The Value of Baseline [18F]FDG-PET in Predicting the Progression-Free Survival in Patients with Thymic Epithelial Tumours: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy
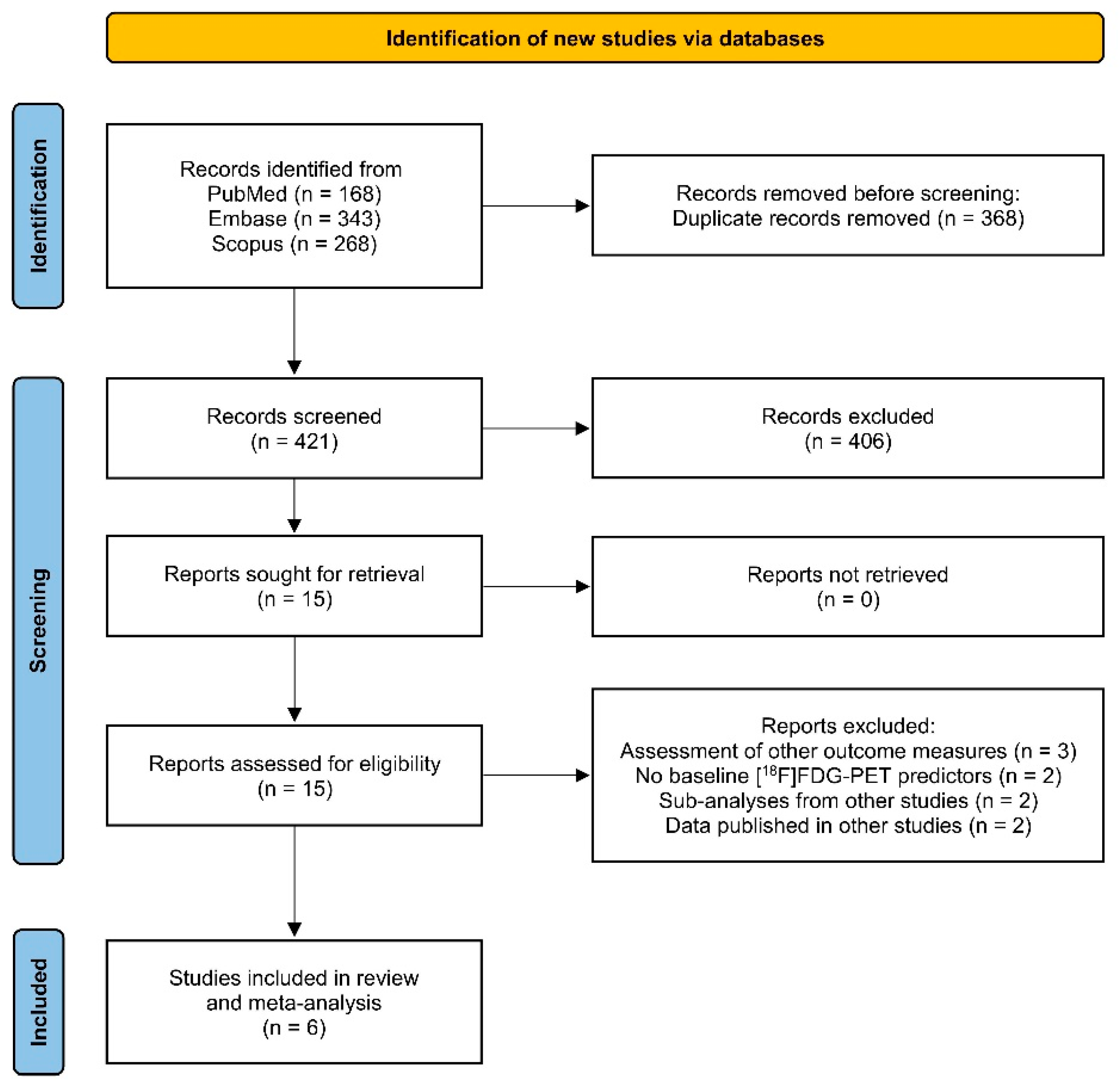
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Risk of Bias (RoB) Assessment
3. Results
3.1. Narrative Description
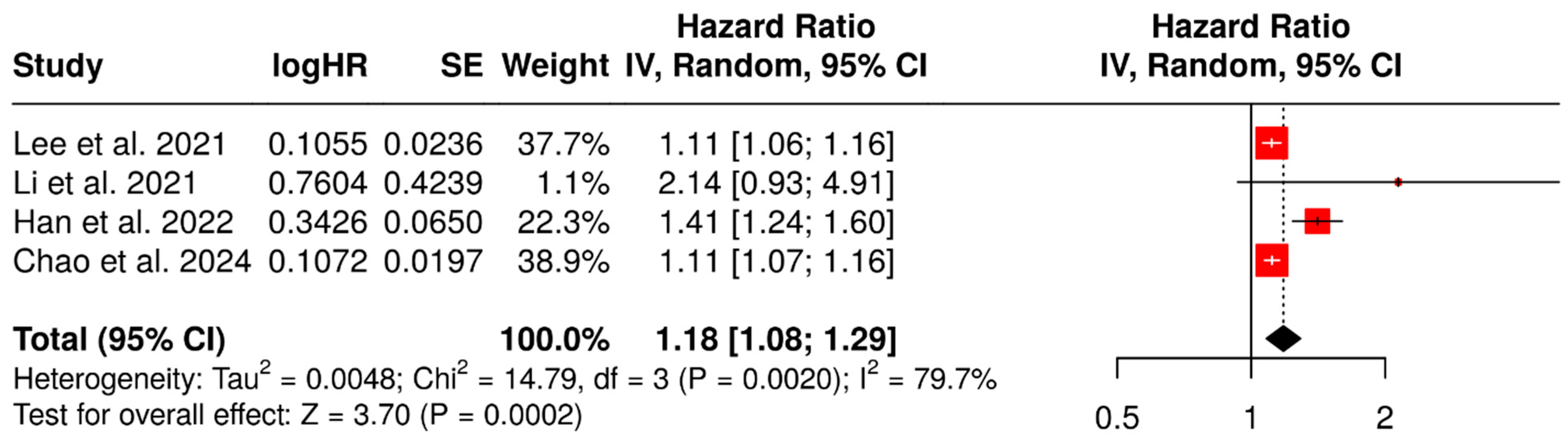
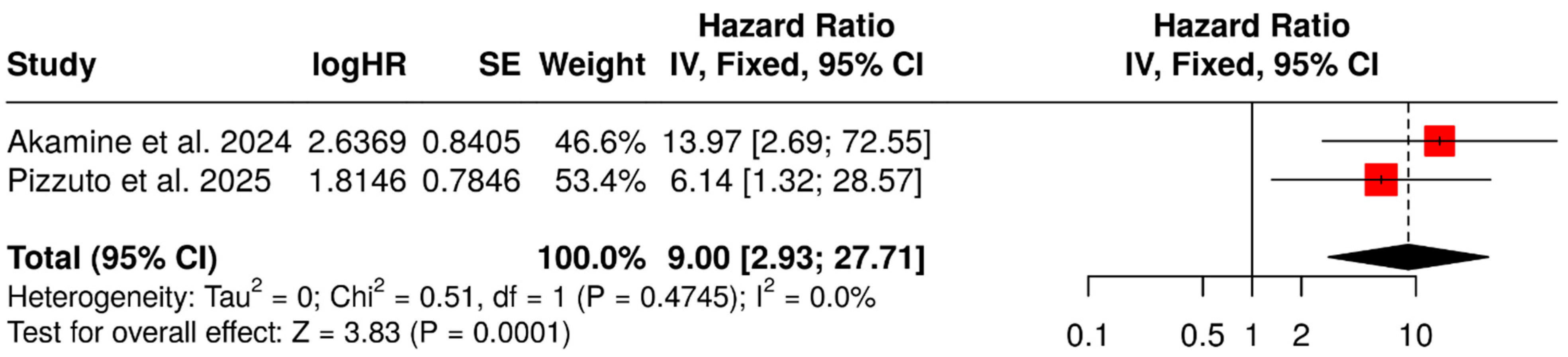
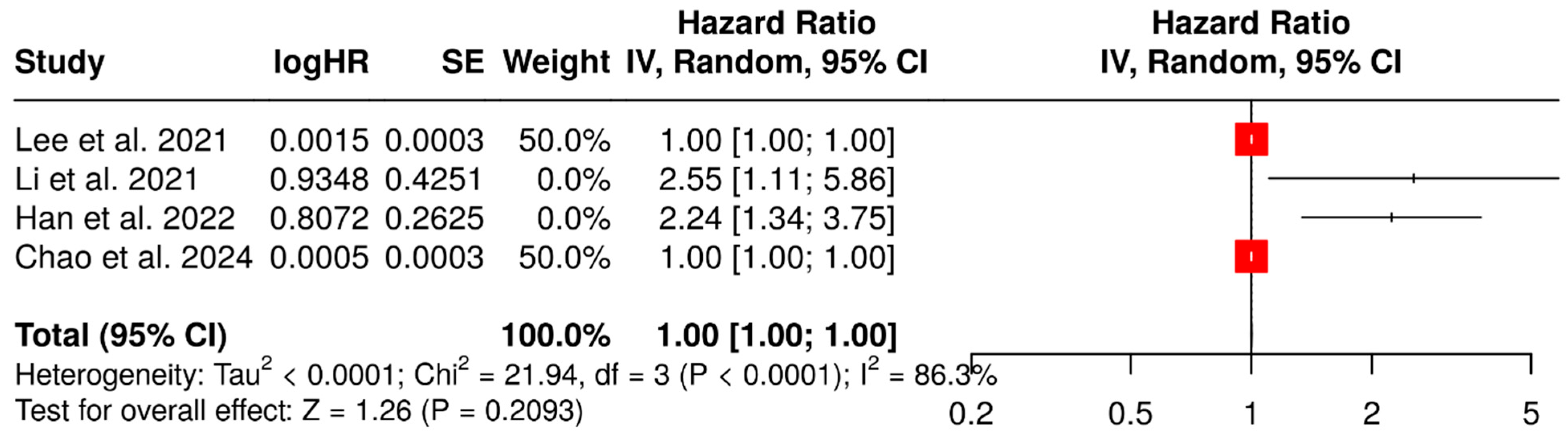
3.2. Prognostic Value of SUVmax
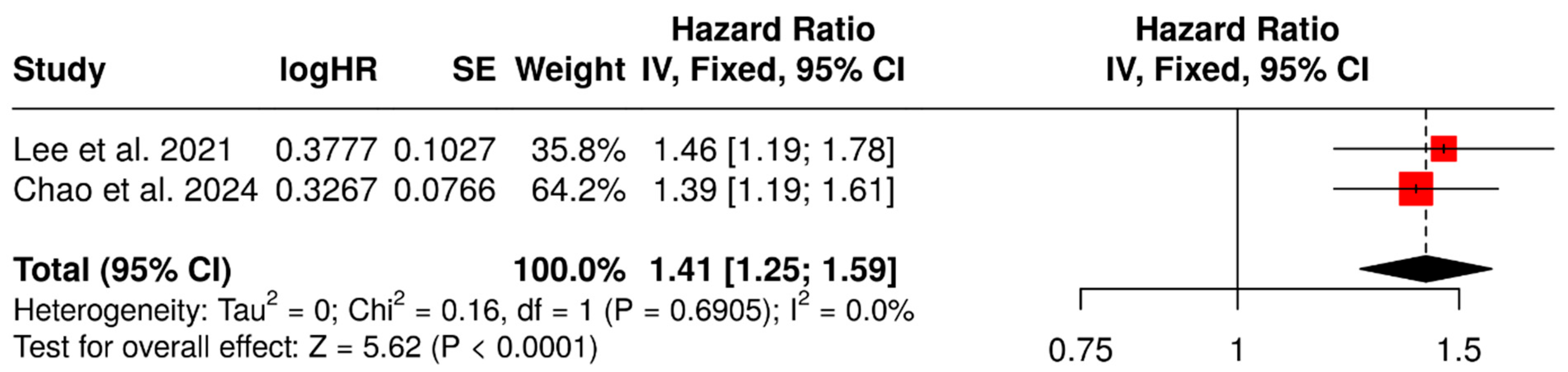
3.3. Prognostic Value of SUVmean
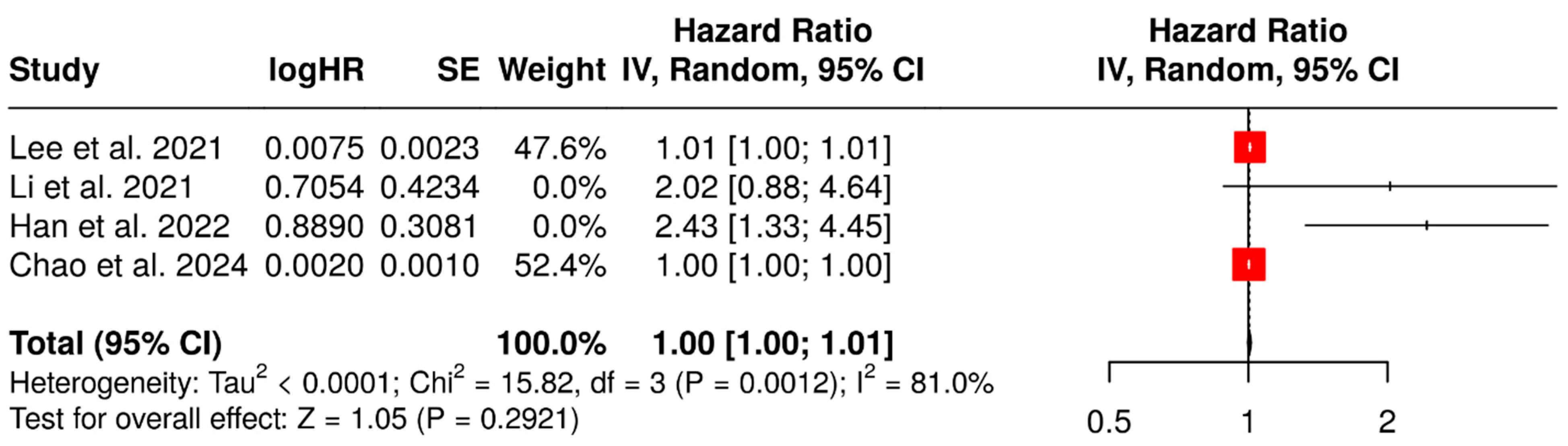
3.4. Prognostic Value of MTV and TLG
4. Quality of the Studies
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumors. Thoracic Tumors, 5th ed.; The International Agency for Research on Cancer (IARC): Lyon, France, 2021. [Google Scholar]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet—a population-based study. Lancet Oncol. 2017, 18, 1022–1039, Erratum in Lancet Oncol. 2017, 18, e433. https://doi.org/10.1016/S1470-2045(17)30531-4. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Bernabé, R.; Diz, P.; Felip, E.; González-Larriba, J.L.; Lázaro, M.; Mielgo-Rubio, X.; Sánchez, A.; Sullivan, I.; Massutti, B. SEOM-GECP-GETTHI Clinical Guidelines for the treatment of patients with thymic epithelial tumours (2021). Clin. Transl. Oncol. 2022, 24, 635–645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rich, A.L. Epidemiology of thymoma. J. Thorac. Dis. 2020, 12, 7531–7535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, Z.-F.; Mo, X.-A.; Qin, C.; Lai, Y.-R.; Hackett, M.L. Incidence of thymoma in myasthenia gravis: A systematic review. J. Clin. Neurol. 2012, 8, 161–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadoux, J.; Lamarca, A.; Grande, E.; Deandreis, D.; Kaltsas, G.; Janson, E.; Tombal, B.; Pavel, M.; Thariat, J.; van Velthuysen, M.; et al. Neuroendocrine neoplasms of head and neck, genitourinary and gynaecological systems, unknown primaries, parathyroid carcinomas and intrathyroid thymic neoplasms: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2024, 9, 103664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zdrojowy-Wełna, A.; Bolanowski, M.; Syrycka, J.; Jawiarczyk-Przybyłowska, A.; Kuliczkowska-Płaksej, J. Case Report: Thymic neuroendocrine tumor with metastasis to the breast causing ectopic Cushing’s syndrome. Front. Oncol. 2025, 15, 1492187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawrence, L.; Zhang, P.; Choi, H.; Ahmad, U.; Arrossi, V.; Purysko, A.; Makin, V. A unique case of ectopic Cushing’s syndrome from a thymic neuroendocrine carcinoma. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agrafiotis, A.C.; Berzenji, L.; Koyen, S.; Vermeulen, D.; Winthagen, R.; Hendriks, J.M.H.; Van Schil, P.E. Surgical treatment of thymic epithelial tumors: A narrative review. Mediastinum 2024, 8, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavese, V.; Carfì, F.M.; Capelletto, E.; Tabbò, F.; Leo, F.; Passiglia, F.; Righi, L.; Novello, S.; Merlini, A.; Bironzo, P. Therapeutic management of patients with advanced thymic malignancies: A review for clinicians. Lung Cancer 2025, 204, 108554. [Google Scholar] [CrossRef] [PubMed]
- Kishi, N.; Matsuo, Y. Postoperative radiotherapy for thymic epithelial tumors: A narrative review. Mediastinum 2024, 8, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, Y.; Iwahori, K.; Shintani, Y. Current immunotherapy for thymic epithelial tumors: A narrative review. Mediastinum 2024, 8, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiesewetter, B.; Melhorn, P.; Fuereder, T. Thymic malignancies: Role of immunotherapy and novel approaches. Curr. Opin. Oncol. 2025, 37, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Nicholson, A.G.; Kondo, K.; Van Schil, P.; Moran, C. The Masaoka-Koga stage classification for thymic malignancies: Clarification and definition of terms. J. Thorac. Oncol. 2011, 6, S1710–S1716. [Google Scholar] [CrossRef]
- Sorin, V.; Kirshenboim, Z.; Klug, M.; Ahuja, J.; Marom, E.M. The Ninth Edition TNM Staging Classification for Thymic Epithelial Tumors. Semin. Ultrasound CT MRI 2024, 45, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.M.; Ahuja, J.; Strange, C.D.; Shroff, G.S.; Gladish, G.W.; Sabloff, B.S.; Truong, M.T. Multimodality imaging of mediastinal masses and mimics. Mediastinum 2023, 7, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohno, Y.; Kishida, Y.; Seki, S.; Koyama, H.; Yui, M.; Aoyagi, K.; Yoshikawa, T. Comparison of Interobserver Agreement and Diagnostic Accuracy for IASLC/ITMIG Thymic Epithelial Tumor Staging Among Co-registered FDG-PET/MRI, Whole-body MRI, Integrated FDG-PET/CT, and Conventional Imaging Examination with and without Contrast Media Administrations. Acad. Radiol. 2022, 29 (Suppl. S3), S122–S131. [Google Scholar] [CrossRef] [PubMed]
- Heeger, A.P.; Ackman, J.B. Added Value of Magnetic Resonance Imaging for the Evaluation of Mediastinal Lesions. Radiol Clin. North Am. 2021, 59, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.; Ratanaprasatporn, L.; Ratanaprasatporn, L.; Carter, B.W.; Ackman, J.B. Cystic mediastinal masses and the role of MRI. Clin. Imaging 2018, 50, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S. ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v40–v55. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Wang, J.; Fu, Q.; Liu, J.; Ma, W.; Xu, W.; Chen, W. Value of metabolic parameters in distinguishing primary mediastinal lymphomas from thymic epithelial tumors. Cancer Biol. Med. 2020, 17, 468–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nappi, A.G.; Dondi, F.; Lazzarato, A.; Jonghi-Lavarini, L.; Gorica, J.; La Torre, F.; Santo, G.; Miceli, A. Primary Mediastinal B-Cell Lymphoma and [18F]FDG PET/CT: What We Learned and What Is New. Hematol. Rep. 2025, 17, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, G.; Du, L.; Lu, X.; Liu, J.; Zhang, M.; Pan, Y.; Meng, X.; Xu, X.; Guan, Z.; Yang, J. Multiparameter diagnostic model based on 18F-FDG PET and clinical characteristics can differentiate thymic epithelial tumors from thymic lymphomas. BMC Cancer 2022, 22, 895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferdinand, B.; Gupta, P.; Kramer, E.L. Spectrum of thymic uptake at 18F-FDG PET. Radiographics 2004, 24, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Jerushalmi, J.; Frenkel, A.; Bar-Shalom, R.; Khoury, J.; Israel, O. Physiologic thymic uptake of 18F-FDG in children and young adults: A PET/CT evaluation of incidence, patterns, and relationship to treatment. J. Nucl. Med. 2009, 50, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.-Y.; Ao, Y.-Q.; Jiang, J.-H.; Lin, M.; Wang, S.; Shi, H.-C.; Ding, J.-Y. Clinical significance of positron emission tomography-computed tomography in the classification of thymic tumors. Interdiscip. Cardiovasc. Thorac. Surg. 2025, 40, ivaf065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, Y.M.; Lee, K.S.; Kim, B.-T.; Choi, J.Y.; Shim, Y.M.; A Yi, C. 18F-FDG PET/CT of thymic epithelial tumors: Usefulness for distinguishing and staging tumor subgroups. J. Nucl. Med. 2006, 47, 1628–1634. [Google Scholar] [PubMed]
- Lococo, F.; Cesario, A.; Okami, J.; Cardillo, G.; Cavuto, S.; Tokunaga, T.; Apolone, G.; Margaritora, S.; Granone, P. Role of combined 18F-FDG-PET/CT for predicting the WHO malignancy grade of thymic epithelial tumors: A multicenter analysis. Lung Cancer 2013, 82, 245–251. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Giovanella, L.; Cafarotti, S.; Filosso, P.; Lococo, F. Is 18F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer 2014, 86, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, M.; Mendogni, P.; Cattaneo, M.; Evangelista, J.; Farina, P.; Pizzuto, D.A.; Annunziata, S.; Castello, A.; Congedo, M.T.; Tabacco, D.; et al. Is PET/CT Able to Predict Histology in Thymic Epithelial Tumours? A Narrative Review. Diagnostics 2022, 13, 98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morita, T.; Tatsumi, M.; Ishibashi, M.; Isohashi, K.; Kato, H.; Honda, O.; Shimosegawa, E.; Tomiyama, N.; Hatazawa, J. Assessment of Mediastinal Tumors Using SUVmax and Volumetric Parameters on FDG-PET/CT. Asia Ocean. J. Nucl. Med. Biol. 2017, 5, 22–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakajo, M.; Takeda, A.; Katsuki, A.; Jinguji, M.; Ohmura, K.; Tani, A.; Sato, M.; Yoshiura, T. The efficacy of 18F-FDG-PET-based radiomic and deep-learning features using a machine-learning approach to predict the pathological risk subtypes of thymic epithelial tumors. Br. J. Radiol. 2022, 95, 20211050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.S.; Oh, J.S.; Park, Y.S.; Jang, S.J.; Choi, I.S.; Ryu, J.-S. Differentiating the grades of thymic epithelial tumor malignancy using textural features of intratumoral heterogeneity via 18F-FDG PET/CT. Ann. Nucl. Med. 2016, 30, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, P.; Wang, Z.; Liu, Y.; Fan, B.; Dong, W. Radiomics in thymic epithelial tumors: A scoping review of current status and advances. BMC Cancer 2025, 25, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid. Implement. 2015, 13, 196–207. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias Due to Missing Evidence in a Meta-Analysis. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Hamilton, ON, Canada, 2024; version 6.5. [Google Scholar]
- Review Manager (RevMan) [Computer Program], Version 5.3; The Nordic Cochrane Centre: Copenhagen, Denmark; The Cochrane Collaboration: London, UK. Available online: https://revman.cochrane.org/info (accessed on 22 May 2025).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2012. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 May 2025).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.S.; Kim, J.; Shim, Y.M.; Lee, K.-H.; Choi, J.Y. Prognostic Significance of Metabolic Parameters by 18F-FDG PET/CT in Thymic Epithelial Tumors. Cancers 2021, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Huang, Y.; Wu, X.; Yang, Z.; Wu, C.; Jiang, L. Usefulness of 18F-FDG PET/CT in treatment-naive patients with thymic squamous cell carcinoma. Ann. Nucl. Med. 2021, 35, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Oh, J.S.; Kim, Y.I.; Seo, S.Y.; Lee, G.D.; Park, M.J.; Choi, S.; Kim, H.R.; Kim, Y.H.; Kim, D.K.; et al. Fully Automatic Quantitative Measurement of 18F-FDG PET/CT in Thymic Epithelial Tumors Using a Convolutional Neural Network. Clin. Nucl. Med. 2022, 47, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Akamine, T.; Nakagawa, K.; Ito, K.; Watanabe, H.; Yotsukura, M.; Yoshida, Y.; Yatabe, Y.; Kusumoto, M.; Watanabe, S.-I. Impact of 18F-FDG PET on TNM Staging and Prognosis in Thymic Epithelial Tumors. Ann. Surg. Oncol. 2024, 31, 192–200. [Google Scholar] [CrossRef]
- Chao, F.; Wang, R.; Han, X.; Huang, W.; Wang, R.; Yu, Y.; Lin, X.; Yuan, P.; Yang, M.; Gao, J. Intratumoral metabolic heterogeneity by 18F-FDG PET/CT to predict prognosis for patients with thymic epithelial tumors. Thorac. Cancer 2024, 15, 1437–1445. [Google Scholar] [CrossRef]
- Pizzuto, D.A.; Castello, A.; Chiappetta, M.; Castellani, M.; Annunziata, S.; Campanella, A.; Calabrese, G.; Cattaneo, M.; Rosso, L.; Cusumano, G.; et al. The Role of [18F]F-FDG PET/CT for Predicting Histology and Prognosis in Patients with Thymic Lesions. Mol. Diagn. Ther. 2025, 29, 239–248. [Google Scholar] [CrossRef]
- Berghmans, T.; Dusart, M.; Paesmans, M.; Hossein-Foucher, C.; Buvat, I.; Castaigne, C.; Scherpereel, A.; Mascaux, C.; Moreau, M.; Roelandts, M.; et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): A systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J. Thorac. Oncol. 2008, 3, 6–12. [Google Scholar] [CrossRef]
- Dong, M.; Liu, J.; Sun, X.; Xing, L. Prognositc significance of SUVmax on pretreatment 18F-FDG PET/CT in early-stage non-small cell lung cancer treated with stereotactic body radiotherapy: A meta-analysis. J. Med. Imaging Radiat. Oncol. 2017, 61, 652–659. [Google Scholar] [CrossRef]
- Wen, W.; Xu, D.; Piao, Y.; Li, X. Prognostic value of maximum standard uptake value, metabolic tumour volume, and total lesion glycolysis of 18F-FDG PET/CT in patients with malignant pleural mesothelioma: A systematic review and meta-analysis. Cancer Cell Int. 2022, 22, 60. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Zhang, H.; Chen, J.; Wang, Y.; Byanju, S.; Liao, M. Prognostic value of 18F-FDG-PET/CT parameters in patients with pancreatic carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e7813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Zhang, J.; Cheng, W.; Zhu, C.; Chen, L.; Xia, F.; Wang, M.; Yang, F.; Ma, X. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e8084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tosi, D.; Pieropan, S.; Cattoni, M.; Bonitta, G.; Franzi, S.; Mendogni, P.; Imperatori, A.; Rotolo, N.; Castellani, M.; Cuzzocrea, M.; et al. Prognostic Value of 18F-FDG PET/CT Metabolic Parameters in Surgically Treated Stage I Lung Adenocarcinoma Patients. Clin. Nucl. Med. 2021, 46, 621–626. [Google Scholar] [CrossRef]
- Amrane, K.; Thuillier, P.; Bourhis, D.; Le Meur, C.; Quere, C.; Leclere, J.C.; Ferec, M.; Jestin-Le Tallec, V.; Doucet, L.; Alemany, P.; et al. Prognostic value of pre-therapeutic FDG-PET radiomic analysis in gastro-esophageal junction cancer. Sci. Rep. 2023, 13, 5789, Erratum in Sci. Rep. 2023, 13, 6665. https://doi.org/10.1038/s41598-023-33877-7. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, J.W.; Kang, B.; Song, S.Y.; Lee, J.D.; Lee, J.-H. Prognostic significance of volume-based FDG PET/CT parameters in patients with locally advanced pancreatic cancer treated with chemoradiation therapy. Yonsei Med. J. 2014, 55, 1498–1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dholakia, A.S.; Chaudhry, M.; Leal, J.P.; Chang, D.T.; Raman, S.P.; Hacker-Prietz, A.; Su, Z.; Pai, J.; Oteiza, K.E.; Griffith, M.E.; et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 539–546. [Google Scholar] [CrossRef]
- Pak, K.; Cheon, G.J.; Nam, H.-Y.; Kim, S.-J.; Kang, K.W.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: A systematic review and meta-analysis. J. Nucl. Med. 2014, 55, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Rebuzzi, S.E.; Signori, A.; Donegani, M.I.; Murianni, V.; Miceli, A.; Borea, R.; Raffa, S.; Damassi, A.; Ponzano, M.; et al. The Prognostic Role of Baseline Metabolic Tumor Burden and Systemic Inflammation Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223: A Proof of Concept Study. Cancers 2020, 12, 3213. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Wehrle, C.J.; Satish, S.; Mahajan, P.; Kamath, S.; Koyfman, S.; Ma, W.W.; Linganna, M.; Esfeh, J.M.; Miller, C.; et al. PET-Assessed Metabolic Tumor Volume Across the Spectrum of Solid-Organ Malignancies: A Review of the Literature. Biomedicines 2025, 13, 123. [Google Scholar] [CrossRef]
- Cook, G.J.; Yip, C.; Siddique, M.; Goh, V.; Chicklore, S.; Roy, A.; Marsden, P.; Ahmad, S.; Landau, D. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J. Nucl. Med. 2013, 54, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Goh, V.; Mandeville, H.C.; Ng, Q.S.; Hoskin, P.J.; Miles, K.A. Non-small cell lung cancer: Histopathologic correlates for texture parameters at CT. Radiology 2013, 266, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K. Practical value of fluorodeoxyglucose positron emission tomography in treatment strategies for thymic epithelial tumors: Implications for more specific use in routine clinical practice. Mediastinum 2025, 9, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Database | Research String |
|---|---|
| PubMed | ((thymoma) OR ((thymic) AND (cancer OR carcinoma OR adenocarcinoma OR neoplasia OR neoplasm OR tumour OR tumour)) AND ((positron emission tomography OR pet) AND (fdg OR fluorodeoxyglucose)) AND (survival OR progression OR recurrence OR relapse OR prognosis OR prognostic)) |
| Embase | ((‘thymoma’) OR ((‘thymic’) AND (‘cancer’ OR ‘carcinoma’ OR ‘adenocarcinoma’ OR ‘neoplasia’ OR ‘neoplasm’ OR ‘tumour’ OR ‘tumour’)) AND ((‘positron emission tomography’ OR ‘pet’) AND (‘fdg’ OR ‘fluorodeoxyglucose’)) AND (‘survival’ OR ‘progression’ OR ‘recurrence’ OR ‘relapse’ OR ‘prognosis’ OR ‘prognostic’)) |
| Scopus | ((thymoma) OR ((thymic) AND (cancer OR carcinoma OR adenocarcinoma OR neoplasia OR neoplasm OR tumour OR tumour)) AND ((“positron emission tomography” OR pet) AND (fdg OR fluorodeoxyglucose)) AND (survival OR progression OR recurrence OR relapse OR prognosis OR prognostic)) |
| Author, Year | Study Design | Enrolled Patients | Follow-Up (Months) | TETs Classification | Event for PFS |
|---|---|---|---|---|---|
| Lee et al. 2021 [45] | Retrospective, monocentric | 83 | Mean: 28.6 SD: 22.2 Range: 0.0–79.0 | Thymoma Carcinoma | Disease recurrence, progression, or death |
| Li et al. 2021 [46] | Retrospective, monocentric | 42 | Mean: 21 SD: 12 Range: 7–60 | Carcinoma | Disease progression |
| Han et al., 2022 [47] | Retrospective, monocentric | 114 | Median: 39 IQR: 25–58 | Thymoma Carcinoma | Disease recurrence |
| Akamine et al., 2024 [48] | Retrospective, monocentric | 177 | Median: 35 IQR: 12–59 | Thymoma Carcinoma Neuroendocrine neoplasm | Disease recurrence, progression, or death |
| Chao et al., 2024 [49] | Retrospective, monocentric | 100 | Mean: 25.7 SD: 19.8 Range 1–97 | Thymoma Carcinoma | Disease recurrence, progression, or death |
| Pizzuto et al., 2025 [50] | Retrospective, bi-centric | 77 | Median: 38 Range 14–72 | Hyperplasia Thymoma Carcinoma | Disease recurrence |
| Author, Year | [18F]FDG-PET Parameter | Variable Type | Variable Computation |
|---|---|---|---|
| Lee et al., 2021 [45] | SUVmax | Continuous | |
| SUVmean | Continuous | ||
| MTV | Continuous | Fixed SUV threshold of 2.5 | |
| TLG | Continuous | ||
| Li et al., 2021 [46] | SUVmax | Continuous | |
| Primary tumour MTV | Continuous | Threshold at 40% of SUVmax | |
| Primary tumour TLG | Continuous | ||
| Metastases MTV | Continuous | Threshold at 40% of SUVmax | |
| Metastases TLG | Continuous | ||
| Han et al., 2022 [47] | SUVmax | Continuous | |
| MTV | Continuous | Fixed SUV threshold of 2.5 | |
| TLG | Continuous | ||
| SUVmax | Continuous | Automatic segmentation | |
| MTV | Continuous | Automatic segmentation Fixed SUV threshold of 2.5 | |
| TLG | Continuous | Automatic segmentation | |
| Akamine et al., 2024 [48] | SUVmax | Binarized | |
| Chao et al., 2024 [49] | SUVmax | Continuous | |
| SUVmean | Continuous | ||
| MTV | Continuous | Fixed SUV threshold of 2.5 | |
| TLG | Continuous | ||
| Heterogeneity index-1 | Continuous | ||
| Heterogeneity index-2 | Continuous | ||
| Pizzuto et al., 2025 [50] | SUVmax | Binarized | |
| SUVmean | Binarized | ||
| SUVpeak | Binarized | ||
| MTV | Binarized | Threshold at 40% of SUVmax | |
| TLG | Binarized | ||
| rPET | Binarized | ||
| qPET | Binarized | ||
| T/M | Binarized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, A.; Librando, M.; Dondi, F.; Jonghi-Lavarini, L.; D’Antonio, A.; Mura, A.; Nappi, A.G.; Rovera, G.; De Feo, M.S.; Santo, G.; et al. The Value of Baseline [18F]FDG-PET in Predicting the Progression-Free Survival in Patients with Thymic Epithelial Tumours: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 2458. https://doi.org/10.3390/diagnostics15192458
Miceli A, Librando M, Dondi F, Jonghi-Lavarini L, D’Antonio A, Mura A, Nappi AG, Rovera G, De Feo MS, Santo G, et al. The Value of Baseline [18F]FDG-PET in Predicting the Progression-Free Survival in Patients with Thymic Epithelial Tumours: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(19):2458. https://doi.org/10.3390/diagnostics15192458
Chicago/Turabian StyleMiceli, Alberto, Maria Librando, Francesco Dondi, Lorenzo Jonghi-Lavarini, Adriana D’Antonio, Antonio Mura, Anna Giulia Nappi, Guido Rovera, Maria Silvia De Feo, Giulia Santo, and et al. 2025. "The Value of Baseline [18F]FDG-PET in Predicting the Progression-Free Survival in Patients with Thymic Epithelial Tumours: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 19: 2458. https://doi.org/10.3390/diagnostics15192458
APA StyleMiceli, A., Librando, M., Dondi, F., Jonghi-Lavarini, L., D’Antonio, A., Mura, A., Nappi, A. G., Rovera, G., De Feo, M. S., Santo, G., & Lanfranchi, F. (2025). The Value of Baseline [18F]FDG-PET in Predicting the Progression-Free Survival in Patients with Thymic Epithelial Tumours: A Systematic Review and Meta-Analysis. Diagnostics, 15(19), 2458. https://doi.org/10.3390/diagnostics15192458