Serum P-Cresyl Sulfate Is Associated with Peripheral Arterial Stiffness in Chronic Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design and Patient Enrollment
2.2. Anthropometric Data Collection
2.3. Biochemical Determinations
2.4. Carotid-Ankle Vascular Index Measurement
2.5. Quantification of Serum p-Cresyl Sulfate by High Performance Liquid Chromatography Mass Spectrometry (HPLC-MS)-Based Measurement
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| baPWV | Brachial-ankle pulse wave velocity |
| BMI | Body mass index |
| BP | Blood pressure |
| CAVI | Cardio-ankle vascular index |
| cfPWV | Carotid-femoral pulse wave velocity |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| CV | Cardiovascular |
| DBP | Diastolic blood pressure |
| ESRD | End-stage renal disease |
| Kt/V | The fractional clearance of urea, adjusted for the patient’s total body water. |
| HD | Hemodialysis |
| LC-MS | Liquid chromatography-mass spectrometry |
| PAS | Peripheral arterial stiffness |
| PCS | p-Cresyl sulfate |
| PWV | Pulse wave velocity |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
References
- Guo, J.; Liu, Z.; Wang, P.; Wu, H.; Fan, K.; Jin, J.; Zheng, L.; Liu, Z.; Xie, R.; Li, C. Global, regional, and national burden inequality of chronic kidney disease, 1990–2021: A systematic analysis for the global burden of disease study 2021. Front. Med. 2025, 11, 1501175. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Echefu, G.; Stowe, I.; Burka, S.; Basu-Ray, I.; Kumbala, D. Pathophysiological concepts and screening of cardiovascular disease in dialysis patients. Front. Nephrol. 2023, 3, 1198560. [Google Scholar] [CrossRef]
- Ren, S.C.; Mao, N.; Yi, S.; Ma, X.; Zou, J.Q.; Tang, X.; Fan, J.M. Vascular calcification in chronic kidney disease: An update and perspective. Aging Dis. 2022, 13, 673–697. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, L.; Dong, J.; Zhang, X.; Yan, S. Arterial stiffness and increased cardiovascular risk in chronic kidney disease. Int. Urol. Nephrol. 2015, 47, 1157–1164. [Google Scholar] [CrossRef]
- Go, A.S. Cardiovascular disease consequences of CKD. Semin. Nephrol. 2016, 36, 293–304. [Google Scholar] [CrossRef]
- Beros, A.L.; Sluyter, J.D.; Scragg, R. Association of arterial stiffness with chronic kidney disease: A systematic review. Kidney Blood Press. Res. 2024, 49, 763–772. [Google Scholar] [CrossRef]
- Nagayama, D.; Fujishiro, K.; Suzuki, K.; Shirai, K. Comparison of predictive ability of arterial stiffness parameters including cardio-ankle vascular index, pulse wave velocity and cardio-ankle vascular Index. Vasc. Health Risk Manag. 2022, 18, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Saiki, A.; Watanabe, Y.; Yamaguchi, T.; Ohira, M.; Nagayama, D.; Sato, N.; Kanayama, M.; Takahashi, M.; Shimizu, K.; Moroi, M.; et al. Cavilowering effect of pitavastatin may be involved in the prevention of cardiovascular disease: Subgroup analysis of the TOHO-LIP. J. Atheroscler. Thromb. 2021, 28, 1083–1094. [Google Scholar] [CrossRef]
- Sumin, A.N.; Shcheglova, A.V.; Zhidkova, I.I.; Ivanov, S.V.; Barbarash, O.L. Assessment of arterial stiffness by cardio-ankle vascular index for prediction of five-year cardiovascular events after coronary artery bypass surgery. Glob. Heart 2021, 16, 90. [Google Scholar] [CrossRef]
- Watanabe, K.; Yoshihisa, A.; Sato, Y.; Hotsuki, Y.; Anzai, F.; Ichijo, Y.; Kimishima, Y.; Yokokawa, T.; Misaka, T.; Sato, T.; et al. Cardio-ankle vascular index reflects impaired exercise capacity and predicts adverse prognosis in patients with heart failure. Front. Cardiovasc. Med. 2021, 8, 631807. [Google Scholar] [CrossRef]
- Rerkasem, A.; Tangmunkongvorakul, A.; Aurpibul, L.; Sripan, P.; Parklak, W.; Nantakool, S.; Srithanaviboonchai, K.; Rerkasem, K. Association of cardio-ankle vascular index and future major adverse cardiovascular events in older adults living with HIV. AIDS Care 2023, 35, 591–599. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. P-Cresyl sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Enoki, Y.; Ishima, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Tanaka, M.; Matsushita, K.; Mori, Y.; et al. P-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015, 3, e00092. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl sulfate and p-Cresyl sulfate promote vascular calcification and associate with glucose intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Wang, C.H.; Kuo, C.H.; Lin, Y.L.; Tsai, J.P.; Hsu, B.G. Serum p-Cresyl sulfate is a predictor of central arterial stiffness in patients on maintenance hemodialysis. Toxins 2019, 12, 10. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, Y.L.; Lai, Y.H.; Wang, C.H.; Hsu, B.G. Serum p-Cresyl sulfate level is an independent marker of peripheral arterial stiffness as assessed using brachial-ankle pulse wave velocity in patients with non-dialysis chronic kidney disease stage 3 to 5. Toxins 2022, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, H. Assessment of arterial stiffness using the cardio-ankle vascular index. Pulse 2016, 4, 11–23. [Google Scholar] [CrossRef]
- Hayashi, K.; Handa, H.; Nagasawa, S.; Okumura, A.; Moritake, K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J. Biomech. 1980, 13, 175–184. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Giraldo-Grueso, M.; Echeverri, D. From endothelial dysfunction to arterial stiffness in diabetes mellitus. Curr. Diabetes Rev. 2020, 16, 230–237. [Google Scholar] [CrossRef]
- Partalidou, S.; Patoulias, D.; Pantekidis, I.; Kefas, A.; Doumas, M.; Gkaliagkousi, E.; Rizzo, M.; Dimitroulas, T.; Anyfanti, P. The cross-talk between arterial stiffness and microvascular complications in diabetes mellitus: A systematic review of the literature. J. Diabetes Metab. Disord. 2025, 24, 144. [Google Scholar] [CrossRef]
- Kim, H.L. Arterial stiffness and hypertension. Clin. Hypertens. 2023, 29, 31. [Google Scholar] [CrossRef]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of arterial stiffening: From mechanotransduction to epigenetics. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ning, C.; Liu, J.; Yao, T.; Zhang, L.; Zhao, L.; Wei, X.; Zhang, X.; Gao, Y.; Zhang, R.; et al. The association between cumulative C-reactive protein and brachial–ankle pulse wave velocity. Aging Clin. Exp. Res. 2020, 32, 789–796. [Google Scholar] [CrossRef]
- Koppe, L.; Croze, M.L.; Monteiro, E.B.; Benoit, B.; Bres, E.; Guebre-Egziabher, F.; Daleprane, J.B.; Fouque, D.; Soulage, C.O. The protein-bound uremic toxin p-Cresyl-sulfate promotes intracellular ROS production and lipid peroxidation in 3T3-L1 adipose cells. Biochimie 2021, 189, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Kuo, H.L.; Liu, S.H.; Hsieh, C.Y.; Hsu, C.P.; Hung, K.C.; Wang, T.M.; Wu, C.C.; Lu, K.C.; Lin, W.N.; et al. Translational medicine in uremic vascular calcification: Scavenging ROS attenuates p-Cresyl sulfate-activated caspase-1, NLRP3 inflammasome and eicosanoid inflammation in human arterial smooth muscle cells. Life 2022, 12, 769. [Google Scholar] [CrossRef]
- Viaene, L.; Thijs, L.; Jin, Y.; Liu, Y.; Gu, Y.; Meijers, B.; Claes, K.; Staessen, J.; Evenepoel, P. Heritability and clinical determinants of serum indoxyl sulfate and p-Cresyl sulfate, candidate biomarkers of the human microbiome enterotype. PLoS ONE 2014, 9, e79682. [Google Scholar] [CrossRef]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Giebultowicz, J.; Wroczynski, P.; Paczek, L. Age-dependent increase in serum levels of indoxyl sulphate and p-Cresol sulphate is not related to their precursors: Tryptophan and tyrosine. Geriatr. Gerontol. Int. 2017, 17, 1022–1026. [Google Scholar] [CrossRef]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M., 3rd; Sen, R.; Ferrucci, L. Aging and inflammation. Cold Spring Harb. Perspect. Med. 2024, 14, a041197. [Google Scholar] [CrossRef] [PubMed]
- Kubozono, T.; Miyata, M.; Ueyama, K.; Nagaki, A.; Otsuji, Y.; Kusano, K.; Kubozono, O.; Tei, C. Clinical significance and reproducibility of new arterial distensibility index. Circ. J. 2007, 71, 89–94. [Google Scholar] [CrossRef]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Ibata, J.; Sasaki, H.; Kakimoto, T.; Matsuno, S.; Nakatani, M.; Kobayashi, M.; Tatsumi, K.; Nakano, Y.; Wakasaki, H.; Furuta, H.; et al. Cardio-ankle vascular index measures arterial wall stiffness independent of blood pressure. Diabetes Res. Clin. Pract. 2008, 80, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Song, M.; Suzuki, J.; Kurosu, T.; Oyama, T.; Nagayama, D.; Miyashita, Y.; Yamamura, S.; Takahashi, M. Contradictory Effects of β1- and α1-adrenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI). J. Atheroscler. Thromb. 2011, 18, 49–55. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 110) | Control Group (n = 73) | PAS Group (n = 37) | p Value |
|---|---|---|---|---|
| Age (years) | 60.47 ± 11.16 | 58.78 ± 11.56 | 63.81 ± 9.61 | 0.025 * |
| HD vintage (months) | 65.70 (30.81–101.85) | 67.80 (31.50–108.54) | 53.88 (27.96–86.10) | 0.374 |
| Pre-HD body weight (Kg) | 70.73 ± 16.95 | 70.16 ± 17.68 | 71.84 ± 15.57 | 0.625 |
| Post-HD body weight (Kg) | 68.50 ± 16.23 | 68.03 ± 17.01 | 69.42 ± 14.75 | 0.672 |
| Body mass index (Kg/m2) | 26.63 ± 5.27 | 26.70 ± 5.32 | 26.50 ± 5.25 | 0.850 |
| Left CAVI | 7.59 ± 2.71 | 6.06 ± 1.73 | 10.59 ± 1.52 | <0.001 * |
| Right CAVI | 7.56 ± 2.70 | 6.01 ± 1.71 | 10.62 ± 1.30 | <0.001 * |
| Systolic blood pressure (mmHg) | 149.52 ± 27.04 | 145.86 ± 21.17 | 156.78 ± 25.10 | 0.018 * |
| Diastolic blood pressure (mmHg) | 79.84 ± 12.98 | 78.73 ± 12.72 | 82.03 ± 13.39 | 0.209 |
| White blood cell (×103 μL) | 6.51 ± 1.80 | 6.55 ± 1.79 | 6.41 ± 1.83 | 0.706 |
| Hemoglobin (g/dL) | 10.72 ± 1.27 | 10.72 ± 1.45 | 10.74 ± 0.83 | 0.926 |
| Albumin (mg/dL) | 4.34 ± 0.54 | 4.39 ± 0.52 | 4.24 ± 0.57 | 0.153 |
| Total cholesterol (mg/dL) | 150.14 ± 44.58 | 149.58 ± 46.41 | 151.24 ± 41.34 | 0.854 |
| Triglyceride (mg/dL) | 147.50 (93.75–220.25) | 148.00 (95.00–122.50) | 147.00 (90.00–205.50) | 0.569 |
| Glucose (mg/dL) | 153.50 (114.00–209.50) | 148.00 (95.00–222.50) | 144.00 (113.00–232.00) | 0.952 |
| Blood urea nitrogen (mg/dL) | 64.96 ± 17.69 | 65.82 ± 18.67 | 63.27 ± 15.68 | 0.477 |
| Creatinine (mg/dL) | 10.34 ± 2.67 | 10.42 ± 2.63 | 10.17 ± 2.76 | 0.650 |
| Total calcium (mg/dL) | 9.27 ± 0.91 | 9.25 ± 0.80 | 9.32 ± 1.11 | 0.711 |
| Phosphorus (mg/dL) | 4.76 ± 1.35 | 4.67 ± 1.38 | 4.94 ± 1.29 | 0.331 |
| iPTH (pg/mL) | 329.55 (203.33–512.25) | 334.30 (222.95–508.95) | 315.50 (195.70–601.10) | 0.922 |
| Uric acid (mg/dL) | 6.66 ± 1.51 | 6.76 ± 1.57 | 6.46 ± 1.40 | 0.336 |
| Total p-Cresyl sulfate (mg/L) | 21.70 ± 12.02 | 16.68 ± 8.03 | 31.61 ± 12.52 | <0.001 * |
| C-reactive protein (mg/dL) | 0.31 (0.15–0.57) | 0.29 (0.13–0.55) | 0.41 (0.24–0.87) | 0.007 * |
| Urea reduction rate | 0.72 ± 0.05 | 0.72 ± 0.05 | 0.72 ± 0.05 | 0.961 |
| Kt/V (Gotch) | 1.28 ± 0.20 | 1.28 ± 0.19 | 1.28 ± 0.21 | 0.994 |
| Female, n (%) | 50 (45.50) | 33 (45.20) | 17 (45.90) | 0.941 |
| Diabetes mellitus, n (%) | 56 (50.90) | 32 (43.80) | 24 (64.90) | 0.037 * |
| Hypertension, n (%) | 66 (60.00) | 39 (53.40) | 27 (73.00) | 0.048 * |
| Causes of uremia | ||||
| Diabetic nephropathy, n (%) | 56 (50.90) | 37 (50.70) | 19 (51.40) | 0.947 |
| Glomerulonephritis, n (%) | 31 (28.20) | 23 (31.50) | 8 (21.60) | 0.276 |
| Hypertensive nephrosclerosis, n (%) | 18 (16.40) | 11 (15.10) | 7 (18.90) | 0.606 |
| p-Cresyl Sulfate | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | aOR (95% CI) | p Value | aOR (95% CI) | p Value | |
| Per 1 mg/L increase | 1.178 (1.106–1.255) | <0.001 * | 1.199 (1.112–1.292) | <0.001 * | 1.238 (1.119–1.371) | <0.001 * |
| Variables | Left CAVI | Right CAVI | PCS (mg/L) | |||
|---|---|---|---|---|---|---|
| Spearman Correlation Coefficient | p Value | Spearman Correlation Coefficient | p Value | Spearman Correlation Coefficient | p Value | |
| Left CAVI | — | — | 0.934 | <0.001 * | 0.557 | <0.001 * |
| Right CAVI | 0.934 | <0.001 * | — | — | 0.555 | <0.001 * |
| PCS (mg/L) | 0.557 | <0.001 * | 0.555 | <0.001 * | — | — |
| Age (years) | 0.219 | 0.022 * | 0.206 | 0.031 * | 0.244 | 0.010 * |
| Log-HD vintage (months) | −0.056 | 0.561 | −0.031 | 0.748 | 0.001 | 0.989 |
| SBP (mmHg) | 0.197 | 0.039 * | 0.180 | 0.059 | 0.085 | 0.376 |
| DBP (mmHg) | 0.081 | 0.401 | 0.060 | 0.532 | 0.068 | 0.480 |
| White blood cell (×103 μL) | −0.038 | 0.692 | −0.062 | 0.523 | 0.071 | 0.462 |
| Hemoglobin (g/dL) | −0.031 | 0.749 | −0.114 | 0.238 | −0.039 | 0.686 |
| Albumin (mg/dL) | −0.123 | 0.199 | −0.129 | 0.180 | −0.141 | 0.142 |
| Total cholesterol (mg/dL) | 0.076 | 0.430 | 0.042 | 0.662 | −0.076 | 0.431 |
| Log-Triglyceride (mg/dL) | −0.017 | 0.857 | −0.045 | 0.644 | −0.104 | 0.279 |
| Log-Glucose (mg/dL) | 0.039 | 0.684 | 0.019 | 0.840 | −0.088 | 0.363 |
| BUN (mg/dL) | −0.007 | 0.939 | −0.021 | 0.826 | 0.029 | 0.762 |
| Creatinine (mg/dL) | −0.020 | 0.837 | −0.074 | 0.441 | −0.047 | 0.625 |
| Total calcium (mg/dL) | 0.072 | 0.457 | 0.089 | 0.353 | −0.102 | 0.291 |
| Phosphorus (mg/dL) | 0.101 | 0.293 | 0.014 | 0.886 | 0.061 | 0.525 |
| Log-iPTH (pg/mL) | −0.012 | 0.900 | −0.019 | 0.845 | 0.049 | 0.611 |
| Uric acid (mg/dL) | −0.070 | 0.464 | −0.106 | 0.270 | −0.167 | 0.080 |
| Log-CRP (mg/dL) | 0.205 | 0.032 * | 0.226 | 0.018 * | 0.277 | 0.003 * |
| Urea reduction rate | 0.113 | 0.238 | 0.117 | 0.224 | 0.001 | 0.990 |
| Kt/V (Gotch) | 0.118 | 0.219 | 0.128 | 0.184 | 0.023 | 0.814 |
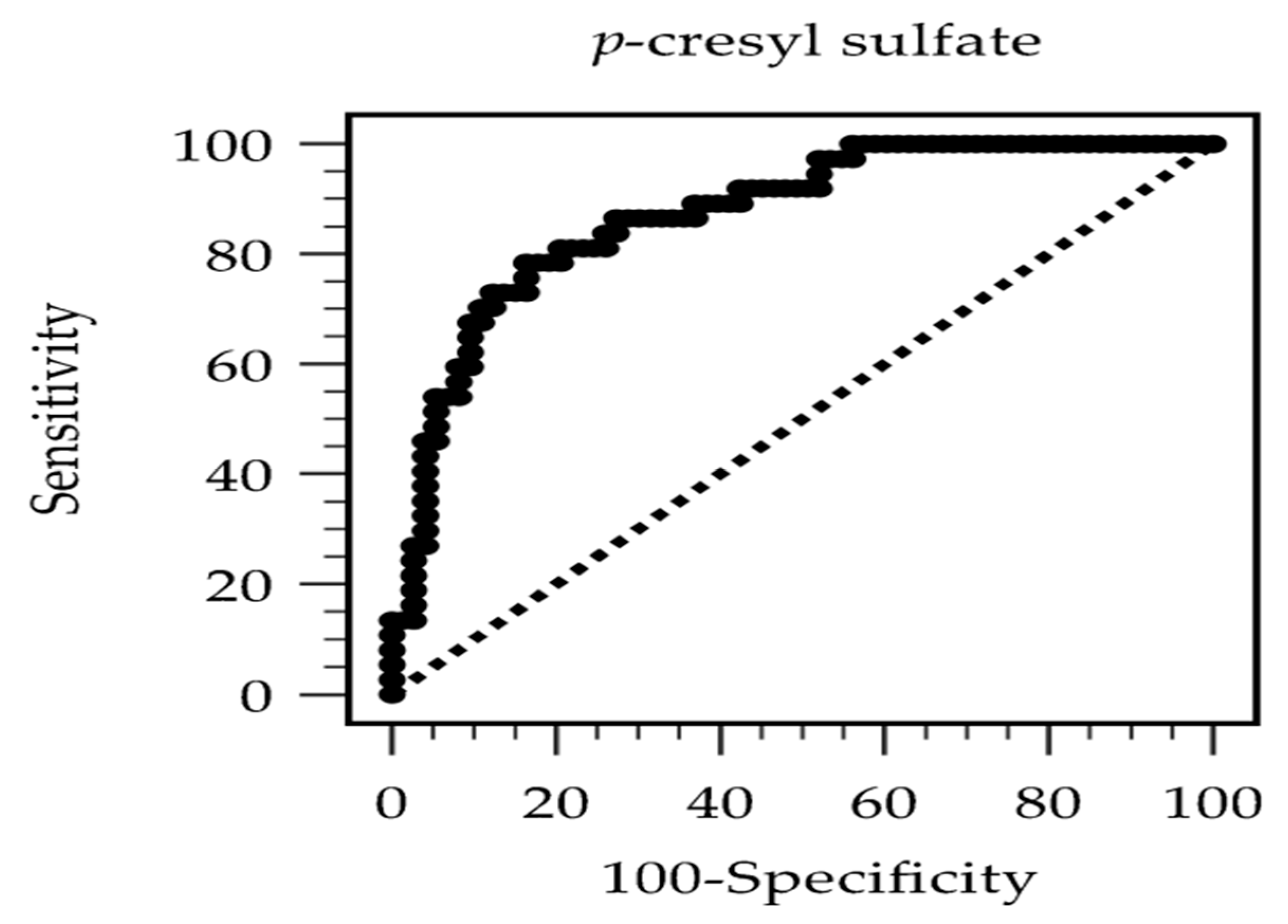
| Study (Author, Year) | Population | N | Arterial Stiffness Measurement | Key Association Metric | AUC (95% CI) | Optimal Cutoff (mg/L) |
|---|---|---|---|---|---|---|
| Current study | HD | 110 | CAVI | aOR: 1.238 per 1 mg/L increase | 0.872 (0.805–0.939) | 24.29 |
| Lai et al. (2019) [17] | HD | 118 | cfPWV | aOR: 1.067 per 1 mg/L increase | 0.661 (0.568–0.746) | 18.99 |
| Chang et al. (2022) [18] | Non-dialysis CKD (stage 3–5) | 160 | baPWV | aOR: 1.098 per 1 mg/L increase | 0.628 (0.531–0.725) | 20.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-B.; Wang, C.-H.; Liu, C.-H.; Liou, H.-H.; Tsai, J.-P.; Hsu, B.-G. Serum P-Cresyl Sulfate Is Associated with Peripheral Arterial Stiffness in Chronic Hemodialysis Patients. Diagnostics 2025, 15, 2353. https://doi.org/10.3390/diagnostics15182353
Chern Y-B, Wang C-H, Liu C-H, Liou H-H, Tsai J-P, Hsu B-G. Serum P-Cresyl Sulfate Is Associated with Peripheral Arterial Stiffness in Chronic Hemodialysis Patients. Diagnostics. 2025; 15(18):2353. https://doi.org/10.3390/diagnostics15182353
Chicago/Turabian StyleChern, Yahn-Bor, Chih-Hsien Wang, Chin-Hung Liu, Hung-Hsiang Liou, Jen-Pi Tsai, and Bang-Gee Hsu. 2025. "Serum P-Cresyl Sulfate Is Associated with Peripheral Arterial Stiffness in Chronic Hemodialysis Patients" Diagnostics 15, no. 18: 2353. https://doi.org/10.3390/diagnostics15182353
APA StyleChern, Y.-B., Wang, C.-H., Liu, C.-H., Liou, H.-H., Tsai, J.-P., & Hsu, B.-G. (2025). Serum P-Cresyl Sulfate Is Associated with Peripheral Arterial Stiffness in Chronic Hemodialysis Patients. Diagnostics, 15(18), 2353. https://doi.org/10.3390/diagnostics15182353








