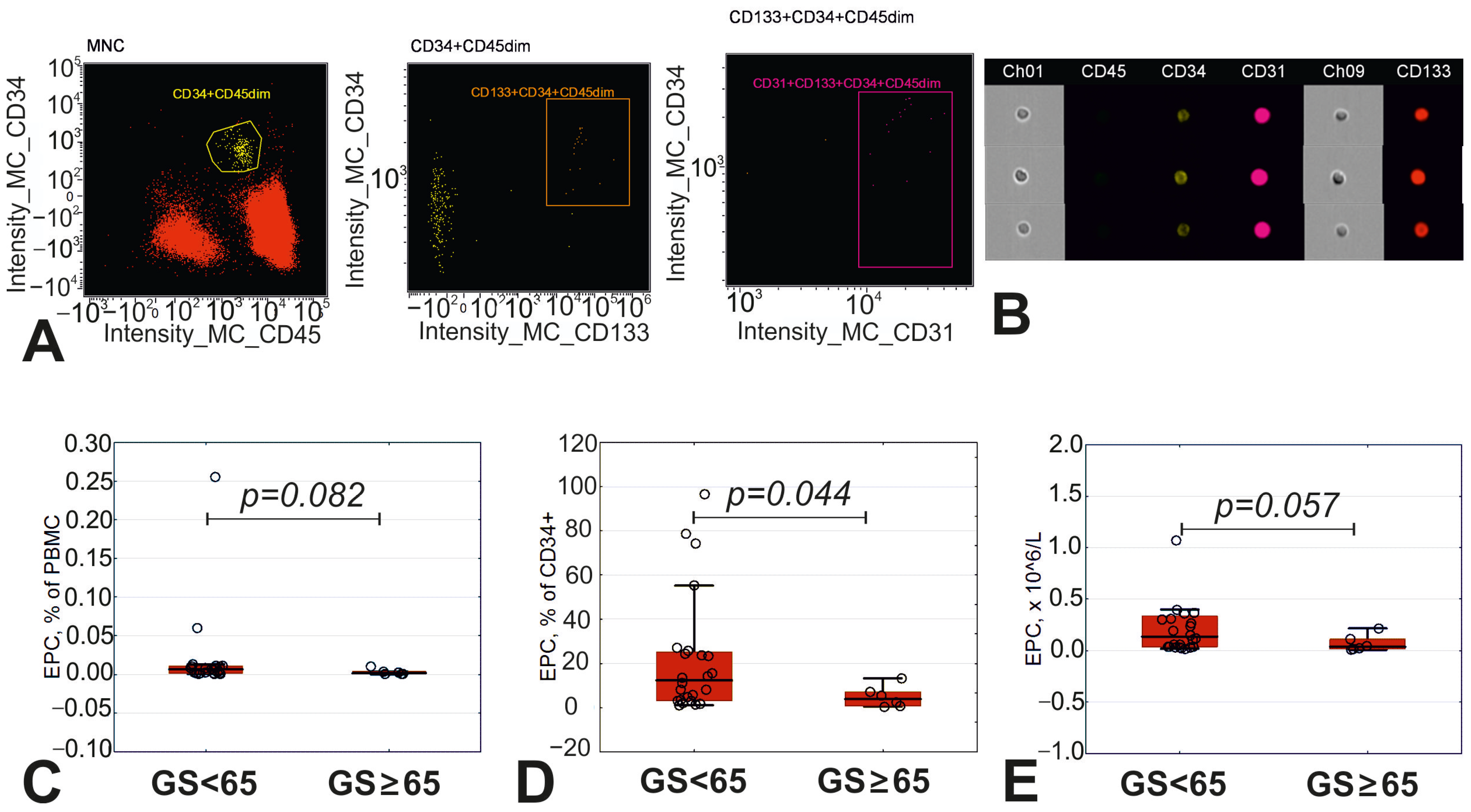
2.4. Imaging Flow Cytometry
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Biochemical Analysis
2.7. Multiplex Analysis of Cardiovascular Biomarkers
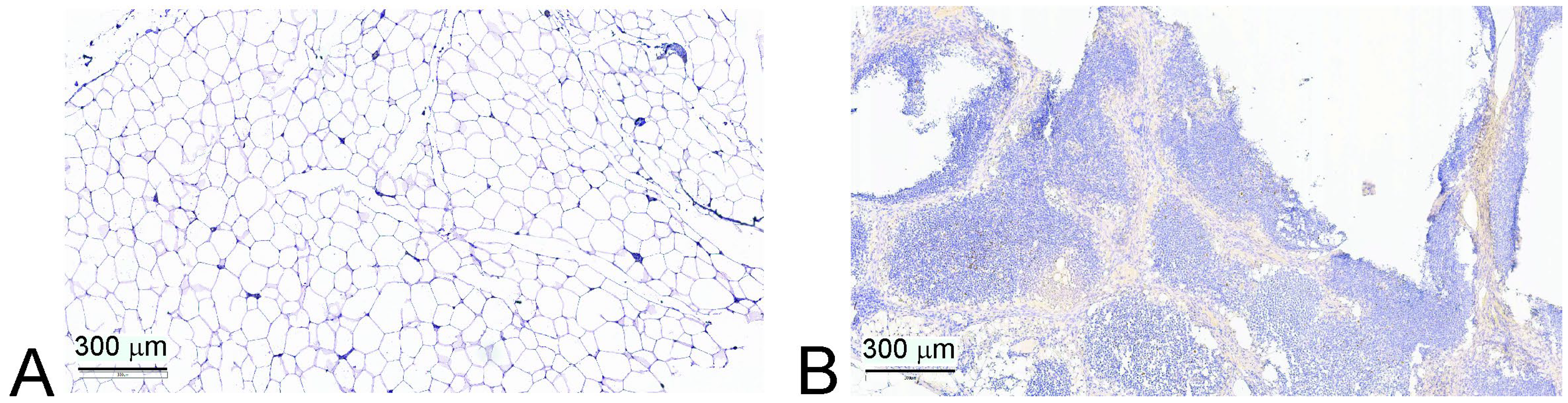
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Immune Regulatory Cells in Peripheral Blood
3.3. Circulating Endothelial Precursor Cells in Peripheral Blood
3.4. Immune Composition of Atrophied Thymus
3.5. Senescence Associated Molecular Pattern in Peripheral Blood
3.6. Degree of Atherosclerosis and Cells of Immune System in Patients with CAD
3.7. Vascular Aging Index and Immune Cells in Patients with CAD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CD | cluster of differentiation |
| cfPWV | carotid–femoral pulse wave velocity |
| EDTA | ethylenediamine tetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| eMDSC | early myeloid-derived suppressor cell |
| EPC | endothelial precursor cell |
| FoxP3 | Forkhead Box P3 |
| GS | Gensini score |
| IGF | insulin-like growth factor |
| IL | interleukin |
| IMT | intima–media thickness |
| MDSC | myeloid-derived suppressor cell |
| Me | median |
| M-MDSC | monocytic myeloid-derived suppressor cell |
| NK | natural killer |
| NKT | natural killer T lymphocyte |
| PBMC | peripheral blood mononuclear cell |
| PMN-MDSC | polymorphonuclear myeloid-derived suppressor cell |
| SVF | stromal vascular fraction |
| TCR | T cell receptor |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| Treg | T regulatory lymphocyte |
| VAI | vascular aging index |
References
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.Y.; Climie, R.E.; Shu, M.; Grieve, S.M.; Kozor, R.; Figtree, G.A. Vascular aging and cardiovascular disease: Pathophysiology and measurement in the coronary arteries. Front. Cardiovasc. Med. 2023, 10, 1206156. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: Moving from bench towards bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117, Erratum in Eur. J. Prev. Cardiol. 2023, 30, 1165. https://doi.org/10.1093/eurjpc/zwad134. [Google Scholar] [CrossRef]
- Wankumbu, S.C.; Ji, X.-M.; Xu, M. Decoding vascular aging: Implications for atherosclerosis progression and clinical intervention. Explor. Drug Sci. 2024, 2, 449–472. [Google Scholar] [CrossRef]
- Patino-Alonso, C.; Gómez-Sánchez, M.; Gómez-Sánchez, L.; Rodríguez-Sánchez, E.; Agudo-Conde, C.; García-Ortiz, L.; Gómez-Marcos, M.A. Diagnosing Vascular Aging Based on Macro and Micronutrients Using Ensemble Machine Learning. Mathematics 2023, 11, 1645. [Google Scholar] [CrossRef]
- Wadström, B.N.; Fatehali, A.H.; Engström, G.; Nilsson, P.M. A Vascular Aging Index as Independent Predictor of Cardiovascular Events and Total Mortality in an Elderly Urban Population. Angiology 2019, 70, 929–937. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial Stiffness and Hypertension in the Elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef]
- Barcena, M.L.; Aslam, M.; Pozdniakova, S.; Norman, K.; Ladilov, Y. Cardiovascular Inflammaging: Mechanisms and Translational Aspects. Cells 2022, 11, 1010. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. The role of myeloid-derived suppressor cells (MDSC) in the inflammaging process. Ageing Res. Rev. 2018, 48, 1–10. [Google Scholar] [CrossRef]
- Kologrivova, I.V.; Suslova, T.E.; Koshelskaya, O.A.; Kharitonova, O.A.; Trubacheva, O.A.; Kravchenko, E.S. Circulating FoxP3+ T-lymphocytes in chronic coronary artery disease: Associations with the severity of atherosclerosis and lipid metabolism. Sib. J. Clin. Exp. Med. 2021, 36, 45–51. [Google Scholar] [CrossRef]
- Pawelec, G.; Picard, E.; Bueno, V.; Verschoor, C.P.; Ostrand-Rosenberg, S. MDSCs, ageing and inflammageing. Cell Immunol. 2021, 62, 104297. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Palatella, M.; Guillaume, S.M.; Linterman, M.A.; Huehn, J. The dark side of Tregs during aging. Front. Immunol. 2022, 13, 940705. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, D.; Wang, C.; Wu, Z.; Liang, C. The Pivotal Role of Thymus in Atherosclerosis Mediated by Immune and Inflammatory Response. Int. J. Med. Sci. 2018, 15, 1555–1563. [Google Scholar] [CrossRef]
- Kologrivova, I.V.; Naryzhnaya, N.V.; Suslova, T.E. Thymus in Cardiometabolic Impairments and Atherosclerosis: Not a Silent Player? Biomedicines 2024, 12, 1408. [Google Scholar] [CrossRef]
- Srinivasan, J.; Lancaster, J.N.; Singarapu, N.; Hale, L.P.; Ehrlich, L.I.R.; Richie, E.R. Age-Related Changes in Thymic Central Tolerance. Front. Immunol. 2021, 12, 676236. [Google Scholar] [CrossRef]
- Gensini, G.G. A More Meaningful Scoring System for Determining the Severity of Coronary Heart Disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef]
- Kologrivova, I.V.; Dmitriukov, A.A.; Naryzhnaya, N.V.; Koshelskaya, O.A.; Kharitonova, O.A.; Vyrostkova, A.I.; Evtushenko, V.V.; Krapivina, A.S.; Riabchenko, P.E.; Suslova, T.E. Lymphocyte subsets in epicardial, thymic and subcutaneous adipose tissue during advanced coronary atherosclerosis in patients with coronary artery disease. Russ. J. Immunol. 2024, 27, 243–252. [Google Scholar] [CrossRef]
- Naryzhnaya, N.V.; Koshelskaya, O.A.; Kologrivova, I.V.; Suslova, T.E.; Kharitonova, O.A.; Andreev, S.L.; Gorbunov, A.S.; Kurbatov, B.K.; Boshchenko, A.A. Production of Reactive Oxygen Species by Epicardial Adipocytes Is Associated with an Increase in Postprandial Glycemia, Postprandial Insulin, and a Decrease in Serum Adiponectin in Patients with Severe Coronary Atherosclerosis. Biomedicines 2022, 10, 2054. [Google Scholar] [CrossRef]
- Pérez, M.R.; Vandenabeele, P.; Tougaard, P. The thymus road to a T cell: Migration, selection, and atrophy. Front. Immunol. 2024, 15, 1443910. [Google Scholar] [CrossRef]
- Huang, S.; Ding, R.; Lin, Y.; He, Z.; Wu, F.; Dai, X.; Chen, Y.; Gui, Y.; Huang, Z.; Wu, Z.; et al. Reduced T cell Thymic Export Reflected by sj-TREC in Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 632–643. [Google Scholar] [CrossRef]
- Baran-Gale, J.; Morgan, M.D.; Maio, S.; Dhalla, F.; Calvo-Asensio, I.; Deadman, M.E.; Handel, A.E.; Maynard, A.; Chen, S.; Green, F.; et al. Ageing Compromises Mouse Thymus Function and Remodels Epithelial Cell Differentiation. eLife 2020, 9, e56221. [Google Scholar] [CrossRef]
- Hester, A.K.; Semwal, M.K.; Cepeda, S.; Xiao, Y.; Rueda, M.; Wimberly, K.; Venables, T.; Dileepan, T.; Kraig, E.; Griffith, A.V. Redox Regulation of Age-Associated Defects in Generation and Maintenance of T Cell Self-Tolerance and Immunity to Foreign Antigens. Cell Rep. 2022, 38, 110363. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, R.; Gerdes, N.; Badeau, R.M.; Gisterå, A.; Strodthoff, D.; Ketelhuth, D.F.; Lundberg, A.M.; Rudling, M.; Nilsson, S.K.; Olivecrona, G.; et al. Depletion of FOXP3+ Regulatory T Cells Promotes Hypercholesterolemia and Atherosclerosis. J. Clin. Investig. 2013, 123, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, F.; Hu, X.; Hu, Y.; Wang, Y.; Zhang, W.; Peng, Y.; Cheng, L. Decreased Helios Expression in Regulatory T Cells in Acute Coronary Syndrome. Dis. Markers 2017, 2017, 7909407. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Park, H.J.; Choi, E.A.; Jung, K.C.; Lee, J.I. Cellular heterogeneity of circulating CD4+CD8+ double-positive T cells characterized by single-cell RNA sequencing. Sci. Rep. 2021, 11, 23607. [Google Scholar] [CrossRef]
- Kreslavsky, T. Thymflammation: The Role of a Constitutively Active Inflammatory Network and “Ectopic” Cell Types in the Thymus in the Induction of T Cell Tolerance and Beyond. Immunol. Rev. 2025, 332, e70037. [Google Scholar] [CrossRef]
- Kyaw, T.; Peter, K.; Li, Y.; Tipping, P.; Toh, B.H.; Bobik, A. Cytotoxic lymphocytes and atherosclerosis: Significance, mechanisms and therapeutic challenges. Br. J. Pharmacol. 2017, 174, 3956–3972. [Google Scholar] [CrossRef]
- de Jong, M.J.M.; Schaftenaar, F.H.; Depuydt, M.A.C.; Vigario, F.L.; Janssen, G.M.C.; Peeters, J.A.H.M.; Goncalves, L.; Wezel, A.; Smeets, H.J.; Kuiper, J.; et al. Virus-Associated CD8+ T-Cells Are Not Activated Through Antigen-Mediated Interaction Inside Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1302–1314. [Google Scholar] [CrossRef]
- Quinn, K.M.; Zaloumis, S.G.; Cukalac, T.; Kan, W.T.; Sng, X.Y.; Mirams, M.; Watson, K.A.; McCaw, J.M.; Doherty, P.C.; Thomas, P.G.; et al. Heightened self-reactivity associated with selective survival, but not expansion, of naïve virus-specific CD8+ T cells in aged mice. Proc. Natl. Acad. Sci. USA 2016, 113, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Pei, X.Y.; Shen, H.; Gao, Y.N.; Sun, X.Y.; Wang, W.; Ge, Q.; Zhang, Y. Thymic homing of activated CD4+ T cells induces degeneration of the thymic epithelium through excessive RANK signaling. Sci. Rep. 2017, 7, 2421. [Google Scholar] [CrossRef] [PubMed]
- Berahovich, R.D.; Lai, N.L.; Wei, Z.; Lanier, L.L.; Schall, T.J. Evidence for NK cell subsets based on chemokine receptor expression. J. Immunol. 2006, 177, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Calderón, L.; Boehm, T. Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proc. Natl. Acad. Sci. USA 2011, 108, 7517–7522. [Google Scholar] [CrossRef]
- Zhang, W.C.; Wang, J.; Shu, Y.W.; Tang, T.T.; Zhu, Z.F.; Xia, N.; Nie, S.F.; Liu, J.; Zhou, S.F.; Li, J.J.; et al. Impaired Thymic Export and Increased Apoptosis Account for Regulatory T Cell Defects in Patients with Non-ST Segment Elevation Acute Coronary Syndrome. J. Biol. Chem. 2012, 287, 34157–34166. [Google Scholar] [CrossRef]
- Zhan, Y.; Bourges, D.; Dromey, J.A.; Harrison, L.C.; Lew, A.M. The origin of thymic CD4+CD25+ regulatory T cells and their co-stimulatory requirements are determined after elimination of recirculating peripheral CD4+ cells. Int. Immunol. 2007, 19, 455–463. [Google Scholar] [CrossRef]
- Yang, E.; Zou, T.; Leichner, T.M.; Zhang, S.L.; Kambayashi, T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur. J. Immunol. 2014, 44, 2712–2720. [Google Scholar] [CrossRef]
- Santamaria, J.C.; Borelli, A.; Irla, M. Regulatory T Cell Heterogeneity in the Thymus: Impact on Their Functional Activities. Front. Immunol. 2021, 12, 643153. [Google Scholar] [CrossRef]
- Ao, Y.Q.; Jiang, J.H.; Gao, J.; Wang, H.K.; Ding, J.Y. Recent thymic emigrants as the bridge between thymoma and autoimmune diseases. Biochim. Biophys. Acta Rev. Cancer. 2022, 1877, 188730. [Google Scholar] [CrossRef]
- Magg, T.; Mannert, J.; Ellwart, J.W.; Schmid, I.; Albert, M.H. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur. J. Immunol. 2012, 42, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Kou, W.; Gu, J.; Wei, P.; Wu, X.; Peng, H.; Tao, J.; Yan, W.; Yang, X.; Lebid, A.; et al. TRAF6 directs FOXP3 localization and facilitates regulatory T-cell function through K63-linked ubiquitination. EMBO J. 2019, 38, e99766. [Google Scholar] [CrossRef] [PubMed]
- Kologrivova, I.V.; Dmitriukov, A.A.; Naryzhnaya, N.V.; Kravchenko, E.S.; Kharitonova, O.A.; Vyrostkova, A.I.; Krapivina, A.S.; Andreev, S.L.; Koshelskaya, O.A.; Suslova, T.E. Production of the Key Immunoregulatory Cytokines and the Content of FoxP3+ T-Regulatory Lymphocytes in the Epicardial and Thymus Adipose Tissue in Patients with Coronary Heart Disease. Bull. Exp. Biol. Med. 2024, 178, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, X.; Ji, C.; Liu, P.; Zhou, L.; Xu, D.; Wang, D.; Li, J.; Yu, J. Early myeloid-derived suppressor cells accelerate epithelial-mesenchymal transition by downregulating ARID1A in luminal A breast cancer. Front. Bioeng. Biotechnol. 2022, 10, 973731. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Yang, C.; Rong, R. The Crosstalk between Myeloid Derived Suppressor Cells and Immune Cells: To Establish Immune Tolerance in Transplantation. J. Immunol. Res. 2016, 2016, 4986797. [Google Scholar] [CrossRef]
- Bhat, D.K.; Olkhanud, P.B.; Gangaplara, A.; Seifuddin, F.; Pirooznia, M.; Biancotto, A.; Fantoni, G.; Pittman, C.; Francis, B.; Dagur, P.K.; et al. Early Myeloid Derived Suppressor Cells (eMDSCs) Are Associated With High Donor Myeloid Chimerism Following Haploidentical HSCT for Sickle Cell Disease. Front. Immunol. 2021, 30, 757279. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, M.; Wang, D.; Wang, Y.; Cheng, L. Th17/Treg balance is regulated by myeloid-derived suppressor cells in experimental autoimmune myocarditis. Immun. Inflamm. Dis. 2023, 11, e872. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, X.; Zhang, Z.; Zhang, Q.; Zhao, Y. Age-related thymic involution: Mechanisms and functional impact. Aging Cell 2022, 21, e13671. [Google Scholar] [CrossRef]
- Lee, C.R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.H.; Ye, M.B.; Lee, W.; Sim, K.Y.; Kang, J.A.; Kim, Y.C.; et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-β during Murine Colitis. Cell Rep. 2016, 17, 3219–3232. [Google Scholar] [CrossRef]
- Lauder, S.N.; Smart, K.; Bart, V.M.T.; Pires, A.; Scott, J.; Milutinovic, S.; Godkin, A.; Vanhasebroeck, B.; Gallimore, A. Treg-driven tumour control by PI3Kδ inhibition limits myeloid-derived suppressor cell expansion. Br. J. Cancer. 2022, 127, 1595–1602. [Google Scholar] [CrossRef]
- Pierini, A.; Nishikii, H.; Baker, J.; Kimura, T.; Kwon, H.S.; Pan, Y.; Chen, Y.; Alvarez, M.; Strober, W.; Velardi, A.; et al. Foxp3+ regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat. Commun. 2017, 8, 15068. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ke, Z.; Lyu, M.A.; Masarova, L.; Sadeghi, T.; Flowers, C.R.; Parmar, S. CXCR4-enriched T regulatory cells preferentially home to bone marrow and resolve inflammation. iScience 2024, 27, 110830. [Google Scholar] [CrossRef] [PubMed]
- Dudakov, J.A.; Khong, D.M.; Boyd, R.L.; Chidgey, A.P. Feeding the fire: The role of defective bone marrow function in exacerbating thymic involution. Trends Immunol. 2010, 31, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt-Clermont, P.J. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. Am. Heart J. 2003, 146, S5–S12. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Liu, T.; Zhu, X.; Pan, X. The multifaceted role of the SASP in atherosclerosis: From mechanisms to therapeutic opportunities. Cell Biosci. 2022, 12, 74. [Google Scholar] [CrossRef]
- Bach, L.A. Endothelial cells and the IGF system. J. Mol. Endocrinol. 2015, 54, R1–R13. [Google Scholar] [CrossRef]
- Wen, H.J.; Liu, G.F.; Xiao, L.Z.; Wu, Y.G. (Corrigendum) Involvement of endothelial nitric oxide synthase pathway in IGF-1 protects endothelial progenitor cells against injury from oxidized LDLs. Mol. Med. Rep. 2020, 21, 2284. [Google Scholar] [CrossRef]
- Veeranki, S.; Gandhapudi, S.K.; Tyagi, S.C. Interactions of hyperhomocysteinemia and T cell immunity in causation of hypertension. Can. J. Physiol. Pharmacol. 2017, 95, 239–246. [Google Scholar] [CrossRef]
- Brescia, C.; Audia, S.; Pugliano, A.; Scaglione, F.; Iuliano, R.; Trapasso, F.; Perrotti, N.; Chiarella, E.; Amato, R. Metabolic drives affecting Th17/Treg gene expression changes and differentiation: Impact on immune-microenvironment regulation. APMIS 2024, 132, 1026–1045. [Google Scholar] [CrossRef]
- Flores, R.R.; Clauson, C.L.; Cho, J.; Lee, B.C.; McGowan, S.J.; Baker, D.J.; Niedernhofer, L.J.; Robbins, P.D. Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-κB-dependent mechanism. Aging Cell 2017, 16, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lin, Y.C.; Chang, L.Y.; Huang, S.K.; Huang, C.H.; Yang, C.K.; Huang, C.T.; Lin, C.Y. Therapeutic Role of Inducible Nitric Oxide Synthase Expressing Myeloid-Derived Suppressor Cells in Acetaminophen-Induced Murine Liver Failure. Front. Immunol. 2020, 11, 574839. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Huang, Y.; Wang, H.; Zhao, J.; Gaskin, F.; Yang, N.; Fu, S.M. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clin. Immunol. 2015, 157, 175–186. [Google Scholar] [CrossRef]
- Keltsch, E.; Greiner, J.; Wahl, L.; Knape, I.; Tews, D.; Denkinger, M.; Debatin, K.-M.; Strauss, G. Aging modulates the immunosuppressive, polarizing and metabolic functions of blood-derived myeloid-derived suppressor cells (MDSCs). Immun. Ageing 2025, 22, 29. [Google Scholar] [CrossRef]
- Limpijankit, T.; Jongjirasiri, S.; Meemook, K.; Unwanatham, N.; Thakkinstian, A.; Laothamatas, J. Predictive values of coronary artery calcium and arterial stiffness for long-term cardiovascular events in patients with stable coronary artery disease. Clin. Cardiol. 2023, 46, 171–183. [Google Scholar] [CrossRef]
- Strömberg, S.; Stomby, A.; Engvall, J.; Östgren, C.J. Systematic Coronary Risk Evaluation 2 (SCORE2), arterial stiffness, and subclinical coronary atherosclerosis in a popula-tion-based study. Scand. J. Prim. Health Care 2025, 43, 455–462. [Google Scholar] [CrossRef]
- Lakhonina, N.; Goloviznin, M.; Donetskova, A.; Nikonova, M.; Yarilin, A.; Buldakova, Y.; Tektova, A. T cell receptor rearrangement excision circles (TREC) study as an approach to “in vivo” thymus gland function investigation. Arthritis Res. Ther. 2012, 14 (Suppl. S1), P37. [Google Scholar] [CrossRef]
- Aranda, J.F.; Ramírez, C.M.; Mittelbrunn, M. Inflammageing, a targetable pathway for preventing cardiovascular diseases. Cardiovasc. Res. 2025, 121, 1537–1550. [Google Scholar] [CrossRef]

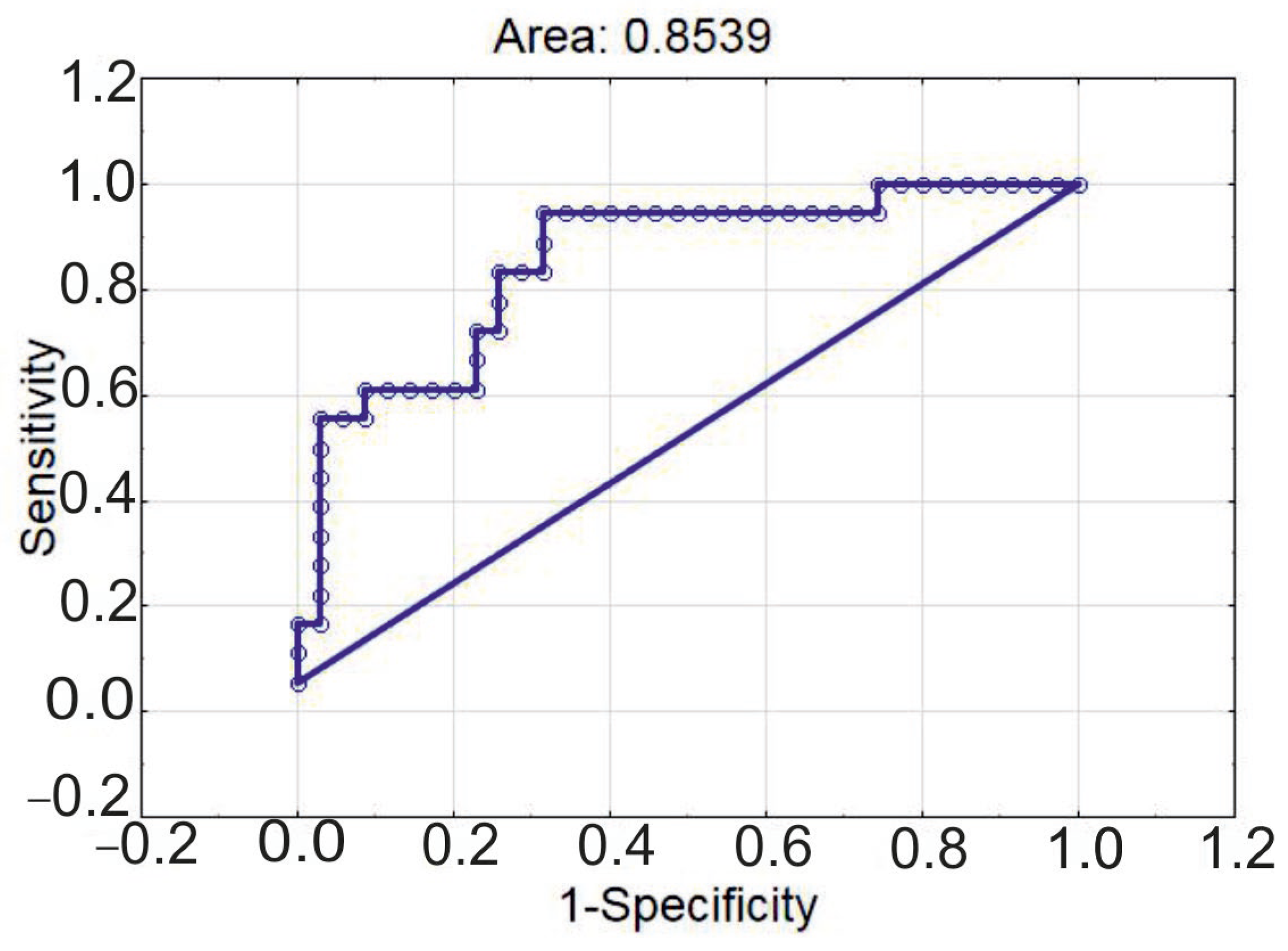
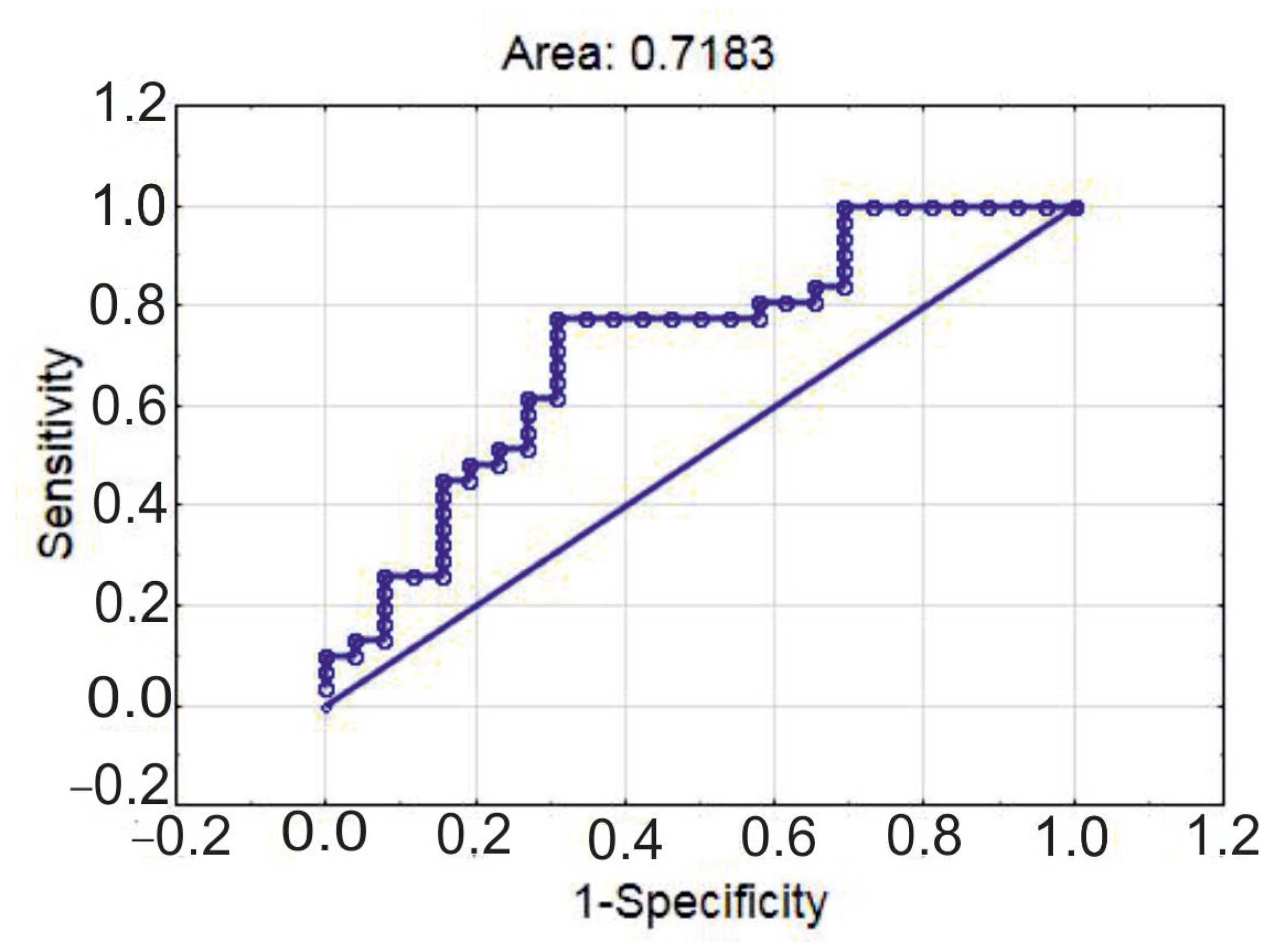
| Characteristics | CAD GS < 65 (n = 37) | CAD GS ≥ 65 (n = 19) | p |
|---|---|---|---|
| Gender (m), n (%) | 29 (78.4) | 18 (94.7) | 0.146 |
| Age, years | 65 (58; 69) | 65 (58; 68) | 0.918 |
| History of myocardial infarction, n (%) | 13 (35.1) | 16 (84.2) | <0.001 |
| Arterial hypertension, n (%) | 34 (91.9) | 19 (100) | 0.544 |
| Diabetes mellitus type 2, n (%) | 13 (35) | 7 (36.8) | 0.999 |
| Duration of coronary artery disease, years | 2.5 (1.0; 9.0) | 3.0 (1.0; 12.0) | 0.428 |
| Systolic blood pressure, mm Hg | 125 (118; 137) | 119 (112; 132) | 0.066 |
| Diastolic blood pressure, mm Hg | 70 (65; 77) | 70 (67; 76) | 0.849 |
| Smoking, n (%) | 15 (40.5) | 6 (31.6) | 0.572 |
| Body mass index, kg/m2 | 28.7 (26.4; 31.5) | 28.7 (27.0; 31.8) | 0.693 |
| Waist circumference, cm | 104 (97; 112) | 103 (97; 111) | 0.963 |
| Gensini score, points | 42.5 (12.5; 52) | 77.0 (71.0; 101.5) | <0.001 |
| cfPWV, m/s | 10.0 (9.2; 11.2) | 9.7 (9.3; 10.0) | 0.139 |
| IMT, mm | 0.85 (0.75; 0.95) | 0.87 (0.75; 1.00) | 0.837 |
| Vascular aging index | 84.6 (79.5; 91.4) | 82.8 (77.7; 89.69) | 0.484 |
| Oral hypoglycemic drugs, n (%) | 7 (18.9) | 7 (36.8) | 0.195 |
| Insulin, n (%) | 4 (10.8) | 1 (5.26) | 0.652 |
| RAAS inhibitors, n (%) | 26 (70.3) | 11 (57.9) | 0.550 |
| Calcium channels antagonists, n (%) | 19 (51.4) | 8 (42.1) | 0.580 |
| Beta-blockers, n (%) | 30 (81.1) | 15 (78.9) | 0.999 |
| Diuretics, n (%) | 13 (35.1) | 9 (47.4) | 0.401 |
| Statins, n (%) | 34 (91.9) | 18 (94.7) | 0.998 |
| Parameters | CAD GS < 65 (n = 37) | CAD GS ≥ 65 (n = 19) | p |
|---|---|---|---|
| PMN-MDSC, % | 0.03 (0.02; 0.05) | 0.03 (0.02; 0.05) | 0.888 |
| PMN-MDSC, ×106/L | 0.79 (0.47; 1.46) | 0.64 (0.28; 1.17) | 0.464 |
| M-MDSC, % | 0.11 (0.04; 0.47) | 0.11 (0.06; 0.20) | 0.845 |
| M-MDSC, ×106/L | 3.01 (1.22; 10.96) | 2.85 (1.18; 5.69) | 0.706 |
| eMDSC, % | 0.31 (0.17; 0.61) | 0.22 (0.10; 0.32) | 0.154 |
| eMDSC, ×106/L | 8.24 (5.08; 14.23) | 4.02 (2.6 2; 8.70) | 0.041 |
| CD4 + CD25hiFoxP3+, % | 6.18 (5.08; 7.44) | 5.72 (4.57; 6.69) | 0.166 |
| CD4 + CD25lowFoxP3+, % | 1.19 (0.70; 1.74) | 0.91 (0.74; 1.23) | 0.097 |
| CD4 + CD25hiFoxP3+, ×107/L | 5.91 (4.41; 8.67) | 3.99 (3.38; 6.56) | 0.049 |
| CD4 + CD25lowFoxP3+, ×107/L | 1.15 (0.69; 1.53) | 0.76 (0.50; 0.94) | 0.007 |
| CD25hi FoxP3 nuclear, % | 96.5 (84.1; 98.1) | 89.5 (73.1; 97.3) | 0.191 |
| CD25low FoxP3 nuclear, % | 89.7 (76.5; 93.6) | 76.2 (63.8; 96.8) | 0.312 |
| CD25hi FoxP3 nuclear, ×107/L | 5.20 (3.97; 7.47) | 3.73 (2.30; 6.00) | 0.031 |
| CD25low FoxP3 nuclear, ×107/L | 0.91 (0.59; 1.26) | 0.56 (0.31; 0.85) | 0.009 |
| Parameters | CAD GS < 65 (n = 15) | CAD GS ≥ 65 (n = 7) | p |
|---|---|---|---|
| Preserved thymic parenchyma, % | 2 (1; 10) | 2 (0; 15) | 0.636 |
| FoxP3+ cells, n (%) | 6 (40.0) | 2 (28.5) | 0.999 |
| Parameters | CAD GS < 65 (n = 17) | CAD GS ≥ 65 (n = 11) | p |
|---|---|---|---|
| CD4+ T lymphocytes, % | 33.6 (30.5; 45.5) | 29.6 (25.4; 31.6) | 0.113 |
| CD8+ T lymphocytes, % | 19.5 (15.8; 27.2) | 30.3 (29.9; 31.0) | 0.026 |
| CD4 + CD8+ T lymphocytes, % | 1.3 (0.7; 2.4) | 0.3 (0.2; 0.6) | 0.036 |
| CD4hiCD8low T lymphocytes, % | 0.5 (0.3; 1.2) | 0.2 (0.1; 0.4) | 0.067 |
| CD4lowCD8hi T lymphocytes, % | 0.3 (0.2; 0.4) | 0.2 (0; 0.5) | 0.529 |
| NK cells, % | 3.1 (1.8; 6.0) | 14.2 (10.9; 16.7) | 0.018 |
| NKT cells, % | 9.9 (4.7; 14.6) | 10.9 (5.8; 18.9) | 0.607 |
| B lymphocytes, % | 5.7 (3.8; 8.5) | 3.3 (2.6; 4.2) | 0.328 |
| CD4 + CD25hiFoxP3+, % | 7.5 (5.6; 13.9) | 7.0 (4.7; 16.3) | 0.853 |
| CD4 + CD25lowFoxP3+, % | 3.1 (2.2; 6.6) | 4.5 (1.0; 6.8) | 0.735 |
| CD25hi FoxP3 nuclear, % | 33.1 (22.6; 44.5) | 19.4 (12.5; 26.0) | 0.134 |
| CD25low FoxP3 nuclear, % | 20.2 (7.3; 28.8) | 10.8 (0; 18.7) | 0.122 |
| CD4 + CD25hiFoxP3 + CD31+, % | 1.7 (0.3; 11.4) | 0 | 0.085 |
| CD4 + CD25lowFoxP3 + CD31+, % | 0 (0; 1.2) | 0 | 0.517 |
| Parameters | CAD GS < 65 (n = 37) | CAD GS ≥ 65 (n = 19) | p |
|---|---|---|---|
| IL-1β, pg/mL | 0.82 (0.54; 1.17) | 0.79 (0.51; 1.15) | 0.887 |
| IL-10, pg/mL | 1.93 (1.53; 3.21) | 2.34 (1.66; 2.63) | 0.745 |
| IL-6, pg/mL | 1.53 (0.87; 2.39) | 1.50 (1.27; 1.87) | 0.530 |
| TNF, pg/mL | 2.02 (0.71; 2.78) | 2.38 (1.46; 2.62) | 0.652 |
| TGFβ, ng/mL | 32.08 (25.62; 38.34) | 32.57 (19.05; 37.17) | 0.562 |
| IGF, μg/mL | 77.27 (58.26; 106.00) | 66.11 (42.59; 100.00) | 0.411 |
| Homocysteine, μmol/L | 11.51 (9.91; 14.75) | 12.10 (9.73; 14.26) | 0.951 |
| Endocan-1, pg/mL | 1893.5 (1518.5; 2571.0) | 2304.0 (1922.0; 2603.0) | 0.114 |
| Endothelin-1, fmol/mL | 0.38 (0.31; 0.78) | 0.44 (0.37; 0.56) | 0.568 |
| Resistin, ng/mL | 4.71 (3.85; 6.33) | 4.40 (3.76; 5.62) | 0.540 |
| Sortilin, ng/mL | 11.73 (4.54; 116.08) | 9.37 (5.44; 36.21) | 0.835 |
| Estimates | 95% CI (Lower) | 95% CI (Upper) | p | |
|---|---|---|---|---|
| Intercept | −0.678 | 0.05938 | 4.34305 | 0.536 |
| Infarction | 2.752 | 2.85114 | 86.24346 | 0.002 |
| eMDSC, ×106/L | −0.081 | 0.82418 | 1.03149 | 0.922 |
| CD25low FoxP3 nuclear, ×107/L | −1.514 | 0.02624 | 1.84440 | 0.163 |
| Estimates | 95% CI (Lower) | 95% CI (Upper) | p | |
|---|---|---|---|---|
| eMDSC, ×106/L | 1.104639 | 1.001398 | 1.218523 | 0.047 |
| age | 1.050619 | 0.984182 | 1.121541 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kologrivova, I.; Dmitriukov, A.; Naryzhnaya, N.; Koshelskaya, O.; Kharitonova, O.; Vyrostkova, A.; Kravchenko, E.; Stepanov, I.; Andreev, S.; Evtushenko, V.; et al. Associations Between Regulatory Immune Cells, Thymus Cellular Remodeling, and Vascular Aging in Advanced Coronary Atherosclerosis: A Pilot Study. Diagnostics 2025, 15, 2494. https://doi.org/10.3390/diagnostics15192494
Kologrivova I, Dmitriukov A, Naryzhnaya N, Koshelskaya O, Kharitonova O, Vyrostkova A, Kravchenko E, Stepanov I, Andreev S, Evtushenko V, et al. Associations Between Regulatory Immune Cells, Thymus Cellular Remodeling, and Vascular Aging in Advanced Coronary Atherosclerosis: A Pilot Study. Diagnostics. 2025; 15(19):2494. https://doi.org/10.3390/diagnostics15192494
Chicago/Turabian StyleKologrivova, Irina, Alexey Dmitriukov, Natalia Naryzhnaya, Olga Koshelskaya, Olga Kharitonova, Alexandra Vyrostkova, Elena Kravchenko, Ivan Stepanov, Sergey Andreev, Vladimir Evtushenko, and et al. 2025. "Associations Between Regulatory Immune Cells, Thymus Cellular Remodeling, and Vascular Aging in Advanced Coronary Atherosclerosis: A Pilot Study" Diagnostics 15, no. 19: 2494. https://doi.org/10.3390/diagnostics15192494
APA StyleKologrivova, I., Dmitriukov, A., Naryzhnaya, N., Koshelskaya, O., Kharitonova, O., Vyrostkova, A., Kravchenko, E., Stepanov, I., Andreev, S., Evtushenko, V., Gusakova, A., Ogurkova, O., & Suslova, T. (2025). Associations Between Regulatory Immune Cells, Thymus Cellular Remodeling, and Vascular Aging in Advanced Coronary Atherosclerosis: A Pilot Study. Diagnostics, 15(19), 2494. https://doi.org/10.3390/diagnostics15192494









