AI-Based 3D-Segmentation Quantifies Sarcopenia in Multiple Myeloma Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Clinical Data Selection and Study Design
2.3. Computed Tomography Acquisition
2.4. Segmentation
2.5. Statistical Analysis
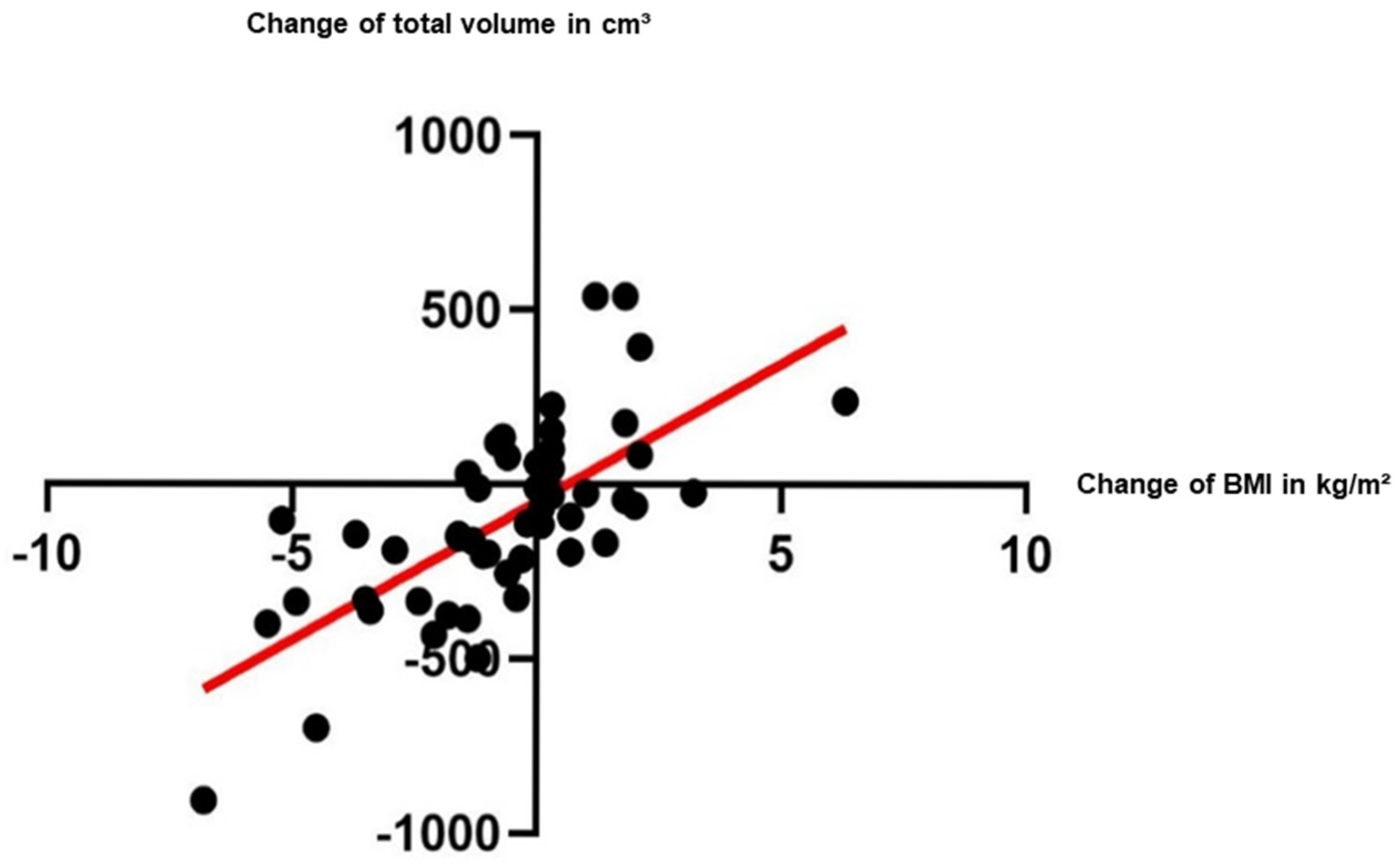
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| BCA | Body composition analysis |
| BMI | Body-Mass-Index |
| BOA | Body and organ analysis |
| CT | Computed tomography |
| CTDIvol | Volumetric computed tomography dose index |
| HU | Hounsfield Unit |
| IMAT | Intramuscular adipose tissue volume |
| IQR | Interquartile range |
| MM | Multiple myeloma |
| MV | Muscle volume |
| nnU-Net | No New U-Net |
| SD | Standard deviation |
| T1 | Initial examination |
| T2 | Follow-up examination |
| TV | Total volume |
References
- Colloca, G.; Di Capua, B.; Bellieni, A.; Cesari, M.; Marzetti, E.; Valentini, V.; Calvani, R. Muscoloskeletal aging, sarcopenia and cancer. J. Geriatr. Oncol. 2019, 10, 504–509. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 2016, 54, 2–10. [Google Scholar] [CrossRef]
- Jung, J.; Lee, E.; Shim, H.; Park, J.H.; Eom, H.S.; Lee, H. Prediction of clinical outcomes through assessment of sarcopenia and adipopenia using computed tomography in adult patients with acute myeloid leukemia. Int. J. Hematol. 2021, 114, 44–52. [Google Scholar] [CrossRef]
- Guo, J.; Cai, P.; Li, P.; Cao, C.; Zhou, J.; Dong, L.; Yang, Y.; Xuan, Q.; Wang, J.; Zhang, Q. Body Composition as a Predictor of Toxicity and Prognosis in Patients with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy. Curr. Oncol. 2021, 28, 1325–1337. [Google Scholar] [CrossRef]
- Kirsten, J.; Wais, V.; Schulz, S.V.W.; Sala, E.; Treff, G.; Bunjes, D.; Steinacker, J.M. Sarcopenia Screening Allows Identifying High-Risk Patients for Allogenic Stem Cell Transplantation. Cancers 2021, 13, 1771. [Google Scholar] [CrossRef] [PubMed]
- Zilioli, V.R.; Albano, D.; Arcari, A.; Merli, F.; Coppola, A.; Besutti, G.; Marcheselli, L.; Gramegna, D.; Muzi, C.; Manicone, M.; et al. Clinical and prognostic role of sarcopenia in elderly patients with classical Hodgkin lymphoma: A multicentre experience. J. Cachexia Sarcopenia Muscle 2021, 12, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Baruah, D.; Patel, J.; Szabo, A.; Chhabra, S.; Dhakal, B.; Hari, P.; Janz, S.; Stolley, M.; D’Souza, A. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone Marrow Transpl. 2021, 56, 225–231. [Google Scholar] [CrossRef]
- Abdallah, N.H.; Nagayama, H.; Takahashi, N.; Gonsalves, W.; Fonder, A.; Dispenzieri, A.; Dingli, D.; Buadi, F.K.; Lacy, M.Q.; Hobbs, M.; et al. Muscle and fat composition in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2023, 13, 185. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Goldschmidt, H. Das Multiple Myelom—Diagnose und Therapie, 3rd ed.; UNI-MED: Bremen, Germany, 2023. [Google Scholar]
- O’Donnell, E.K.; Raje, N.S. Myeloma bone disease: Pathogenesis and treatment. Clin. Adv. Hematol. Oncol. 2017, 15, 285–295. [Google Scholar]
- West, J.; Dahlqvist Leinhard, O.; Romu, T.; Collins, R.; Garratt, S.; Bell, J.D.; Borga, M.; Thomas, L. Feasibility of MR-Based Body Composition Analysis in Large Scale Population Studies. PLoS ONE 2016, 11, e0163332. [Google Scholar] [CrossRef]
- Karlsson, A.; Rosander, J.; Romu, T.; Tallberg, J.; Gronqvist, A.; Borga, M.; Dahlqvist Leinhard, O. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J. Magn. Reson. Imaging 2015, 41, 1558–1569. [Google Scholar] [CrossRef]
- Wald, D.; Teucher, B.; Dinkel, J.; Kaaks, R.; Delorme, S.; Boeing, H.; Seidensaal, K.; Meinzer, H.P.; Heimann, T. Automatic quantification of subcutaneous and visceral adipose tissue from whole-body magnetic resonance images suitable for large cohort studies. J. Magn. Reson. Imaging 2012, 36, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Heimann, T.; Meinzer, H.P. Statistical shape models for 3D medical image segmentation: A review. Med. Image Anal. 2009, 13, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Hedstrom, A.; Morwald, K.; Weghuber, D.; Forslund, A.; Bergsten, P.; Ahlstrom, H.; Kullberg, J. Fully convolutional networks for automated segmentation of abdominal adipose tissue depots in multicenter water-fat MRI. Magn. Reson. Med. 2019, 81, 2736–2745. [Google Scholar] [CrossRef]
- Kustner, T.; Hepp, T.; Fischer, M.; Schwartz, M.; Fritsche, A.; Haring, H.U.; Nikolaou, K.; Bamberg, F.; Yang, B.; Schick, F.; et al. Fully Automated and Standardized Segmentation of Adipose Tissue Compartments via Deep Learning in 3D Whole-Body MRI of Epidemiologic Cohort Studies. Radiol. Artif. Intell. 2020, 2, e200010. [Google Scholar] [CrossRef] [PubMed]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Haubold, J.; Baldini, G.; Parmar, V.; Schaarschmidt, B.M.; Koitka, S.; Kroll, L.; van Landeghem, N.; Umutlu, L.; Forsting, M.; Nensa, F.; et al. BOA: A CT-Based Body and Organ Analysis for Radiologists at the Point of Care. Investig. Radiol. 2023, 59, 433–441. [Google Scholar] [CrossRef]
- Wasserthal, J.; Breit, H.C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust Segmentation of 104 Anatomic Structures in CT Images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef]
- Koitka, S.; Kroll, L.; Malamutmann, E.; Oezcelik, A.; Nensa, F. Fully automated body composition analysis in routine CT imaging using 3D semantic segmentation convolutional neural networks. Eur. Radiol. 2021, 31, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Sinatti, G.; Citro, V.; Santini, S.J.; Balsano, C. Sarcopenia, a condition shared by various diseases: Can we alleviate or delay the progression? Intern. Emerg. Med. 2023, 18, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.Y.; Richardson, D.; Cregor, M.; Davis, H.M.; Au, E.D.; McAndrews, K.; Zimmers, T.A.; Organ, J.M.; Peacock, M.; Plotkin, L.I.; et al. Glucocorticoids Induce Bone and Muscle Atrophy by Tissue-Specific Mechanisms Upstream of E3 Ubiquitin Ligases. Endocrinology 2017, 158, 664–677. [Google Scholar] [CrossRef]
- Minetto, M.A.; Botter, A.; Lanfranco, F.; Baldi, M.; Ghigo, E.; Arvat, E. Muscle fiber conduction slowing and decreased levels of circulating muscle proteins after short-term dexamethasone administration in healthy subjects. J. Clin. Endocrinol. Metab. 2010, 95, 1663–1671. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Rossi, F.; Bignotti, B.; Torri, L.; Bonsignore, A.; Belgioia, L.; Domineitto, A. CT-derived relationship between low relative muscle mass and bone damage in patients with multiple myeloma undergoing stem cells transplantation. Br. J. Radiol. 2022, 95, 20210923. [Google Scholar] [CrossRef]
- Hillengass, J.; Hillengass, M.; Joseph, J.M.; Attwood, K.; Cannioto, R.; Jacobson, H.; Miller, C.; Wittmeyer, B.; Moysich, K. Effects on the Physical Functioning of Two Exercise Interventions in Patients with Multiple Myeloma: A Pilot Feasibility Study. Cancers 2024, 16, 1774. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Land, J.; Hackshaw, A.; Heinrich, M.; McCourt, O.; Beeken, R.J.; Philpott, S.; DeSilva, D.; Rismani, A.; Rabin, N.; et al. Fatigue, quality of life and physical fitness following an exercise intervention in multiple myeloma survivors (MASCOT): An exploratory randomised Phase 2 trial utilising a modified Zelen design. Br. J. Cancer 2020, 123, 187–195. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvao, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
- Nandakumar, B.; Baffour, F.; Abdallah, N.H.; Kumar, S.K.; Dispenzieri, A.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; et al. Sarcopenia identified by computed tomography imaging using a deep learning-based segmentation approach impacts survival in patients with newly diagnosed multiple myeloma. Cancer 2023, 129, 385–392. [Google Scholar] [CrossRef]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Singh, M.A. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef]
- Drey, M. Sarcopenia—Pathophysiology and clinical relevance. Wien. Med. Wochenschr. 2011, 161, 402–408. [Google Scholar] [CrossRef]
- Tuttle, L.J.; Sinacore, D.R.; Mueller, M.J. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J. Aging Res. 2012, 2012, 172957. [Google Scholar] [CrossRef]
- Waters, D.L. Intermuscular Adipose Tissue: A Brief Review of Etiology, Association with Physical Function and Weight Loss in Older Adults. Ann. Geriatr. Med. Res. 2019, 23, 3–8. [Google Scholar] [CrossRef]
- Mallard, J.; Hucteau, E.; Bender, L.; Charlot, A.; Debrut, L.; Pflumio, C.; Trensz, P.; Schott, R.; Favret, F.; Pivot, X.; et al. Development of skeletal muscle atrophy and intermuscular adipose tissue in patients with early breast cancer treated with chemotherapy. Am. J. Physiol. Cell Physiol. 2022, 323, C1325–C1332. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.S.; Long, S.S.; Zoga, A.C.; Parker, L.; Morrison, W.B. Association of Gluteus Medius and Minimus Muscle Atrophy and Fall-Related Hip Fracture in Older Individuals Using Computed Tomography. J. Comput. Assist. Tomogr. 2016, 40, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshige, Y.; Watanabe, E. Fatty degeneration of gluteus minimus muscle as a predictor of falls. Arch. Gerontol. Geriatr. 2015, 60, 59–61. [Google Scholar] [CrossRef] [PubMed]



| Patients’ Clinical Data | n = 51 |
|---|---|
| Age (years) at initial diagnosis | Median 58 |
| IQR 52–66 | |
| Range: 26–72 | |
| Gender | |
| Male | 38 |
| Female | 13 |
| Time until follow-up CT-scan (months) | Mean 11.8 (SD ± 3) |
| International Staging System (ISS) | |
| 1 | 22 |
| 2 | 8 |
| 3 | 19 |
| Not available | 2 |
| High-dose chemotherapy/autologous stem cell transplantation | |
| Single | 24 |
| Tandem | 27 |
| Response assessment at T2 | |
| ≥VGPR | 44 |
| PR, MR, SD | 5 |
| Not available | 2 |
| Body-Mass-Index (BMI) kg/m2 | |
| Weight loss | n = 28 |
| T1 | 27.6 (SD ± 6.2) |
| T2 | 25.4 (SD ± 5.8) |
| Weight gain | n = 20 |
| T1 | 24.9 (SD ± 3.8) |
| T2 | 26.3 (SD ± 3.8) |
| No weight change | n = 3 |
| ABM | IM | GMAX | GMED | GMIN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | |
| Total volume in cm3 | ||||||||||
| T1 | 525 (473–599) | 539 (448–604) | 314 * (264–364) (p = 0.001) | 303 * (250–373) (p = 0.0004) | 601 * (479–717) (p = 0.022) | 640 * (503–754) (p = 0.002) | 265 * (237–303) (p = 0.02) | 267 * (225–315) (p = 0.005) | 64 (56–73) | 70 (60–80) |
| T2 | 521 (453–597) | 526 (436–601) | 304 (255–357) | 281 (245–344) | 576 (515–665) | 600 (513–718) | 257 (227–297) | 261 (229–304) | 65 (55–75) | 71 (61–82) |
| Muscle volume in cm3 | ||||||||||
| T1 | 481 (409–561) | 478 (400–559) | 290 * (243–336) (p = 0.003) | 282 * (233–359) (p = 0.0004) | 568 * (463–675) (p = 0.008) | 609 * (486–718) (p = 0.0004) | 253 * (219–290) (p = 0.003) | 258 * (218–298) (p = 0.001) | 61 (52–69) | 66 (55–75) |
| T2 | 483 (402–536) | 468 (401–549) | 277 (236–333) | 260 (223–327) | 546 (465–636) | 559 (457–686) | 240 (213–282) | 246 (220–290) | 61 (46–68) | 66 (55–78) |
| IMAT in cm3 | ||||||||||
| T1 | 38 (28–57) | 41 (28–58) | 6 (4–12) | 7 (5–12) | 19 (9–43) | 18 * (9–41) (p = 0.018) | 9 * (6–19) (p = 0.03) | 8 * (5–18) (p = 0.037) | 2 * (1–4) (p = 0.001) | 3 * (1–5) (p = 0.018) |
| T2 | 42 (30–60) | 46 (30–62) | 7 (5–13) | 8 (5–14) | 22 (12–43) | 23 (12–41) | 11 (8–16) | 9 (6–15) | 3 (2–5) | 3 (2–5) |
| n = 51 | −BMI (n = 28) | +BMI (n = 20) | |
|---|---|---|---|
| Total volume in cm3 | |||
| T1 | 3653 * (3037–4066) (p = 0.005) | 3742 * (3393–4213) (p < 0.0001) | 3340 (2863–4034) |
| T2 | 3461 (3026–3987) | 3513 (3192–3955) | 3433 (2926–4128) |
| Muscle volume in cm3 | |||
| T1 | 3329 * (2847–3931) (p = 0.0004) | 3419 * (3176–4000) (p < 0.0001) | 3067 (2730–3858) |
| T2 | 3231 (2809–3664) | 3226 (3014–3662) | 3257 (2737–3845) |
| IMAT in cm3 | |||
| T1 | 161 * (99–254) (p = 0.021) | 191 (113.8–396.3) | 122 * (96.8–202.8) (p = 0.0002) |
| T2 | 179 (117–267) | 184.5 (117.8–395.8) | 145.5 (115–248.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.-D.; Nonnenmacher, T.; Burghardt, M.; Zschaebitz, S.; Hajiyianni, M.; Mai, E.K.; Raab, M.-S.; Müller-Tidow, C.; Kauczor, H.-U.; Goldschmidt, H.; et al. AI-Based 3D-Segmentation Quantifies Sarcopenia in Multiple Myeloma Patients. Diagnostics 2025, 15, 2466. https://doi.org/10.3390/diagnostics15192466
Do T-D, Nonnenmacher T, Burghardt M, Zschaebitz S, Hajiyianni M, Mai EK, Raab M-S, Müller-Tidow C, Kauczor H-U, Goldschmidt H, et al. AI-Based 3D-Segmentation Quantifies Sarcopenia in Multiple Myeloma Patients. Diagnostics. 2025; 15(19):2466. https://doi.org/10.3390/diagnostics15192466
Chicago/Turabian StyleDo, Thuy-Duong, Tobias Nonnenmacher, Marieke Burghardt, Stefanie Zschaebitz, Marina Hajiyianni, Elias Karl Mai, Marc-Steffen Raab, Carsten Müller-Tidow, Hans-Ulrich Kauczor, Hartmut Goldschmidt, and et al. 2025. "AI-Based 3D-Segmentation Quantifies Sarcopenia in Multiple Myeloma Patients" Diagnostics 15, no. 19: 2466. https://doi.org/10.3390/diagnostics15192466
APA StyleDo, T.-D., Nonnenmacher, T., Burghardt, M., Zschaebitz, S., Hajiyianni, M., Mai, E. K., Raab, M.-S., Müller-Tidow, C., Kauczor, H.-U., Goldschmidt, H., & Dapunt, U. (2025). AI-Based 3D-Segmentation Quantifies Sarcopenia in Multiple Myeloma Patients. Diagnostics, 15(19), 2466. https://doi.org/10.3390/diagnostics15192466






