A Cost-Effective Screening Inflammation Indicator for Atopic Dermatitis Suitable for Primary Care and Self-Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
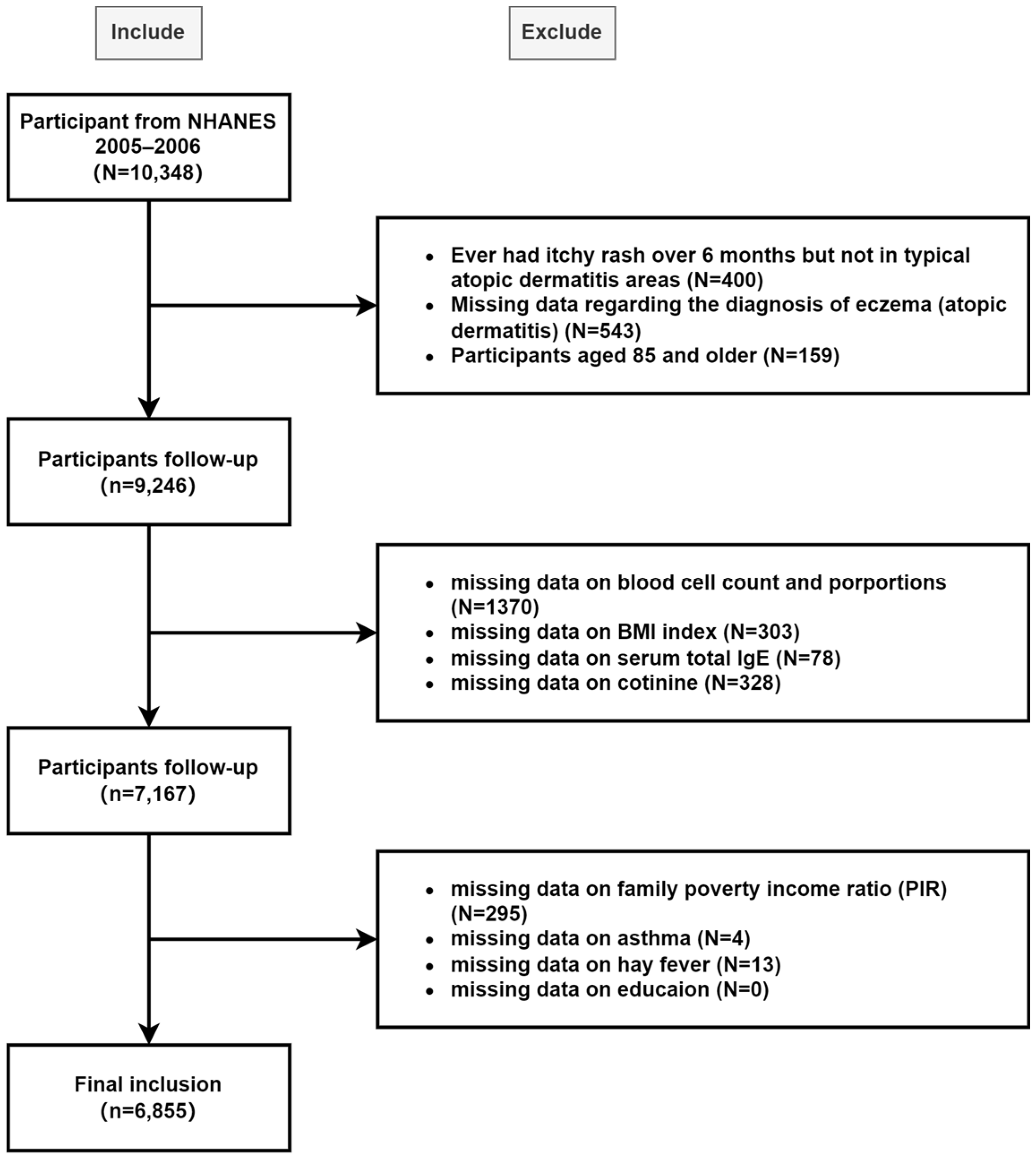
2.2. Study Design and Population
2.3. Definition of Atopic Dermatitis
2.4. Exposure Variable
2.5. Covariates
2.6. Statistical Methods
3. Results
3.1. The Stability and Reliability of AII in Clinical Practice
3.2. The Independent Predictive Role of AII in AD Patients
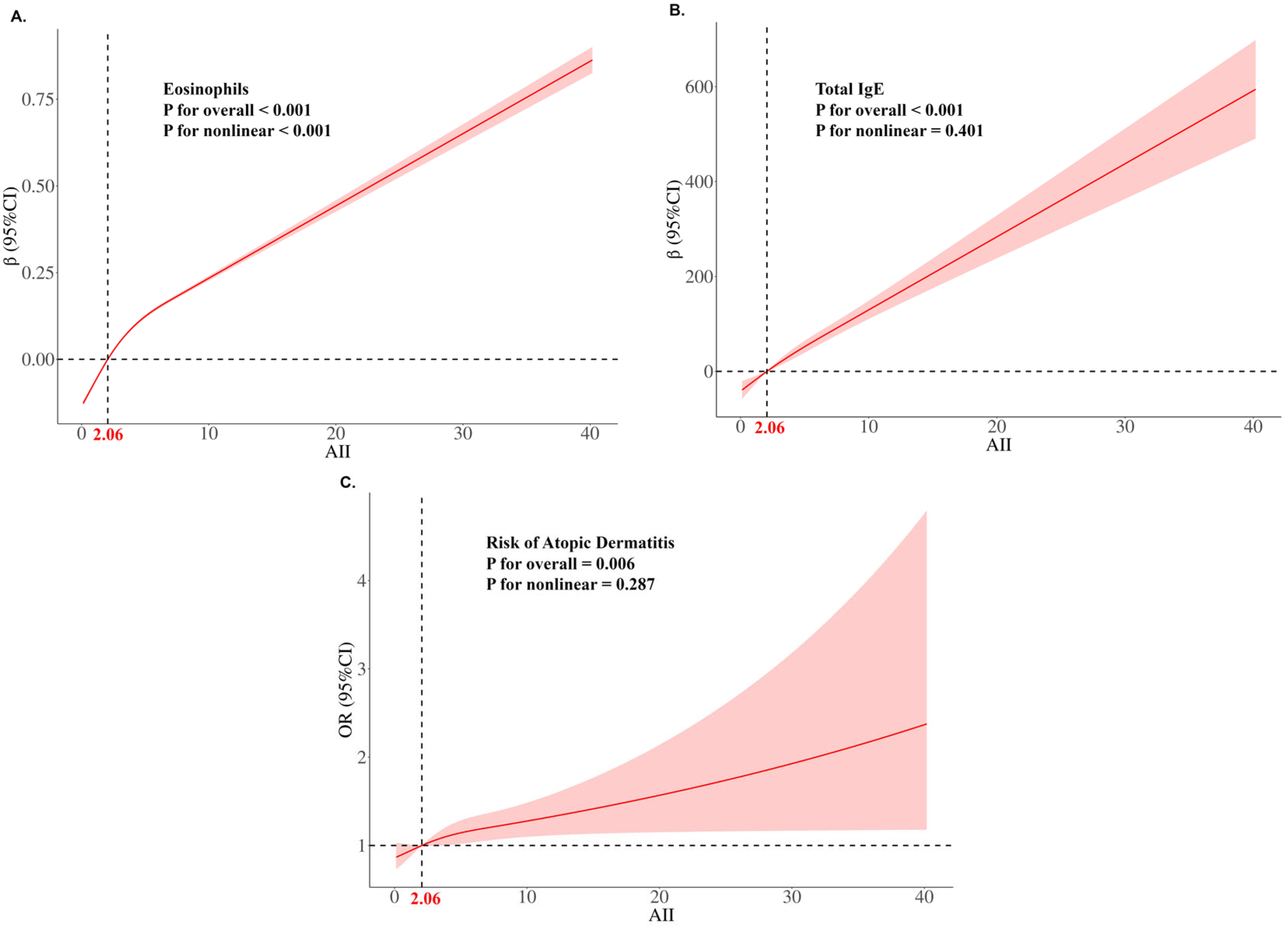
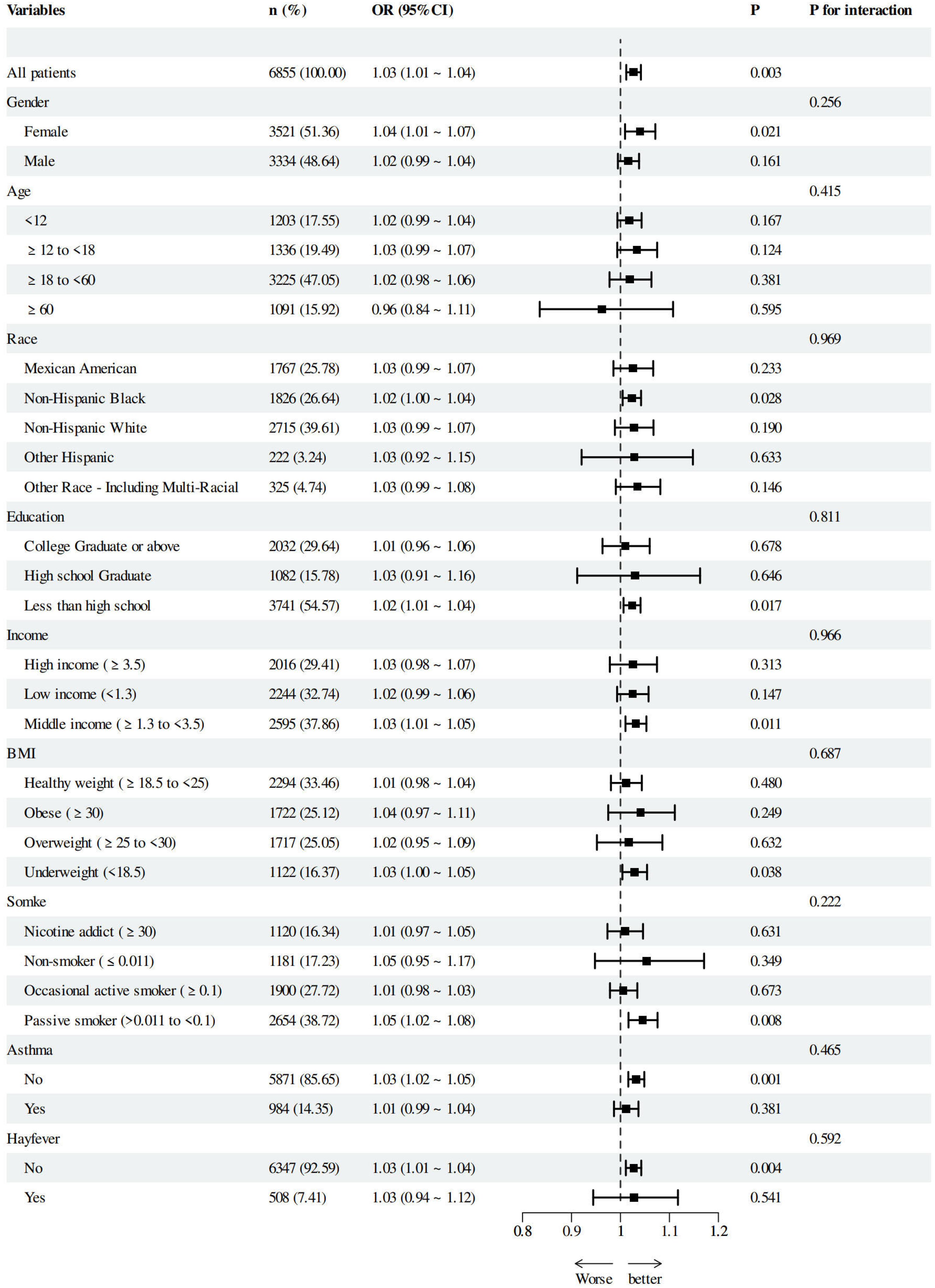
3.3. The Linear Relationship Between AII and AD Risk and Subgroup Analysis
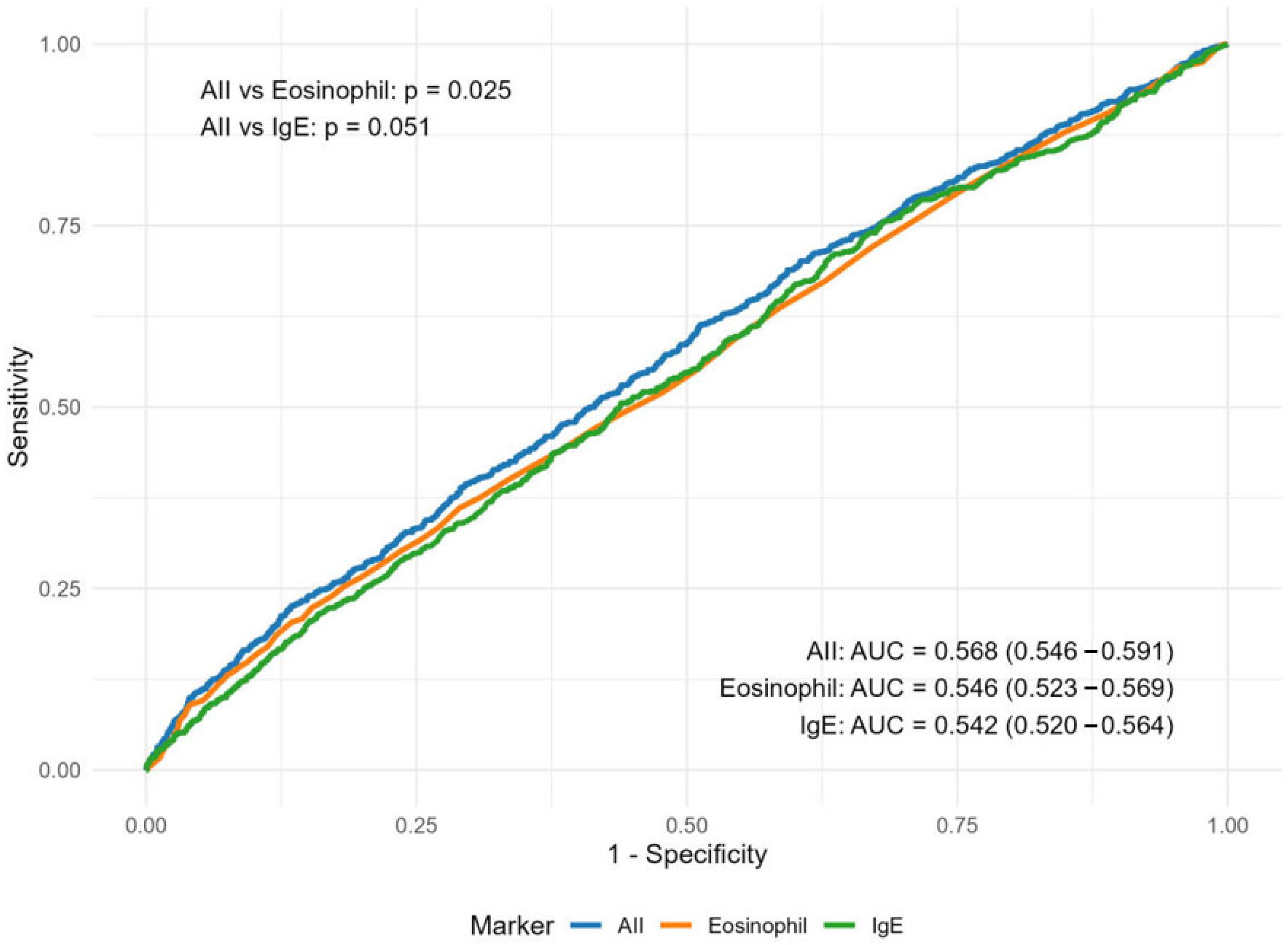
3.4. The Efficacy of AII in AD Screening and Sensitivity Analysis
4. Discussion
4.1. AII Reflects AD-Specific Type 2 Inflammation Through the Evaluation of AD-Related Cells
4.2. AII Detected Mild AD Cases and Did Not Overlap with Other Inflammatory Skin Diseases
4.3. AII Correlates with Disease Severity, Independently Predicts AD and Outperforms Traditional Biomarkers
4.4. Strengths, Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHANES | National Health and Nutrition Examination Survey |
| NCHS | National Center for Health Statistics |
| AD | Atopic Dermatitis |
| AII | Atopic Inflammation Index |
| PIR | Poverty Income Ratio |
| BMI | Body Mass Index |
| SII | Systemic Immune-Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| AISI | Aggregate Index of Systemic Inflammation |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| PLR | Platelet-to-Lymphocyte Ratio |
| PMR | Platelet-to-Monocyte Ratio |
| MLR | Monocyte-to-Lymphocyte Ratio |
| LMR | Lymphocyte-to-Monocyte Ratio |
| ELR | Eosinophil-to-Lymphocyte Ratio |
| ENR | Eosinophil-to-Neutrophil Ratio |
| MCH | Mean Corpuscular Hemoglobin |
| IgE | Immunoglobulin E |
| OR | Odds Ratios |
| CI | Confidence Intervals |
| RCS | Restricted Cubic Spline |
| AIC | Akaike Information Criterion |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| CALLY | C-Reactive Protein–Albumin–Lymphocyte Index |
| HALP | Hemoglobin–Albumin–Lymphocyte–Platelet Index |
References
- Guttman-Yassky, E.; Renert-Yuval, Y.; Brunner, P.M. Atopic Dermatitis. Lancet 2025, 405, 583–596. [Google Scholar] [CrossRef]
- Deckers, I.A.G.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating International Time Trends in the Incidence and Prevalence of Atopic Eczema 1990-2010: A Systematic Review of Epidemiological Studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef]
- Faye, O.; Flohr, C.; Kabashima, K.; Ma, L.; Paller, A.S.; Rapelanoro, F.R.; Steinhoff, M.; Su, J.C.; Takaoka, R.; Wollenberg, A.; et al. Atopic Dermatitis: A Global Health Perspective. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Laughter, M.R.; Maymone, M.B.C.; Mashayekhi, S.; Arents, B.W.M.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The Global Burden of Atopic Dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef]
- Weidinger, S.; Nosbaum, A.; Simpson, E.; Guttman, E. Good Practice Intervention for Clinical Assessment and Diagnosis of Atopic Dermatitis: Findings from the Atopic Dermatitis Quality of Care Initiative. Dermatol. Ther. 2022, 35, e15259. [Google Scholar] [CrossRef]
- Vakharia, P.P.; Chopra, R.; Silverberg, J.I. Systematic Review of Diagnostic Criteria Used in Atopic Dermatitis Randomized Controlled Trials. Am. J. Clin. Dermatol. 2018, 19, 15–22. [Google Scholar] [CrossRef]
- Rudikoff, D.; Lebwohl, M. Atopic Dermatitis. Lancet 1998, 351, 1715–1721. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of Care for the Management of Atopic Dermatitis: Section 1. Diagnosis and Assessment of Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef]
- Tokura, Y.; Hayano, S. Subtypes of Atopic Dermatitis: From Phenotype to Endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Thijs, J.L.; De Bruin-Weller, M.S.; Hijnen, D. Current and Future Biomarkers in Atopic Dermatitis. Immunol. Allergy Clin. N. Am. 2017, 37, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.; De Bruin-Weller, M.; Drylewicz, J.; Van Wijk, F.; Thijs, J. Biomarkers in Atopic Dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, Y.; Mu, Z.-L.; Lu, Q.-J.; Zhang, L.; Yao, X.; Zheng, M.; Tang, Y.-W.; Lu, X.-X.; Xia, X.-J.; et al. Clinical Features of Adult/Adolescent Atopic Dermatitis and Chinese Criteria for Atopic Dermatitis. Chin. Med. J. 2016, 129, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, S.; Liu, P.; Mu, Z.; Zhang, J. Clinical Relevance of Eosinophils, Basophils, Serum Total IgE Level, Allergen-Specific IgE, and Clinical Features in Atopic Dermatitis. J. Clin. Lab. Anal. 2020, 34, e23214. [Google Scholar] [CrossRef]
- Tuzimek, A.; Dziedzic, E.A.; Beck, J.; Kochman, W. Correlations Between Acute Coronary Syndrome and Novel Inflammatory Markers (Systemic Immune-Inflammation Index, Systemic Inflammation Response Index, and Aggregate Index of Systemic Inflammation) in Patients with and without Diabetes or Prediabetes. J. Inflamm. Res. 2024, 17, 2623–2632. [Google Scholar] [CrossRef]
- Ma, F.; Li, L.; Xu, L.; Wu, J.; Zhang, A.; Liao, J.; Chen, J.; Li, Y.; Li, L.; Chen, Z.; et al. The Relationship between Systemic Inflammation Index, Systemic Immune-Inflammatory Index, and Inflammatory Prognostic Index and 90-Day Outcomes in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. J. Neuroinflamm. 2023, 20, 220. [Google Scholar] [CrossRef]
- Zhang, L.; Pi, J.; Wang, J.; Chen, J.; Zhang, Y.; Li, J.; Wang, L.; Li, Y.; Chen, A.; Luo, X.; et al. The Association of Inflammatory Indexes Derived from Peripheral Blood Cell Count and Clinical Signs with Response to Treatment with Dupilumab in Pediatric Patients with Moderate-to-Severe Atopic Dermatitis. J. Inflamm. Res. 2025, 18, 271–282. [Google Scholar] [CrossRef]
- Hagino, T.; Saeki, H.; Fujimoto, E.; Kanda, N. The Eosinophil-to-Lymphocyte Ratio Acts as an Indicator for Improvement of Clinical Signs and Itch by Upadacitinib Treatment in Atopic Dermatitis. J. Clin. Med. 2023, 12, 2201. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Pembroke, A.C.; Hay, R.J. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent Hospital Validation. Br. J. Dermatol. 1994, 131, 406–416. [Google Scholar] [CrossRef]
- Xu, L.; Cao, Y. Association between Atopic Dermatitis with Hyperparathyroidism Not Mediated by Vitamin D in the United States (NHANES 2005-2006). Arch. Dermatol. Res. 2024, 317, 100. [Google Scholar] [CrossRef]
- De, D.; Kanwar, A.J.; Handa, S. Comparative Efficacy of Hanifin and Rajka’s Criteria and the UK Working Party’s Diagnostic Criteria in Diagnosis of Atopic Dermatitis in a Hospital Setting in North India. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Collier, M.R.; Devjani, S.; Han, G.; Wu, J.J. Association between Atopic Dermatitis and Thyroid Disease among U.S. Adults in the 2001-2006 National Health and Nutrition Examination Survey. J. Am. Acad. Dermatol. 2023, 88, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Lin, L.; Li, X.; Xie, X.; Lu, T. Association between Systemic Immune-Inflammation Index and Atopic Dermatitis: A Cross-Sectional Study of NHANES 2001–2006. Front. Med. 2024, 11, 1461596. [Google Scholar] [CrossRef] [PubMed]
- Iype, J.; Fux, M. Basophils Orchestrating Eosinophils’ Chemotaxis and Function in Allergic Inflammation. Cells 2021, 10, 895. [Google Scholar] [CrossRef]
- Zeng-Yun-Ou, Z.; Zhong-Yu, J.; Wei, L. Bidirectional Associations between Eosinophils, Basophils, and Lymphocytes with Atopic Dermatitis: A Multivariable Mendelian Randomization Study. Front. Immunol. 2022, 13, 1001911. [Google Scholar] [CrossRef]
- Pablo-Torres, C.; Izquierdo, E.; Tan, T.J.; Obeso, D.; Layhadi, J.A.; Sánchez-Solares, J.; Mera-Berriatua, L.; Bueno-Cabrera, J.L.; Del Mar Reaño-Martos, M.; Iglesias-Cadarso, A.; et al. Deciphering the Role of Platelets in Severe Allergy by an Integrative Omics Approach. Allergy 2023, 78, 1319–1332. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A Novel Systemic Inflammation Response Index (SIRI) for Predicting the Survival of Patients with Pancreatic Cancer after Chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef]
- Zhou, J.; Du, W.; Huang, H.; Chen, Y.; Chen, L.; Zheng, M. Association of CALLY Index and CLR with COPD Risk in Middle-Aged and Older Americans: Evidence from NHANES 2017-2020. Front. Med. 2025, 12, 1535415. [Google Scholar] [CrossRef]
- Antar, R.; Farag, C.; Xu, V.; Drouaud, A.; Gordon, O.; Whalen, M.J. Evaluating the Baseline Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in the United States Adult Population and Comorbidities: An Analysis of the NHANES. Front. Nutr. 2023, 10, 1206958. [Google Scholar] [CrossRef]
- Ghosh, D.; Bernstein, J.A.; Khurana Hershey, G.K.; Rothenberg, M.E.; Mersha, T.B. Leveraging Multilayered “Omics” Data for Atopic Dermatitis: A Road Map to Precision Medicine. Front. Immunol. 2018, 9, 2727. [Google Scholar] [CrossRef]
- Ruge, I.F.; Halling, A.-S.; Rasmussen Rinnov, M.; Gerner, T.; Knudgaard, M.H.; Ravn, N.H.; Nielsen, M.-L.; Skov, L.; Trautner, S.; Thomsen, S.F.; et al. Infant Palmar Hyperlinearity and Type 2 Inflammatory Markers Predict Atopic Dermatitis at 1 Year of Age. J. Eur. Acad. Dermatol. Venereol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Pesqué, D.; Silvestre-Salvador, J.F.; Figueiredo, A.C.; Pujol, R.M.; Gonçalo, M.; Giménez-Arnau, A.M. A Review of Hand Eczema Subtypes: Clinical Features, Biomarkers and Treatment Strategies. Contact Dermat. 2025, 92, 421–435. [Google Scholar] [CrossRef]
- Bakker, D.S.; Nierkens, S.; Knol, E.F.; Giovannone, B.; Delemarre, E.M.; van der Schaft, J.; van Wijk, F.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Confirmation of Multiple Endotypes in Atopic Dermatitis Based on Serum Biomarkers. J. Allergy Clin. Immunol. 2021, 147, 189–198. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, C.; Wang, S.; Yin, H.; Qiu, Z.; Luo, Y.; Li, Z.; Wang, C.; Yao, X.; Li, W. Serum Biomarker-Based Endotypes of Atopic Dermatitis in China and Prediction for Efficacy of Dupilumab. Br. J. Dermatol. 2023, 188, 649–660. [Google Scholar] [CrossRef]

| Variables | Total | Eosinophil | AII | ||||
|---|---|---|---|---|---|---|---|
| Value, M (Q1, Q3) | OR (95%CI) a | p | Value, M (Q1, Q3) | OR (95%CI) a | p | ||
| Healthy controls | 151 | 0.10 (0.06, 0.15) | 1.00 (Reference) | 1.66 (0.97, 2.64) | 1.00 (Reference) | ||
| Mild AD | 180 | 0.27 (0.14, 0.47) | 37,828.73 (2069.22~691,571.70) | <0.001 * | 4.41 (1.81, 7.97) | 1.62 (1.38~1.90) | <0.001 * |
| Moderate to severe AD | 106 | 0.48 (0.31, 0.66) | 1,243,099.66 (28,801.34~53,653,648.47) | <0.001 * | 4.77 (2.48, 9.39) | 1.90 (1.54~2.34) | <0.001 * |
| Psoriasis vulgaris | 152 | 0.15 (0.08, 0.24) | 84.98 (3.38~2134.56) | 0.007 * | 1.54 (0.98, 2.83) | 1.18 (0.99~1.40) | 0.061 |
| Chronic urticaria | 152 | 0.12 (0.08, 0.22) | 47.78 (4.37~522.10) | 0.002 * | 1.45 (0.83, 2.56) | 1.03 (0.90~1.18) | 0.639 |
| Variable | Total (n = 6855) | Non-Atopic Dermatitis (n = 6135) | Atopic Dermatitis (n = 720) | Statistic | p |
|---|---|---|---|---|---|
| Age, M (Q1, Q3) | 38.00 (21.00, 53.00) | 38.00 (21.00, 53.00) | 36.00 (15.00, 53.00) | Z = −2.06 | 0.040 * |
| Age, n (%) | χ2 = 46.61 | <0.001 * | |||
| <12 | 1203 (11.05) | 1014 (10.23) | 189 (18.05) | ||
| ≥12 to <18 | 1336 (9.23) | 1178 (9.01) | 158 (11.15) | ||
| ≥18 to <60 | 3225 (63.64) | 2943 (64.47) | 282 (56.50) | ||
| ≥60 | 1091 (16.08) | 1000 (16.29) | 91 (14.30) | ||
| Gender, n (%) | χ2 = 2.53 | 0.151 | |||
| Female | 3521 (51.38) | 3138 (51.05) | 383 (54.19) | ||
| Male | 3334 (48.62) | 2997 (48.95) | 337 (45.81) | ||
| Race, n (%) | χ2 = 19.31 | 0.002 * | |||
| Mexican American | 1767 (9.07) | 1662 (9.52) | 105 (5.22) | ||
| Non-Hispanic Black | 1826 (11.83) | 1583 (11.72) | 243 (12.83) | ||
| Non-Hispanic White | 2715 (69.89) | 2396 (69.34) | 319 (74.59) | ||
| Other Hispanic | 222 (3.63) | 200 (3.73) | 22 (2.72) | ||
| Other Race—Including Multi-Racial | 325 (5.58) | 294 (5.69) | 31 (4.65) | ||
| Education, n (%) | χ2 = 17.33 | 0.094 | |||
| College graduate or above | 2032 (45.85) | 1830 (46.16) | 202 (43.14) | ||
| High school graduate | 1082 (19.98) | 1003 (20.43) | 79 (16.12) | ||
| Less than high school | 3741 (34.17) | 3302 (33.41) | 439 (40.74) | ||
| Family income, n (%) | χ2 = 3.67 | 0.314 | |||
| Low | 2244 (19.05) | 2020 (19.03) | 224 (19.24) | ||
| Medium | 2595 (37.62) | 2342 (37.98) | 253 (34.50) | ||
| High | 2016 (43.33) | 1773 (42.99) | 243 (46.27) | ||
| Body Mass Index (BMI), n (%) | χ2 = 27.27 | <0.001 * | |||
| Underweight | 1122 (11.07) | 955 (10.47) | 167 (16.25) | ||
| Healthy weight | 2294 (32.74) | 2057 (32.53) | 237 (34.52) | ||
| Overweight | 1717 (27.65) | 1557 (28.13) | 160 (23.49) | ||
| Obese | 1722 (28.54) | 1566 (28.87) | 156 (25.75) | ||
| Smoke, n (%) | χ2 = 4.57 | 0.291 | |||
| Non-smoker | 1181 (17.10) | 1063 (16.84) | 118 (19.31) | ||
| Passive smoker | 2654 (38.24) | 2367 (38.13) | 287 (39.23) | ||
| Occasional active smoker | 1900 (22.44) | 1691 (22.56) | 209 (21.41) | ||
| Nicotine addict | 1120 (22.22) | 1014 (22.47) | 106 (20.05) | ||
| Asthma, n (%) | χ2 = 33.89 | <0.001 * | |||
| No | 5871 (85.67) | 5326 (86.51) | 545 (78.45) | ||
| Yes | 984 (14.33) | 809 (13.49) | 175 (21.55) | ||
| Hay-fever, n (%) | χ2 = 32.10 | <0.001 * | |||
| No | 6347 (89.07) | 5716 (89.80) | 631 (82.82) | ||
| Yes | 508 (10.93) | 419 (10.20) | 89 (17.18) | ||
| Biomarker, n (%) | |||||
| IgE | 39.40 (14.80, 111.00) | 39.00 (14.60, 110.00) | 43.90 (17.20, 124.00) | Z = −2.40 | 0.016 * |
| Eosinophil | 0.17 (0.11, 0.27) | 0.17 (0.11, 0.27) | 0.18 (0.12, 0.28) | Z = −1.54 | 0.125 |
| AII | 2.06 (1.22, 3.56) | 2.03 (1.19, 3.49) | 2.33 (1.39, 4.09) | Z = −2.70 | 0.007 * |
| Variables a | Model1 b | Model2 c | Model3 d | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| AII | ||||||
| Continuous | 1.04 (1.02~1.05) | <0.001 * | 1.03 (1.02~1.05) | <0.001 * | 1.03 (1.01~1.04) | 0.003 * |
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Q2 | 1.10 (0.73~1.65) | 0.646 | 1.09 (0.71~1.66) | 0.697 | 1.07 (0.71~1.63) | 0.740 |
| Q3 | 1.26 (0.94~1.67) | 0.138 | 1.24 (0.90~1.69) | 0.204 | 1.16 (0.85~1.57) | 0.357 |
| Q4 | 1.59 (1.21~2.08) | 0.004 * | 1.47 (1.04~2.08) | 0.044 * | 1.37 (0.99~1.90) | 0.075 |
| P for trend | 0.006 * | 0.043 * | 0.081 | |||
| IgE | ||||||
| Continuous | 1.01 (1.01~1.01) | 0.025 * | 1.01 (1.01~1.01) | 0.019 * | 1.00 (1.00~1.00) | 0.055 |
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Q2 | 1.18 (0.89~1.55) | 0.266 | 1.24 (0.94~1.62) | 0.146 | 1.22 (0.92~1.61) | 0.187 |
| Q3 | 1.22 (0.97~1.52) | 0.108 | 1.33 (1.05~1.69) | 0.030 * | 1.24 (0.97~1.60) | 0.113 |
| Q4 | 1.27 (1.09~1.49) | 0.009 * | 1.43 (1.22~1.68) | <0.001 * | 1.24 (1.05~1.46) | 0.022 * |
| P for trend | 0.073 | 0.009 * | 0.192 | |||
| Eosinophil | ||||||
| Continuous | 1.96 (1.19~3.23) | 0.018 * | 2.02 (1.23~3.30) | 0.014 * | 1.62 (0.98~2.68) | 0.081 |
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Q2 | 1.15 (0.86~1.53) | 0.354 | 1.18 (0.88~1.59) | 0.281 | 1.16 (0.87~1.55) | 0.324 |
| Q3 | 1.00 (0.73~1.36) | 0.983 | 1.05 (0.76~1.45) | 0.790 | 0.98 (0.71~1.34) | 0.891 |
| Q4 | 1.26 (0.93~1.71) | 0.163 | 1.31 (0.94~1.82) | 0.130 | 1.17 (0.84~1.62) | 0.373 |
| P for trend | 0.316 | 0.269 | 0.620 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Zhou, X.; Zou, Y. A Cost-Effective Screening Inflammation Indicator for Atopic Dermatitis Suitable for Primary Care and Self-Assessment. Diagnostics 2025, 15, 2483. https://doi.org/10.3390/diagnostics15192483
Ye C, Zhou X, Zou Y. A Cost-Effective Screening Inflammation Indicator for Atopic Dermatitis Suitable for Primary Care and Self-Assessment. Diagnostics. 2025; 15(19):2483. https://doi.org/10.3390/diagnostics15192483
Chicago/Turabian StyleYe, Chengbin, Xuyang Zhou, and Ying Zou. 2025. "A Cost-Effective Screening Inflammation Indicator for Atopic Dermatitis Suitable for Primary Care and Self-Assessment" Diagnostics 15, no. 19: 2483. https://doi.org/10.3390/diagnostics15192483
APA StyleYe, C., Zhou, X., & Zou, Y. (2025). A Cost-Effective Screening Inflammation Indicator for Atopic Dermatitis Suitable for Primary Care and Self-Assessment. Diagnostics, 15(19), 2483. https://doi.org/10.3390/diagnostics15192483






