McKittrick–Wheelock Syndrome, a Rare Cause of Nonresponsive Persistent Dyselectrolytemia
Abstract
1. Introduction
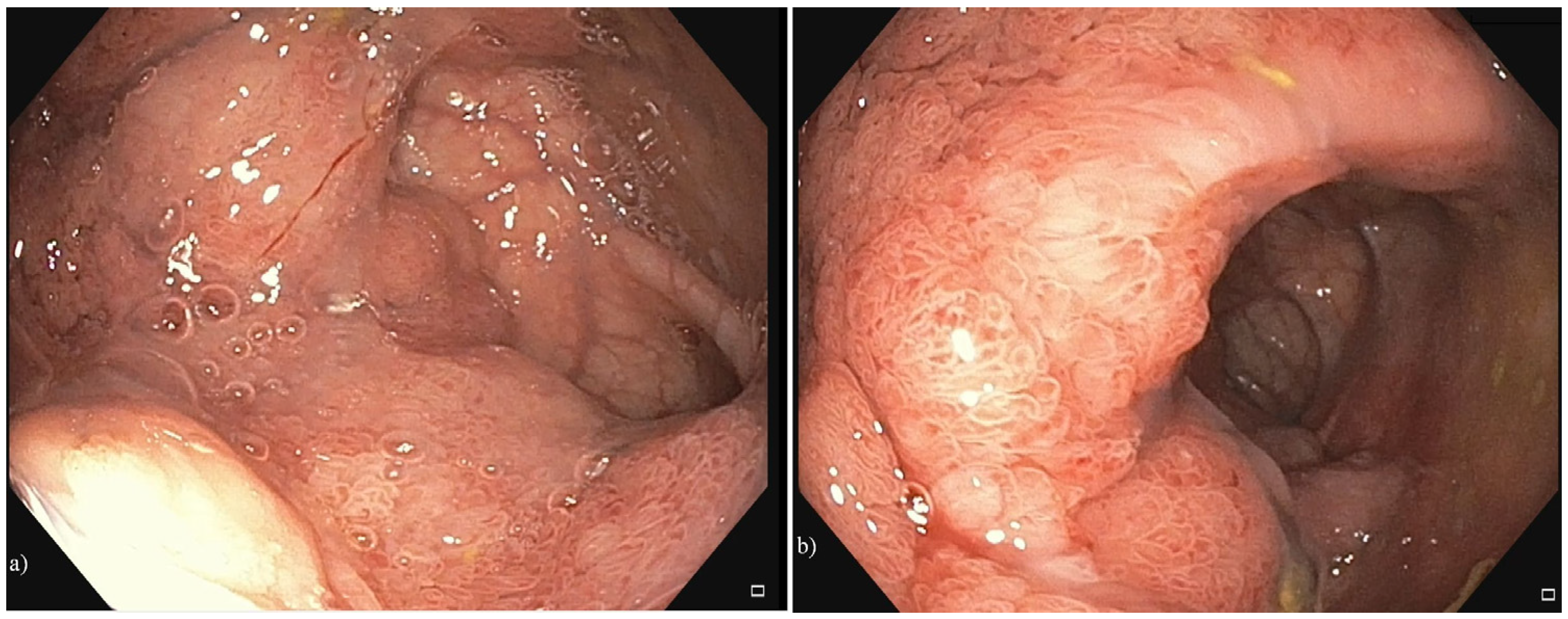
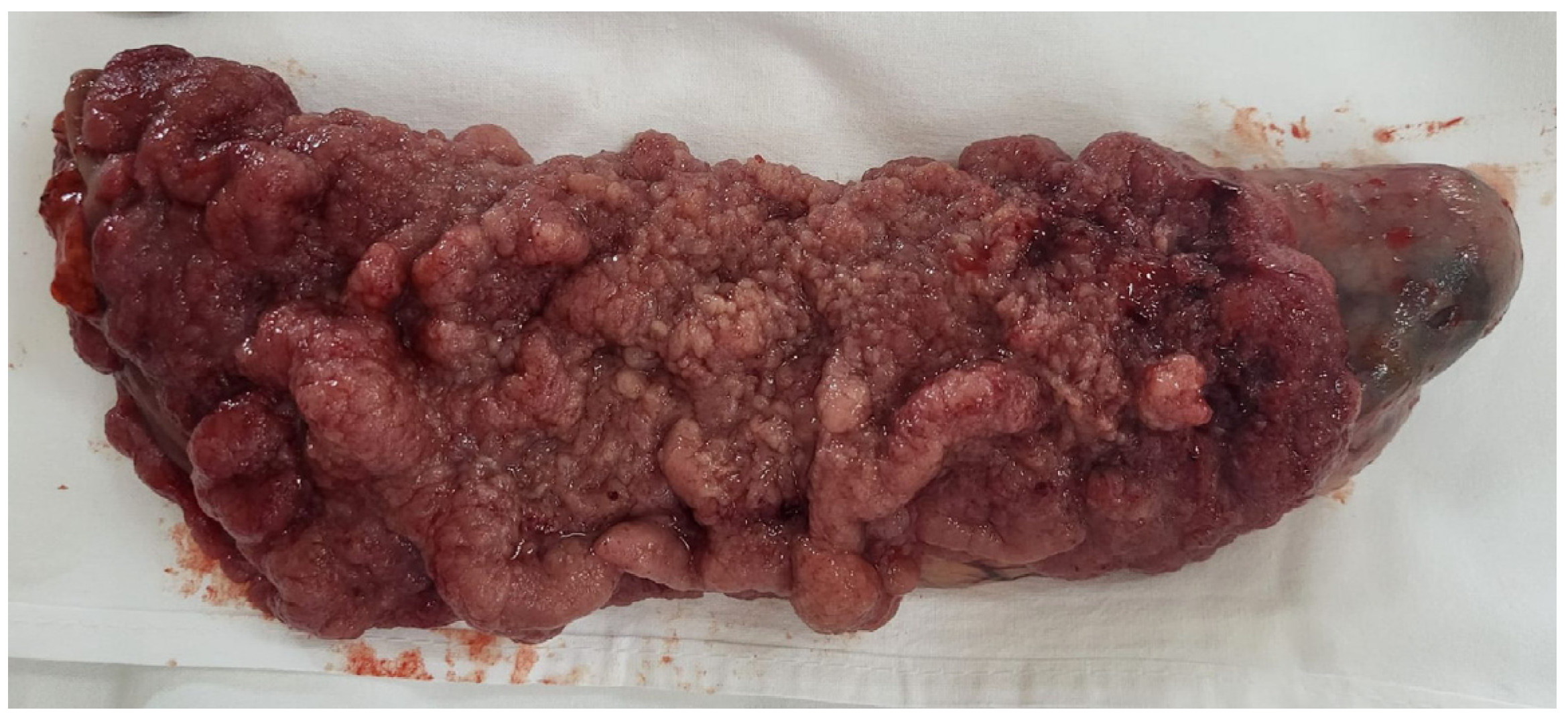
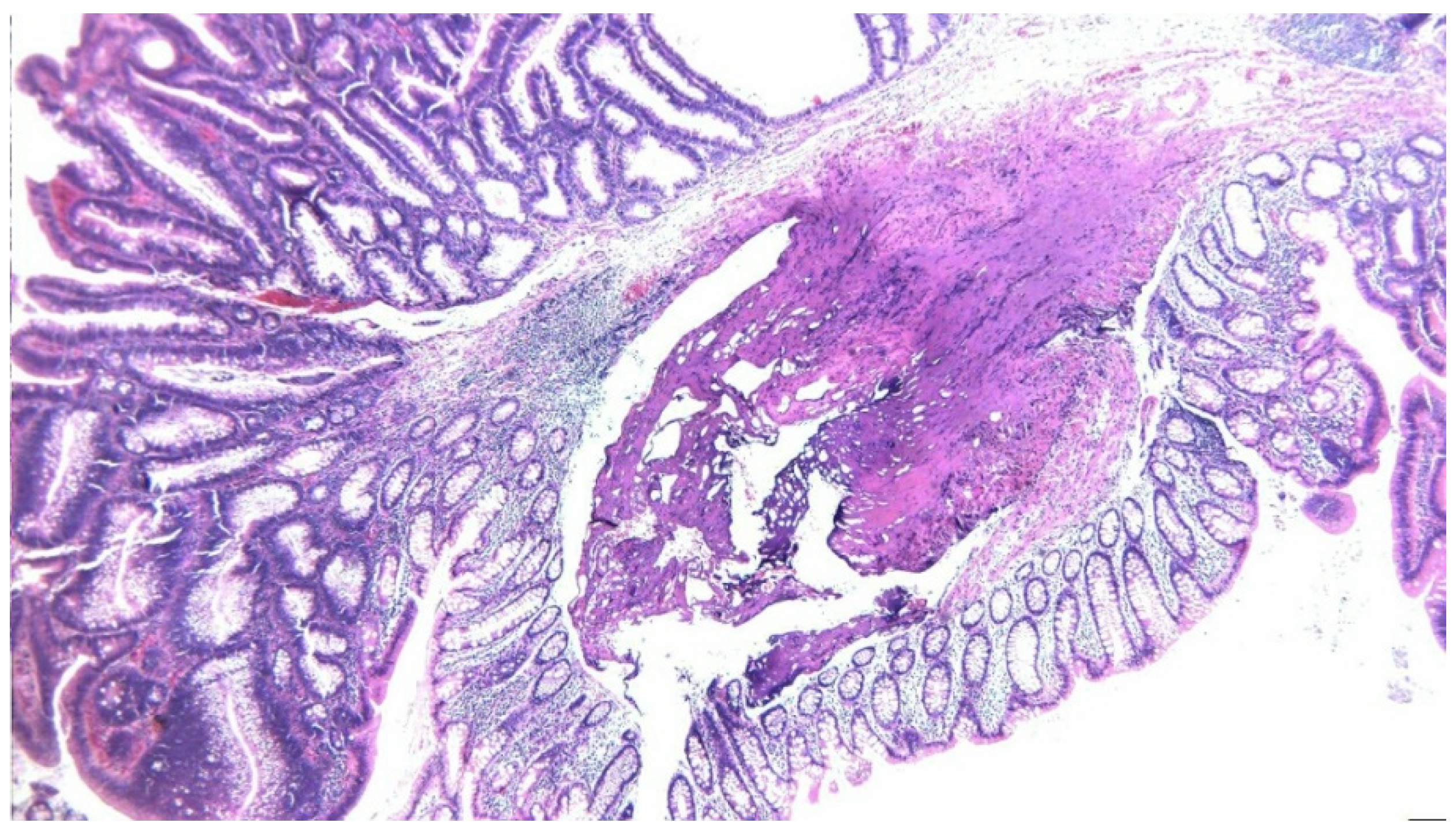
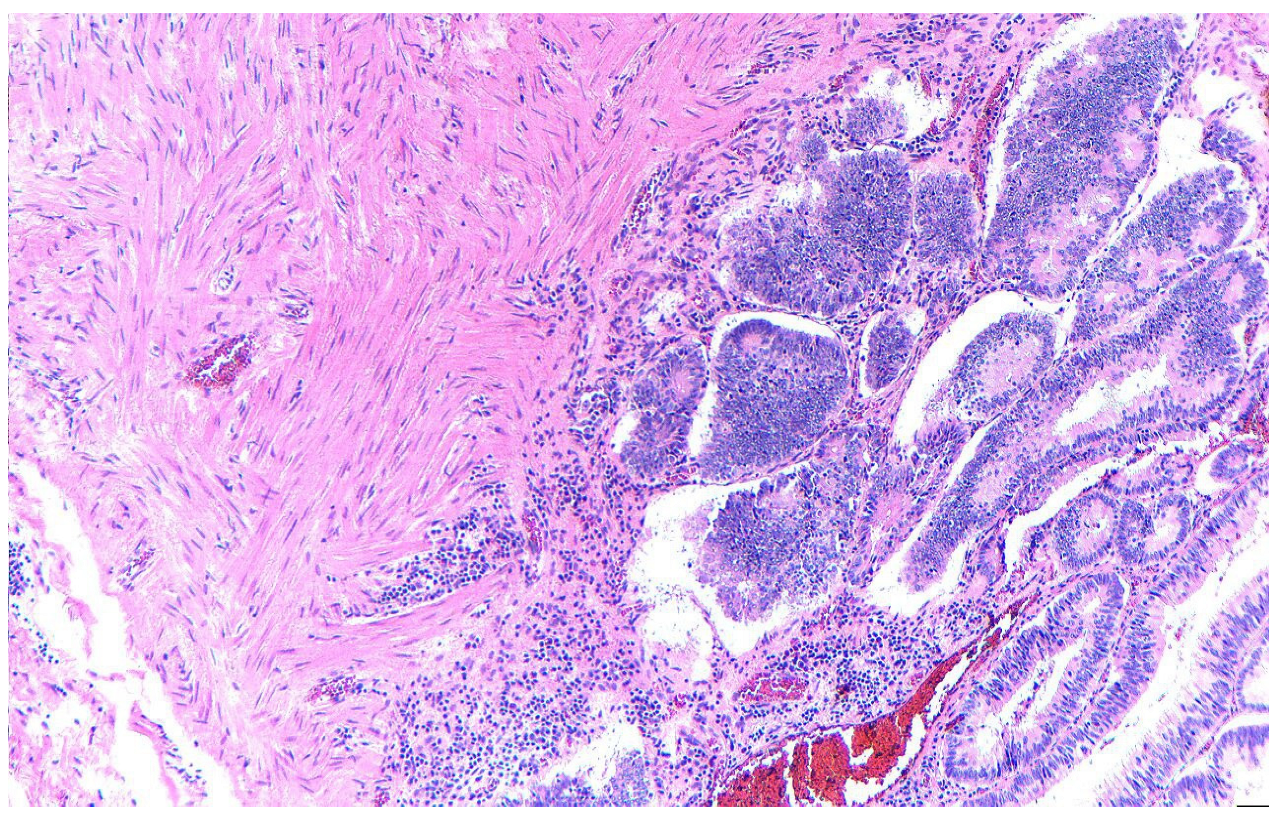
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hureaux, M.; Vargas-Poussou, R. Tubulopathies and Alterations of the RAAS. In Hydro Saline Metabolism: Epidemiology, Genetics, Pathophysiology, Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–43. [Google Scholar]
- Burrello, J.; Monticone, S.; Losano, I.; Cavaglià, G.; Buffolo, F.; Tetti, M.; Covella, M.; Rabbia, F.; Veglio, F.; Pasini, B.; et al. Prevalence of Hypokalemia and Primary Aldosteronism in 5100 Patients Referred to a Tertiary Hypertension Unit. Hypertension 2020, 75, 1025–1033. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Parker, M.D.; Hostler, D.; Pryor, R.R.; Schlader, Z. Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature 2021, 8, 108–159. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.M.; Grossmann, M.; Christ-Crain, M.; Russell, N. Syndrome of Inappropriate Antidiuresis: From Pathophysiology to Management. Endocr. Rev. 2023, 44, 819–861. [Google Scholar] [CrossRef] [PubMed]
- Abdelmageed, M.; Güzelgül, F. Copeptin: Up-to-date diagnostic and prognostic role highlight. Anal. Biochem. 2023, 673, 115181. [Google Scholar] [CrossRef] [PubMed]
- Romel, M.S.H.; Afrin, R. Frequency of Hypomagnesemia in Patients with Hypokalemia Admitted in A Tertiary Care Hospital. Eur. J. Med. Health Sci. 2023, 5, 19–23. [Google Scholar] [CrossRef]
- Schlüter, K.; Cadamuro, J. Erroneous potassium results: Preanalytical causes, detection, and corrective actions. Crit. Rev. Clin. Lab. Sci. 2023, 60, 442–465. [Google Scholar] [CrossRef]
- Negussie, A.B.; Dell, A.C.; Davis, B.A.; Geibel, J.P. Colonic Fluid and Electrolyte Transport 2022: An Update. Cells 2022, 11, 1712. [Google Scholar] [CrossRef]
- Dieng, A.; Ba, M.A.; Lemrabott, A.T.; Diop, M.; Sy, A.; Ndongo, M.; Ndiaye, B.; Diawara, M.S.; Faye, M.; Ba, B.; et al. Metabolic Alkalosis Associated Fluid and Electrolyte Disorders Revealing an Upper Digestive Tract Condition. Open Access Libr. J. 2020, 7, 1. [Google Scholar] [CrossRef]
- Villanueva, M.E.P.; Onglao, M.A.S.; Tampo, M.M.T.; Lopez, M.P.J. McKittrick-Wheelock Syndrome: A Case Series. Ann. Coloproctology 2022, 38, 266. [Google Scholar] [CrossRef]
- Thangaraju, S.G.; Angusamy, S.; Samuel, H.U.; Pawar, S.G.; Ramudu, V.; Thoppalan, B. McKittrick–Wheelock Syndrome. Indian J. Kidney Dis. 2022, 1, 41–44. [Google Scholar]
- Kouladouros, K.; Schneider, K.; Kubicka, S.; Hoerner, C.; Hirth, M. Endoscopic Submucosal Dissection of a Giant Rectal Adenoma Manifesting as McKittrick-Wheelock Syndrome. Z. Gastroenterol. 2023, 62, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Ciortescu, I.; Drug, V.-L.; Bărboi, O.-B.; Pleșca, D.; Livadariu, R.; Ionescu, L. Uncommon Association of Mckittrick-Wheelock Syndrome and Clostridioides difficile Infection in Acute Renal Failure. Diagnostics 2022, 12, 784. [Google Scholar] [CrossRef]
- Orchard, M.R.; Hooper, J.; Wright, J.; McCarthy, K. A systematic review of McKittrick–Wheelock syndrome. Ind. Mark. Manag. 2018, 100, 591–597. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Jessu, R.; Aeddula, N.R. Physiology, sodium potassium pump. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2022. [Google Scholar]
- Khajah, M.A.; Mathew, P.M.; Luqmani, Y.A.; Rishi, A. Na+/K+ ATPase activity promotes invasion of endocrine resistant breast cancer cells. PLoS ONE 2018, 13, e0193779. [Google Scholar] [CrossRef]
- Srisajjakul, S.; Bangchokdee, S. McKittrick-Wheelock Syndrome. Am. J. Roentgenol. 2023, 221, 550. [Google Scholar] [CrossRef]
- Noori, M.; Yadegar, A.; Zali, M.R. A complex scenario of nonsteroidal anti-inflammatory drugs induced prostaglandin E2 production and gut microbiota alteration in clostridium difficile-infected mice. Mbio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Metter, K.; Weißinger, S.E.; Várnai-Händel, A.; Grund, K.-E.; Dumoulin, F.L. Endoscopic Treatment of T1 Colorectal Cancer. Cancers 2023, 15, 3875. [Google Scholar] [CrossRef]
- Tsiamoulos, Z.; Zeidan, S.; Sebastian, J.; Saunders, B. P206 Novel technology and new trans-anal platform to excise a complex left colonic lesion and prevent luminal narrowing. Gut 2023, 72, A160–A161. [Google Scholar] [CrossRef]
- Caron, M.; Dubrûle, C.-E.; Letarte, F.; Lemelin, V.; Lafleur, A. McKittrick-Wheelock syndrome presenting with acute kidney injury and metabolic alkalosis: Case report and narrative review. Case Rep. Gastrointest. Med. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Peress, S.; Bonilla, C.; Yoo, E.; Brinkerhoff, B.; Bakis, G.; Jessica, X.Y. S3343 McKittrick-Wheelock Syndrome: Chronic Diarrhea Due to a Giant Tubulovillous Adenoma With Endoscopic Management. Off. J. Am. Coll. Gastroenterol. ACG 2023, 118, S2215–S2216. [Google Scholar] [CrossRef]
- Chaudhry, H.; Iqbal, H.; Gill, A.; Prajapati, D. McKittrick-Wheelock syndrome: A rare cause of chronic diarrhea treated with endoscopic polypectomy. SAGE Open Med. Case Rep. 2023, 11, 2050313X231177762. [Google Scholar] [CrossRef]
- van der Pool, A.E.M.; de Graaf, E.J.R.; Vermaas, M.; Barendse, R.M.; Doornebosch, P.G. McKittrick Wheelock syndrome treated by transanal minimally invasive surgery: A single-center experience and review of the literature. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 204–208. [Google Scholar] [CrossRef]
- Sumrien, H.; Dadnam, C.; Hewitt, J.; McCarthy, K. Feasibility of transanal minimally invasive surgery (TAMIS) for rectal tumours and its impact on quality of life–the Bristol series. Anticancer Res. 2016, 36, 2005–2009. [Google Scholar] [PubMed]
- Emrich, J.; Niemeyer, C. The secreting villous adenoma as a rare cause of acute renal failure. Med. Klin. 2002, 97, 619–623. [Google Scholar] [CrossRef]
- Pătraşcu, Ş.; Cercelaru, L.; Graure, G.M.; Firuţ, M.A.; Rotaru, I.; Cârţu, D.; Marinescu, D.; Pătraşcu, A.M.; Radu, R.I.; Mitroi, G.; et al. The Histopathological Features and Their Prognostic Impact in the Postoperative Follow-Up of Colorectal Cancer Patients. Rom. J. Morphol. Embryol. 2022, 63, 555–561. [Google Scholar] [CrossRef]


| Parameter | Value | Reference Range |
|---|---|---|
| Sodium (mEq/L) | 125 | 135–145 |
| Potassium (mEq/L) | 2.3 | 3.5–5.1 |
| Chloride (mEq/L) | 77 | 98–107 |
| pH (arterial) | 7.50 | 7.35–7.45 |
| Bicarbonate, HCO3− (mEq/L) | 34 | 22–29 |
| PaCO2 (mmHg) | 47 | 35–45 |
| Creatinine (mg/dL) | 3.4 | <1.2 |
| eGFR (mL/min/1.73 m2) | 19 | >90 |
| Urea (mg/dL) | 209 | 13–43 |
| Serum osmolality (mOsm/kg) | 263 | 275–295 |
| Urine osmolality (mOsm/kg) | 332 | 300–900 |
| Magnesium (mg/dL) | 2.37 | 1.7–2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cana Ruiu, D.; Cheie, M.; Florescu, M.M.; Stanculescu, A.D.; Popescu, C.; Maria, D.-T.; Toma, S.C.; Fota, N.; Calina, D.; Ungureanu, B.S. McKittrick–Wheelock Syndrome, a Rare Cause of Nonresponsive Persistent Dyselectrolytemia. Diagnostics 2025, 15, 2459. https://doi.org/10.3390/diagnostics15192459
Cana Ruiu D, Cheie M, Florescu MM, Stanculescu AD, Popescu C, Maria D-T, Toma SC, Fota N, Calina D, Ungureanu BS. McKittrick–Wheelock Syndrome, a Rare Cause of Nonresponsive Persistent Dyselectrolytemia. Diagnostics. 2025; 15(19):2459. https://doi.org/10.3390/diagnostics15192459
Chicago/Turabian StyleCana Ruiu, Daniela, Mihaela Cheie, Mirela Marinela Florescu, Andreea Doriana Stanculescu, Carmen Popescu, Daniela-Teodora Maria, Sebastian Constantin Toma, Naomi Fota, Daniela Calina, and Bogdan Silviu Ungureanu. 2025. "McKittrick–Wheelock Syndrome, a Rare Cause of Nonresponsive Persistent Dyselectrolytemia" Diagnostics 15, no. 19: 2459. https://doi.org/10.3390/diagnostics15192459
APA StyleCana Ruiu, D., Cheie, M., Florescu, M. M., Stanculescu, A. D., Popescu, C., Maria, D.-T., Toma, S. C., Fota, N., Calina, D., & Ungureanu, B. S. (2025). McKittrick–Wheelock Syndrome, a Rare Cause of Nonresponsive Persistent Dyselectrolytemia. Diagnostics, 15(19), 2459. https://doi.org/10.3390/diagnostics15192459








