The Role of Imaging in Inflammatory Bowel Diseases: From Diagnosis to Individualized Therapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical and Endoscopic Scoring Systems in IBD
3.2. Ultrasound
Intestinal Ultrasounds Imaging Findings and Scoring Systems
3.3. CT and CT Enterography
3.4. Magnetic Resonance Imaging
3.4.1. MRI Techniques and Sequences in IBD
3.4.2. Typical MRI Findings in Crohn’s Disease
3.4.3. Typical MRI Findings in Ulcerative and Indeterminate Colitis
3.4.4. Diagnostic Performance of MRI vs. Endoscopy and Histology
3.4.5. MRI-Based Scoring Systems in IBD
3.4.6. MRI in Assessing Treatment Response
3.5. PET/MR
3.6. Radiomic and Artificial Intelligence in IBD Assessment
4. Intestinal Capsule Endoscopy
5. Role of Imaging in Unclassified IBD
6. Malignancy Risk in IBD and the Role of Imaging
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Calvez, V.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Bartocci, B.; Murgiano, M.; Iaccarino, J.; Parand, E.; Napolitano, D.; Pugliese, D.; et al. Novel Insights into the Pathogenesis of Inflammatory Bowel Diseases. Biomedicines 2025, 13, 305. [Google Scholar] [CrossRef]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD-Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Xue, J.C.; Hou, X.T.; Zhao, Y.W.; Yuan, S. Biological agents as attractive targets for inflammatory bowel disease therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167648. [Google Scholar] [CrossRef]
- Xiao, B.H.; Ma, X.D.; Lv, J.J.; Yang, T.; Liu, X.J.; An, L.Y.; Qi, Y.X.; Lu, M.L.; Duan, Y.Q.; Sun, D.L. Systematic evaluation of the diagnostic approach of inflammatory bowel disease guidelines. Int. J. Clin. Pract. 2021, 75, e14365. [Google Scholar] [CrossRef]
- Rimola, J.; Torres, J.; Kumar, S.; Taylor, S.A.; Kucharzik, T. Recent advances in clinical practice: Advances in cross-sectional imaging in inflammatory bowel disease. Gut 2022, 71, 2587–2597. [Google Scholar] [CrossRef]
- Bohra, A.; Vasudevan, A.; Kutaiba, N.; Van Langenberg, D.R. Replacing Endoscopy with Magnetic Resonance Enterography for Mucosal Activity Assessment in Terminal Ileal Crohn’s Disease: Are We There Yet? Diagnostics 2023, 13, 1061. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Gagliano, C.; Lentini, M.; Masiello, E.; Parisi, F.; Iannella, G.; Cilia, N.D.; Salerno, V.; Cusumano, G.; et al. The Integration of Radiomics and Artificial Intelligence in Modern Medicine. Life 2024, 14, 1248. [Google Scholar] [CrossRef]
- Vitello, A.; Maida, M.; Shahini, E.; Macaluso, F.S.; Orlando, A.; Grova, M.; Ramai, D.; Serviddio, G.; Facciorusso, A. Current Approaches for Monitoring of Patients with Inflammatory Bowel Diseases: A Narrative Review. J. Clin. Med. 2024, 13, 1008. [Google Scholar] [CrossRef]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Sutherland, L.R.; Martin, F.; Greer, S.; Robinson, M.; Greenberger, N.; Saibil, F.; Martin, T.; Sparr, J.; Prokipchuk, E.; Borgen, L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 1987, 92, 1894–1898. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F., Jr. Development of a Crohn’s disease activity index. Natl. Coop. Crohn’s Dis. Study. Gastroenterol. 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Irvine, E.J. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J. Clin. Gastroenterol. 1995, 20, 27–32. [Google Scholar]
- Travis, S.P.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef]
- Mary, J.Y.; Modigliani, R. Development and validation of an endoscopic index of the severity for Crohn’s disease: A prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989, 30, 983–989. [Google Scholar] [CrossRef]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef]
- Turner, D.; Otley, A.R.; Mack, D.; Hyams, J.; de Bruijne, J.; Uusoue, K.; Walters, T.D.; Zachos, M.; Mamula, P.; Beaton, D.E.; et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology 2007, 133, 423–432. [Google Scholar] [CrossRef]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.S.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T.; et al. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 439–447. [Google Scholar] [CrossRef]
- Tan, W.L.; Khoo, E.; Khaing, M.M.; Barr, E.; Fernandes, R.G.; Pham, H.; An, Y.K.; Begun, J. Point-of-Care Intestinal Ultrasound Impacts Inflammatory Bowel Disease Care and Reduces the Need for Additional Investigations. J. Gastroenterol. Hepatol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Allocca, M.; Fiorino, G.; Bonovas, S.; Furfaro, F.; Gilardi, D.; Argollo, M.; Magnoni, P.; Peyrin-Biroulet, L.; Danese, S. Accuracy of Humanitas Ultrasound Criteria in Assessing Disease Activity and Severity in Ulcerative Colitis: A Prospective Study. J. Crohn’s Colitis 2018, 12, 1385–1391. [Google Scholar] [CrossRef]
- Pruijt, M.J.; de Voogd, F.A.E.; Montazeri, N.S.M.; van Etten-Jamaludin, F.S.; D’Haens, G.R.; Gecse, K.B. Diagnostic Accuracy of Intestinal Ultrasound in the Detection of Intra-Abdominal Complications in Crohn’s Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2024, 18, 958–972. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Calabrese, E.; Zorzi, F.; Pallone, F. Ultrasound of the small bowel in Crohn’s disease. Int. J. Inflam. 2012, 2012, 964720. [Google Scholar] [CrossRef]
- Mihai, V.C.; Gheorghe, L.; Rezuș, I.I.; Jucan, A.E.; Andronic, M.C.; Gavrilescu, O.; Dranga, M.; Andronic, A.M.; Prelipcean, C.C.; Rezuș, C.; et al. Novelties and Perspectives of Intestinal Ultrasound in the Personalised Management of Patients with Inflammatory Bowel Diseases-A Systematic Review. Diagnostics 2024, 14, 812. [Google Scholar] [CrossRef]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; Hamlin, P.J.; Hart, A.L.; Higginson, A.; Jacobs, I.; et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): A multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 548–558. [Google Scholar] [CrossRef]
- Moreno, N.; Ripollés, T.; Paredes, J.M.; Ortiz, I.; Martínez, M.J.; López, A.; Delgado, F.; Moreno-Osset, E. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: Changes following treatment with immunomodulators and/or anti-TNF antibodies. J. Crohn’s Colitis 2014, 8, 1079–1087. [Google Scholar] [CrossRef]
- Orlando, S.; Fraquelli, M.; Coletta, M.; Branchi, F.; Magarotto, A.; Conti, C.B.; Mazza, S.; Conte, D.; Basilisco, G.; Caprioli, F. Ultrasound Elasticity Imaging Predicts Therapeutic Outcomes of Patients with Crohn’s Disease Treated with Anti-Tumour Necrosis Factor Antibodies. J. Crohn’s Colitis 2017, 12, 63–70. [Google Scholar] [CrossRef]
- Paredes, J.M.; Moreno, N.; Latorre, P.; Ripollés, T.; Martinez, M.J.; Vizuete, J.; Moreno-Osset, E. Clinical Impact of Sonographic Transmural Healing After Anti-TNF Antibody Treatment in Patients with Crohn’s Disease. Dig. Dis. Sci. 2019, 64, 2600–2606. [Google Scholar] [CrossRef]
- Ungar, B.; Ben-Shatach, Z.; Selinger, L.; Malik, A.; Albshesh, A.; Ben-Horin, S.; Eliakim, R.; Kopylov, U.; Carter, D. Lower adalimumab trough levels are associated with higher bowel wall thickness in Crohn’s disease. United Eur. Gastroenterol. J. 2020, 8, 167–174. [Google Scholar] [CrossRef]
- Albshesh, A.; Ungar, B.; Ben-Horin, S.; Eliakim, R.; Kopylov, U.; Carter, D. Terminal Ileum Thickness During Maintenance Therapy Is a Predictive Marker of the Outcome of Infliximab Therapy in Crohn Disease. Inflamm. Bowel Dis. 2020, 26, 1619–1625. [Google Scholar] [CrossRef]
- de Voogd, F.; Bots, S.; Gecse, K.; Gilja, O.H.; D’Haens, G.; Nylund, K. Intestinal Ultrasound Early on in Treatment Follow-up Predicts Endoscopic Response to Anti-TNFα Treatment in Crohn’s Disease. J. Crohn’s Colitis 2022, 16, 1598–1608. [Google Scholar] [CrossRef]
- Kucharzik, T.; Wilkens, R.; D’Agostino, M.A.; Maconi, G.; Le Bars, M.; Lahaye, M.; Bravatà, I.; Nazar, M.; Ni, L.; Ercole, E.; et al. Early Ultrasound Response and Progressive Transmural Remission After Treatment with Ustekinumab in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 153–163.e112. [Google Scholar] [CrossRef]
- Vaughan, R.; Murphy, E.; Nalder, M.; Gibson, R.N.; Ardalan, Z.; Boussioutas, A.; Christensen, B. Infliximab Trough Levels Are Associated with Transmural Sonographic Healing in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, 1080–1088. [Google Scholar] [CrossRef]
- Hoffmann, T.; Fusco, S.; Blumenstock, G.; Sadik, S.; Malek, N.P.; Froehlich, E. Evaluation of bowel wall thickness by ultrasound as early diagnostic tool for therapeutic response in Crohn’s disease patients treated with ustekinumab. Z. Gastroenterol. 2022, 60, 1212–1220. [Google Scholar] [CrossRef]
- Calabrese, E.; Rispo, A.; Zorzi, F.; De Cristofaro, E.; Testa, A.; Costantino, G.; Viola, A.; Bezzio, C.; Ricci, C.; Prencipe, S.; et al. Ultrasonography Tight Control and Monitoring in Crohn’s Disease During Different Biological Therapies: A Multicenter Study. Clin. Gastroenterol. Hepatol. 2022, 20, e711–e722. [Google Scholar] [CrossRef]
- Guidi, L.; De Franco, A.; De Vitis, I.; Armuzzi, A.; Semeraro, S.; Roberto, I.; Papa, A.; Bock, E.; Gasbarrini, G.; Fedeli, G. Contrast-enhanced ultrasonography with SonoVue after infliximab therapy in Crohn’s disease. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 23–26. [Google Scholar]
- Dolinger, M.T.; Aronskyy, I.; Kellar, A.; Spencer, E.; Pittman, N.; Dubinsky, M.C. Early Intestinal Ultrasound Response to Biologic Therapy Predicts Endoscopic Remission in Children with Ileal Crohn’s Disease: Results from the Prospective Super Sonic Study. J. Crohn’s Colitis 2024, 18, 1002–1011. [Google Scholar] [CrossRef]
- Hoerning, A.; Jüngert, J.; Siebenlist, G.; Knieling, F.; Regensburger, A.P. Ultrasound in Pediatric Inflammatory Bowel Disease-A Review of the State of the Art and Future Perspectives. Children 2024, 11, 156. [Google Scholar] [CrossRef]
- Debnath, P.; Rathi, P. Treat to Target in Patients with Ileocolonic Crohn’s Disease: Endoscopic Remission or Histologic Healing? Clin. Gastroenterol. Hepatol. 2021, 19, 2452. [Google Scholar] [CrossRef]
- Chavannes, M.; Dolinger, M.T.; Cohen-Mekelburg, S.; Abraham, B. AGA Clinical Practice Update on the Role of Intestinal Ultrasound in Inflammatory Bowel Disease: Commentary. Clin. Gastroenterol. Hepatol. 2024, 22, 1790–1795.e1. [Google Scholar] [CrossRef]
- Ripollés, T.; Martínez-Pérez, M.J.; Blanc, E.; Delgado, F.; Vizuete, J.; Paredes, J.M.; Vilar, J. Contrast-enhanced ultrasound (CEUS) in Crohn’s disease: Technique, image interpretation and clinical applications. Insights Imaging 2011, 2, 639–652. [Google Scholar] [CrossRef]
- Novak, K.L.; Nylund, K.; Maaser, C.; Petersen, F.; Kucharzik, T.; Lu, C.; Allocca, M.; Maconi, G.; de Voogd, F.; Christensen, B.; et al. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn’s Disease. J. Crohn’s Colitis 2021, 15, 609–616. [Google Scholar] [CrossRef]
- Bots, S.; Nylund, K.; Löwenberg, M.; Gecse, K.; D’Haens, G. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a novel UC-Ultrasound Index. J. Crohn’s Colitis 2021, 15, 1264–1271. [Google Scholar] [CrossRef]
- Kucharzik, T.; Maaser, C. Intestinal ultrasound and management of small bowel Crohn’s disease. Ther. Adv. Gastroenterol. 2018, 11, 1756284818771367. [Google Scholar] [CrossRef]
- Manzotti, C.; Colombo, F.; Zurleni, T.; Danelli, P.; Maconi, G. Prognostic role of intestinal ultrasound in Crohn’s disease. World J. Gastroenterol. 2023, 29, 3595–3605. [Google Scholar] [CrossRef]
- Rispo, A.; Imperatore, N.; Testa, A.; Nardone, O.M.; Luglio, G.; Caporaso, N.; Castiglione, F. Diagnostic Accuracy of Ultrasonography in the Detection of Postsurgical Recurrence in Crohn’s Disease: A Systematic Review with Meta-analysis. Inflamm. Bowel Dis. 2018, 24, 977–988. [Google Scholar] [CrossRef]
- Furfaro, F.; D’Amico, F.; Zilli, A.; Craviotto, V.; Aratari, A.; Bezzio, C.; Spinelli, A.; Gilardi, D.; Radice, S.; Saibeni, S.; et al. Noninvasive Assessment of Postoperative Disease Recurrence in Crohn’s Disease: A Multicenter, Prospective Cohort Study on Behalf of the Italian Group for Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 3143–3151. [Google Scholar] [CrossRef]
- Frias-Gomes, C.; Torres, J.; Palmela, C. Intestinal Ultrasound in Inflammatory Bowel Disease: A Valuable and Increasingly Important Tool. GE Port. J. Gastroenterol. 2022, 29, 223–239. [Google Scholar] [CrossRef]
- Lin, W.C.; Chang, C.W.; Chen, M.J.; Wang, H.Y. Intestinal Ultrasound in Inflammatory Bowel Disease: A Novel and Increasingly Important Tool. J. Med. Ultrasound 2023, 31, 86–91. [Google Scholar] [CrossRef]
- Cleveland, N.K.; Picker, E.A.; Dolinger, M.T.; Rubin, D.T. The Arrival of Intestinal Ultrasound for Inflammatory Bowel Disease Care in the United States. Gastroenterol. Hepatol. 2023, 19, 147–154. [Google Scholar]
- Barchi, A.; Dal Buono, A.; D’Amico, F.; Furfaro, F.; Zilli, A.; Fiorino, G.; Parigi, T.L.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis. J. Clin. Med. 2023, 12, 7569. [Google Scholar] [CrossRef]
- Hoffmann, J.C.; Ungewitter, T. Role of Intestinal Ultrasound for IBD Care: A Practical Approach. Diagnostics 2024, 14, 1639. [Google Scholar] [CrossRef]
- Celikyay, F.; Yuksekkaya, R.; Yuksekkaya, M.; Kefeli, A. Color Doppler Ultrasound Assessment of Clinical Activity in Inflammatory Bowel Disease. Curr. Med. Imaging 2021, 17, 741–750. [Google Scholar] [CrossRef]
- Limberg, B.; Osswald, B. Diagnosis and differential diagnosis of ulcerative colitis and Crohn’s disease by hydrocolonic sonography. Am. J. Gastroenterol. 1994, 89, 1051–1057. [Google Scholar]
- Wilkens, R.; Novak, K.L.; Maaser, C.; Panaccione, R.; Kucharzik, T. Relevance of monitoring transmural disease activity in patients with Crohn’s disease: Current status and future perspectives. Ther. Adv. Gastroenterol. 2021, 14, 17562848211006672. [Google Scholar] [CrossRef]
- Murphy, M.E.; Bhattacharya, S.; Axelrad, J.E. Diagnosis and Monitoring of Ulcerative Colitis. Clin. Colon. Rectal Surg. 2022, 35, 421–427. [Google Scholar] [CrossRef]
- Bezzio, C.; Vernero, M.; Ribaldone, D.G.; Manes, G.; Saibeni, S. Insights into the role of gastrointestinal ultrasound in ulcerative colitis. Ther. Adv. Gastroenterol. 2021, 14, 17562848211051456. [Google Scholar] [CrossRef]
- Christian, M.; Giovanni, M.; Torsten, K.; Mariangela, A. Ultrasonography in inflammatory bowel disease—So far we are? United Eur. Gastroenterol. J. 2022, 10, 225–232. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Ju, J.Y.; Luo, Y.; Zhuang, H. Validation of intestinal ultrasound scores in assessing endoscopic activity of colonic and small intestinal Crohn’s disease in a southwest Chinese cohort: A retrospective cross-sectional study. Quant. Imaging Med. Surg. 2024, 14, 8374–8386. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Zhang, Y.; Jiang, W.; Ma, J.; Zhang, H. External validation and comparison of simple ultrasound activity score and international bowel ultrasound segmental activity score for Crohn’s disease. Scand. J. Gastroenterol. 2023, 58, 883–889. [Google Scholar] [CrossRef]
- Ripollés, T.; Poza, J.; Suarez Ferrer, C.; Martínez-Pérez, M.J.; Martín-Algíbez, A.; de Las Heras Paez, B. Evaluation of Crohn’s Disease Activity: Development of an Ultrasound Score in a Multicenter Study. Inflamm. Bowel Dis. 2021, 27, 145–154. [Google Scholar] [CrossRef]
- Sævik, F.; Eriksen, R.; Eide, G.E.; Gilja, O.H.; Nylund, K. Development and Validation of a Simple Ultrasound Activity Score for Crohn’s Disease. J. Crohn’s Colitis 2021, 15, 115–124. [Google Scholar] [CrossRef]
- van Wassenaer, E.A.; van Rijn, R.R.; Zwetsloot, S.L.M.; de Voogd, F.A.E.; van Schuppen, J.; Kindermann, A.; de Meij, T.G.J.; van Limbergen, J.E.; Gecse, K.B.; D’Haens, G.R.; et al. Intestinal Ultrasound to Assess Ulcerative Colitis Disease Activity in Children: External Validation and Comparison of 2 Intestinal Ultrasound Activity Indices. Inflamm. Bowel Dis. 2023, 29, 1217–1222. [Google Scholar] [CrossRef]
- Piazza, O.S.N.; Noviello, D.; Filippi, E.; Conforti, F.; Furfaro, F.; Fraquelli, M.; Costantino, A.; Danese, S.; Vecchi, M.; Fiorino, G.; et al. Superior predictive value of transmural over endoscopic severity for colectomy risk in ulcerative colitis: A multicentre prospective cohort study. J. Crohn’s Colitis 2024, 18, 291–299. [Google Scholar] [CrossRef]
- Park, M.J.; Lim, J.S. Computed tomography enterography for evaluation of inflammatory bowel disease. Clin. Endosc. 2013, 46, 327–366. [Google Scholar] [CrossRef]
- Allocca, M.; Danese, S.; Laurent, V.; Peyrin-Biroulet, L. Use of Cross-Sectional Imaging for Tight Monitoring of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1309–1323.e1304. [Google Scholar] [CrossRef]
- Ilangovan, R.; Burling, D.; George, A.; Gupta, A.; Marshall, M.; Taylor, S.A. CT enterography: Review of technique and practical tips. Br. J. Radiol. 2012, 85, 876–886. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Al-Hawary, M.M.; Jagdish, J.; Ganesh, H.S.; Platt, J.F. CT enterography: Principles, trends, and interpretation of findings. Radiographics 2010, 30, 1955–1970. [Google Scholar] [CrossRef]
- Mansour, H.H.; Alajerami, Y.S.; Abushab, K.M.; Najim, A.A.; Quffa, K.M. Diagnostic accuracy of CT enterography correlated to histopathology in the diagnosis of small bowel Crohn’s disease. Ir. J. Med. Sci. 2022, 191, 2605–2610. [Google Scholar] [CrossRef]
- Jensen, M.D.; Nathan, T.; Rafaelsen, S.R.; Kjeldsen, J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin. Gastroenterol. Hepatol. 2011, 9, 124–129. [Google Scholar] [CrossRef]
- Vuyyuru, S.K.; Solitano, V.; Aruljothy, A.; Alkhattabi, M.; Beaton, M.; Gregor, J.; Kassam, Z.; Marshall, H.; Ramsewak, D.; Sedano, R.; et al. Prevalence of stricturing, penetrating complications and extraintestinal manifestations in inflammatory bowel disease detected on cross-sectional imaging in a tertiary care setting. United Eur. Gastroenterol. J. 2024, 12, 870–878. [Google Scholar] [CrossRef]
- Vogel, J.; da Luz Moreira, A.; Baker, M.; Hammel, J.; Einstein, D.; Stocchi, L.; Fazio, V. CT enterography for Crohn’s disease: Accurate preoperative diagnostic imaging. Dis. Colon. Rectum 2007, 50, 1761–1769. [Google Scholar] [CrossRef]
- Wu, Y.W.; Tang, Y.H.; Hao, N.X.; Tang, C.Y.; Miao, F. Crohn’s disease: CT enterography manifestations before and after treatment. Eur. J. Radiol. 2012, 81, 52–59. [Google Scholar] [CrossRef]
- Tong, J.; Feng, Q.; Zhang, C.; Xu, X.; Ran, Z. CT enterography for evaluation of disease activity in patients with ileocolonic Crohn’s disease. BMC Gastroenterol. 2022, 22, 324. [Google Scholar] [CrossRef]
- Lopes, S.; Andrade, P.; Afonso, J.; Cunha, R.; Rodrigues-Pinto, E.; Ramos, I.; Macedo, G.; Magro, F. Monitoring Crohn’s disease activity: Endoscopy, fecal markers and computed tomography enterography. Ther. Adv. Gastroenterol. 2018, 11, 1756284818769075. [Google Scholar] [CrossRef]
- Hara, A.K.; Alam, S.; Heigh, R.I.; Gurudu, S.R.; Hentz, J.G.; Leighton, J.A. Using CT enterography to monitor Crohn’s disease activity: A preliminary study. AJR Am. J. Roentgenol. 2008, 190, 1512–1516. [Google Scholar] [CrossRef]
- Alyami, A.S. The Role of Radiomics in Fibrosis Crohn’s Disease: A Review. Diagnostics 2023, 13, 1623. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V., Jr.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A. Consensus Recommendations for Evaluation, Interpretation, and Utilization of Computed Tomography and Magnetic Resonance Enterography in Patients with Small Bowel Crohn’s Disease. Radiology 2018, 286, 776–799. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Maccioni, F.; Viscido, A.; Marini, M.; Caprilli, R. MRI evaluation of Crohn’s disease of the small and large bowel with the use of negative superparamagnetic oral contrast agents. Abdom. Imaging 2002, 27, 384–393. [Google Scholar] [CrossRef]
- Ajaj, W.; Goyen, M.; Schneemann, H.; Kuehle, C.; Nuefer, M.; Ruehm, S.G.; Goehde, S.C.; Lauenstein, T.C. Oral contrast agents for small bowel distension in MRI: Influence of the osmolarity for small bowel distention. Eur. Radiol. 2005, 15, 1400–1406. [Google Scholar] [CrossRef]
- Gutzeit, A.; Binkert, C.A.; Koh, D.M.; Hergan, K.; von Weymarn, C.; Graf, N.; Patak, M.A.; Roos, J.E.; Horstmann, M.; Kos, S.; et al. Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: Comparison of intravenous and intramuscular drug administration. Eur. Radiol. 2012, 22, 1186–1194. [Google Scholar] [CrossRef]
- Gallego, J.C.; Echarri, A. Role of magnetic resonance imaging in the management of perianal Crohn’s disease. Insights Imaging 2018, 9, 47–58. [Google Scholar] [CrossRef]
- Gage, K.L.; Deshmukh, S.; Macura, K.J.; Kamel, I.R.; Zaheer, A. MRI of perianal fistulas: Bridging the radiological-surgical divide. Abdom. Imaging 2013, 38, 1033–1042. [Google Scholar] [CrossRef]
- Tielbeek, J.A.; Bipat, S.; Boellaard, T.N.; Nio, C.Y.; Stoker, J. Training readers to improve their accuracy in grading Crohn’s disease activity on MRI. Eur. Radiol. 2014, 24, 1059–1067. [Google Scholar] [CrossRef]
- Chalian, M.; Ozturk, A.; Oliva-Hemker, M.; Pryde, S.; Huisman, T.A. MR enterography findings of inflammatory bowel disease in pediatric patients. AJR Am. J. Roentgenol. 2011, 196, W810–W816. [Google Scholar] [CrossRef]
- Guglielmo, F.F.; Anupindi, S.A.; Fletcher, J.G.; Al-Hawary, M.M.; Dillman, J.R.; Grand, D.J.; Bruining, D.H.; Chatterji, M.; Darge, K.; Fidler, J.L.; et al. Small Bowel Crohn Disease at CT and MR Enterography: Imaging Atlas and Glossary of Terms. Radiographics 2020, 40, 354–375. [Google Scholar] [CrossRef] [PubMed]
- Dilauro, S.; Crum-Cianflone, N.F. Ileitis: When it is not Crohn’s disease. Curr. Gastroenterol. Rep. 2010, 12, 249–258. [Google Scholar] [CrossRef]
- Ram, R.; Sarver, D.; Pandey, T.; Guidry, C.L.; Jambhekar, K.R. Magnetic resonance enterography: A stepwise interpretation approach and role of imaging in management of adult Crohn’s disease. Indian. J. Radiol. Imaging 2016, 26, 173–184. [Google Scholar] [CrossRef]
- Sleiman, J.; Chirra, P.; Gandhi, N.S.; Baker, M.E.; Lu, C.; Gordon, I.O.; Viswanath, S.E.; Rieder, F. Crohn’s disease related strictures in cross-sectional imaging: More than meets the eye? United Eur. Gastroenterol. J. 2022, 10, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.L.; Rozenblit, A.; Ricci, Z.; Roberts, J.; Milikow, D.; Chernyak, V.; Wolf, E. Small bowel faeces sign in patients without small bowel obstruction. Clin. Radiol. 2007, 62, 353–357. [Google Scholar] [CrossRef]
- Wnorowski, A.M.; Guglielmo, F.F.; Mitchell, D.G. How to perform and interpret cine MR enterography. J. Magn. Reson. Imaging 2015, 42, 1180–1189. [Google Scholar] [CrossRef]
- Yoon, S.; Park, S.H.; Kim, J.S. Radiologic images of complications of Crohn’s disease. Int. J. Gastrointest. Interv. 2023, 12, 29–36. [Google Scholar] [CrossRef]
- Bousvaros, A.; Antonioli, D.A.; Colletti, R.B.; Dubinsky, M.C.; Glickman, J.N.; Gold, B.D.; Griffiths, A.M.; Jevon, G.P.; Higuchi, L.M.; Hyams, J.S.; et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: Report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Creeping Fat in Crohn’s Disease-Surgical, Histological, and Radiological Approaches. J. Pers. Med. 2023, 13, 1029. [Google Scholar] [CrossRef]
- Gee, M.S.; Harisinghani, M.G. MRI in patients with inflammatory bowel disease. J. Magn. Reson. Imaging 2011, 33, 527–534. [Google Scholar] [CrossRef]
- Zappa, M.; Stefanescu, C.; Cazals-Hatem, D.; Bretagnol, F.; Deschamps, L.; Attar, A.; Larroque, B.; Tréton, X.; Panis, Y.; Vilgrain, V.; et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm. Bowel Dis. 2011, 17, 984–993. [Google Scholar] [CrossRef]
- Alyami, A.S. Imaging of Ulcerative Colitis: The Role of Diffusion-Weighted Magnetic Resonance Imaging. J. Clin. Med. 2024, 13, 5204. [Google Scholar] [CrossRef]
- Ordás, I.; Rimola, J.; García-Bosch, O.; Rodríguez, S.; Gallego, M.; Etchevers, M.J.; Pellisé, M.; Feu, F.; González-Suárez, B.; Ayuso, C.; et al. Diagnostic accuracy of magnetic resonance colonography for the evaluation of disease activity and severity in ulcerative colitis: A prospective study. Gut 2013, 62, 1566–1572. [Google Scholar] [CrossRef]
- Horsthuis, K.; Stokkers, P.C.; Stoker, J. Detection of inflammatory bowel disease: Diagnostic performance of cross-sectional imaging modalities. Abdom. Imaging 2008, 33, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, A.Y.; Yang, S.K.; Chung, J.W.; Kim, S.Y.; Park, S.H.; Ha, H.K. Crohn disease of the small bowel: Comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology 2009, 251, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.; Xiao, W.; Luo, G. A Diagnostic Accuracy Meta-analysis of CT and MRI for the Evaluation of Small Bowel Crohn Disease. Acad. Radiol. 2017, 24, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Khater, N.H.; Fahmy, H.S.; Ali, H.I. Value of MR enterography in assessment of Crohn’s disease: Correlation with capsule endoscopy and colonoscopy. Egypt. J. Radiol. Nucl. Med. 2017, 48, 51–60. [Google Scholar] [CrossRef]
- Rimola, J.; Ordás, I.; Rodriguez, S.; García-Bosch, O.; Aceituno, M.; Llach, J.; Ayuso, C.; Ricart, E.; Panés, J. Magnetic resonance imaging for evaluation of Crohn’s disease: Validation of parameters of severity and quantitative index of activity. Inflamm. Bowel Dis. 2011, 17, 1759–1768. [Google Scholar] [CrossRef]
- Khanna, R.; Nelson, S.A.; Feagan, B.G.; D’Haens, G.; Sandborn, W.J.; Zou, G.Y.; MacDonald, J.K.; Parker, C.E.; Jairath, V.; Levesque, B.G. Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 2016, Cd010642. [Google Scholar] [CrossRef]
- Alfarone, L.; Dal Buono, A.; Craviotto, V.; Zilli, A.; Fiorino, G.; Furfaro, F.; D’Amico, F.; Danese, S.; Allocca, M. Cross-Sectional Imaging Instead of Colonoscopy in Inflammatory Bowel Diseases: Lights and Shadows. J. Clin. Med. 2022, 11, 353. [Google Scholar] [CrossRef]
- Caron, B.; Jairath, V.; Laurent, V.; Stoker, J.; Laghi, A.; D’Haens, G.R.; Danese, S.; Peyrin-Biroulet, L. Defining Magnetic Resonance Imaging Treatment Response and Remission in Crohn’s Disease: A Systematic Review. J. Crohn’s Colitis 2024, 18, 162–170. [Google Scholar] [CrossRef]
- Ordás, I.; Rimola, J.; Alfaro, I.; Rodríguez, S.; Castro-Poceiro, J.; Ramírez-Morros, A.; Gallego, M.; Giner, À.; Barastegui, R.; Fernández-Clotet, A.; et al. Development and Validation of a Simplified Magnetic Resonance Index of Activity for Crohn’s Disease. Gastroenterology 2019, 157, 432–439.e431. [Google Scholar] [CrossRef]
- Tao, Y.; Li, H.; Xu, H.; Tang, W.; Fan, G.; Yang, X. Can the simplified magnetic resonance index of activity be used to evaluate the degree of activity in Crohn’s disease? BMC Gastroenterol. 2021, 21, 409. [Google Scholar] [CrossRef]
- Seo, N. Comprehensive Review of Magnetic Resonance Enterography-Based Activity Scoring Systems for Crohn’s Disease. Investig. Magn. Reson. Imaging 2025, 29, 1–13. [Google Scholar] [CrossRef]
- Lafeuille, P.; Hordonneau, C.; Vignette, J.; Blayac, L.; Dapoigny, M.; Reymond, M.; Rouquette, O.; Sollelis, E.; Boube, M.; Magnin, B.; et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn’s disease. Aliment. Pharmacol. Ther. 2021, 53, 577–586. [Google Scholar] [CrossRef]
- Catalano, O.A.; Wu, V.; Mahmood, U.; Signore, A.; Vangel, M.; Soricelli, A.; Salvatore, M.; Gervais, D.; Rosen, B.R. Diagnostic performance of PET/MR in the evaluation of active inflammation in Crohn disease. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 62–69. [Google Scholar] [PubMed]
- Dalby, S.; Piri, R.; Graumann, O.; Gerke, O.; Andersen, T.L.; Walsted, A.M.; Risby, K.; Nielsen, R.G.; Linnemann, A.; Høilund-Carlsen, P.F.; et al. PET/MRI in paediatric inflammatory bowel disease—A prospective accuracy study. Clin. Physiol. Funct. Imaging 2025, 45, e12903. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Chang, J.H.; Carter, D.; McGovern, D.P.B.; Moore, J.; Wang, P.; Huang, X. Radiomics-Based Analysis of Intestinal Ultrasound Images for Inflammatory Bowel Disease: A Feasibility Study. Crohn’s Colitis 360 2024, 6, otae034. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, H.; Dai, Y.; Zhang, J.; Zhao, S.; Wu, Q. Author Correction: A radiomics nomogram based on MSCT and clinical factors can stratify fibrosis in inflammatory bowel disease. Sci. Rep. 2024, 14, 6073. [Google Scholar] [CrossRef]
- Das, A.; Shukla, T.; Tomita, N.; Richards, R.; Vidis, L.; Ren, B.; Hassanpour, S. Deep Learning for Classification of Inflammatory Bowel Disease Activity in Whole Slide Images of Colonic Histopathology. Am. J. Pathol. 2025, 195, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Chirra, P.; Sleiman, J.; Gandhi, N.S.; Gordon, I.O.; Hariri, M.; Baker, M.; Ottichilo, R.; Bruining, D.H.; Kurowski, J.A.; Viswanath, S.E.; et al. Radiomics to Detect Inflammation and Fibrosis on Magnetic Resonance Enterography in Stricturing Crohn’s Disease. J. Crohn’s Colitis 2024, 18, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Testoni, S.G.G.; Albertini Petroni, G.; Annunziata, M.L.; Dell’Anna, G.; Puricelli, M.; Delogu, C.; Annese, V. Artificial Intelligence in Inflammatory Bowel Disease Endoscopy. Diagnostics 2025, 15, 905. [Google Scholar] [CrossRef] [PubMed]
- Laterza, L.; Boldrini, L.; Tran, H.E.; Votta, C.; Larosa, L.; Minordi, L.M.; Maresca, R.; Pugliese, D.; Zocco, M.A.; Ainora, M.E.; et al. Radiomics could predict surgery at 10 years in Crohn’s disease. Dig. Liver Dis. 2023, 55, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Afzali, A. Artificial intelligence in inflammatory bowel disease: Innovations in diagnosis, monitoring, and personalized care. Ther. Adv. Gastroenterol. 2025, 18, 17562848251357407. [Google Scholar] [CrossRef]
- Pinton, P. Impact of artificial intelligence on prognosis, shared decision-making, and precision medicine for patients with inflammatory bowel disease: A perspective and expert opinion. Ann. Med. 2023, 55, 2300670. [Google Scholar] [CrossRef]
- Labarile, N.; Vitello, A.; Sinagra, E.; Nardone, O.M.; Calabrese, G.; Bonomo, F.; Maida, M.; Iacucci, M. Artificial Intelligence in Advancing Inflammatory Bowel Disease Management: Setting New Standards. Cancers 2025, 17, 2337. [Google Scholar] [CrossRef]
- Stidham, R.W.; Takenaka, K. Artificial Intelligence for Disease Assessment in Inflammatory Bowel Disease: How Will it Change Our Practice? Gastroenterology 2022, 162, 1493–1506. [Google Scholar] [CrossRef]
- Ran, J.; Zhou, M.; Wen, H. Artificial intelligence in inflammatory bowel disease. Saudi J. Gastroenterol. 2025, 31, 197–205. [Google Scholar] [CrossRef]
- Monteiro, S.; Dias de Castro, F.; Boal Carvalho, P.; Rosa, B.; Moreira, M.J.; Pinho, R.; Saraiva, M.M.; Cotter, J. Essential role of small bowel capsule endoscopy in reclassification of colonic inflammatory bowel disease type unclassified. World J. Gastrointest. Endosc. 2017, 9, 34–40. [Google Scholar] [CrossRef]
- Hilmi, I.; Kobayashi, T. Capsule endoscopy in inflammatory bowel disease: When and how. Intest. Res. 2020, 18, 265–274. [Google Scholar] [CrossRef]
- Oliva, S.; Aloi, M.; Viola, F.; Mallardo, S.; Civitelli, F.; Maccioni, F.; Hassan, C.; Papoff, P.; Cucchiara, S.; Cohen, S.A. A Treat to Target Strategy Using Panenteric Capsule Endoscopy in Pediatric Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 2060–2067.e1. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, Y.; Wu, P.; Chen, J.; Shen, Y.; Kamel, I.; Zheng, X.; Wu, B.; Li, Z. Enhancing radiologist’s detection: An imaging-based grading system for differentiating Crohn’s disease from ulcerative colitis. BMC Med. 2024, 22, 441. [Google Scholar] [CrossRef]
- Bots, S.; De Voogd, F.; De Jong, M.; Ligtvoet, V.; Löwenberg, M.; Duijvestein, M.; Ponsioen, C.Y.; D’Haens, G.; Gecse, K.B. Point-of-care Intestinal Ultrasound in IBD Patients: Disease Management and Diagnostic Yield in a Real-world Cohort and Proposal of a Point-of-care Algorithm. J. Crohns Colitis. 2022, 16, 606–615. [Google Scholar] [CrossRef]
- Tontini, G.E.; Vecchi, M.; Pastorelli, L.; Neurath, M.F.; Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: State of the art and future perspectives. World J. Gastroenterol. 2015, 21, 21–46. [Google Scholar] [CrossRef]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef]
- Clarke, W.T.; Feuerstein, J.D. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J. Gastroenterol. 2019, 25, 4148–4157. [Google Scholar] [CrossRef]
- Furfaro, F.; Dal Buono, A.; Sicuso, C.; Allocca, M.; D’Amico, F.; Zilli, A.; Fiorino, G.; Gabbiadini, R.; Danese, S. Carcinomas in inflammatory bowel disease: A narrative review on diagnostic imaging techniques. Chin. Clin. Oncol. 2022, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.; Jaffer, H.; Kajal, D.; Kirsch, R.; Jaffer, N. Imaging Features of Gastrointestinal Neoplasms Complicating Inflammatory Bowel Diseases. Curr. Probl. Diagn. Radiol. 2023, 52, 570–575. [Google Scholar] [CrossRef] [PubMed]
- East, J.E.; Gordon, M.; Nigam, G.B.; Sinopoulou, V.; Bateman, A.C.; Din, S.; Iacucci, M.; Kabir, M.; Lamb, C.A.; Wilson, A.; et al. British Society of Gastroenterology guidelines on colorectal surveillance in inflammatory bowel disease. Gut 2025. Online ahead of print. [Google Scholar] [CrossRef]
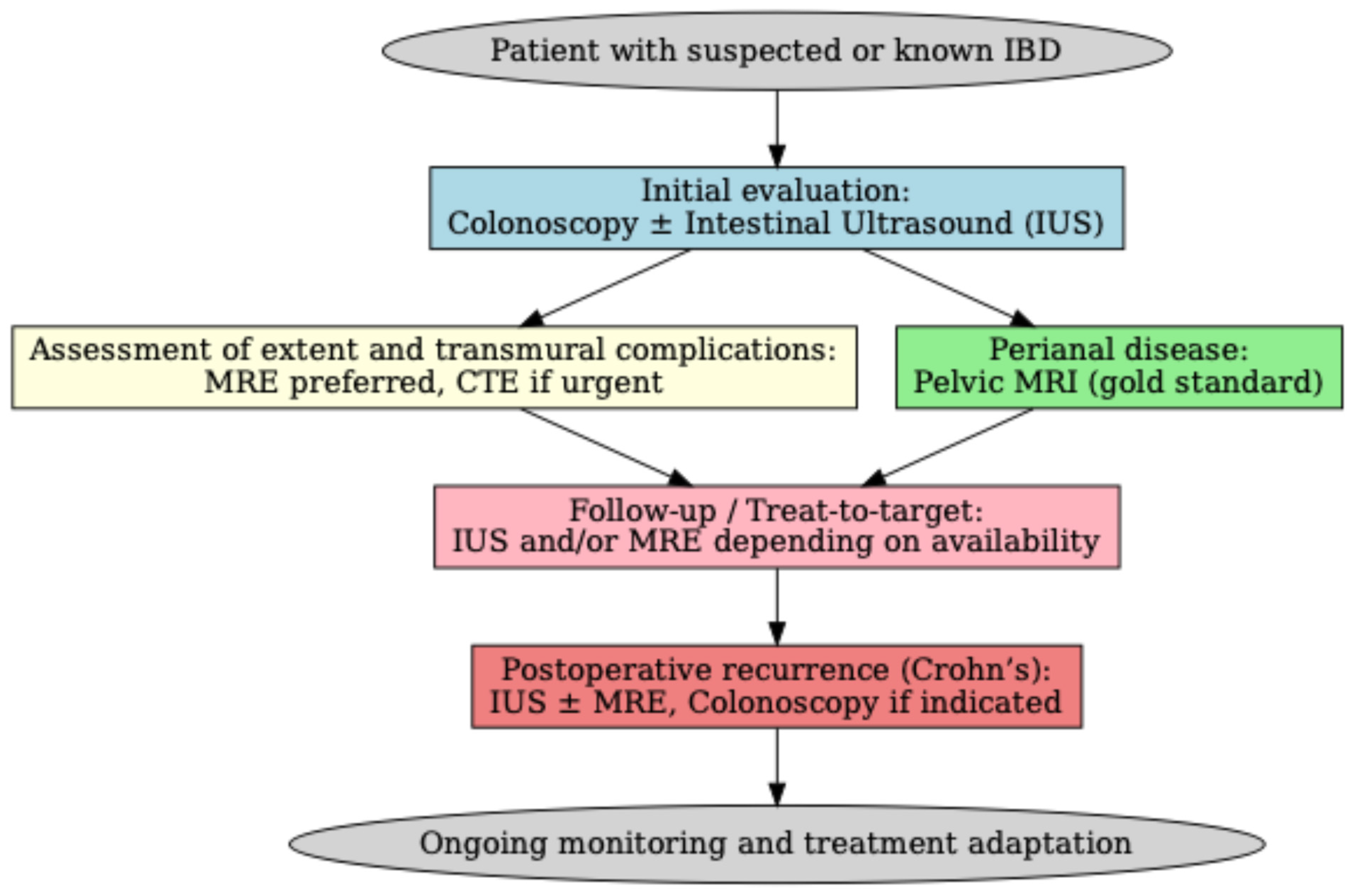
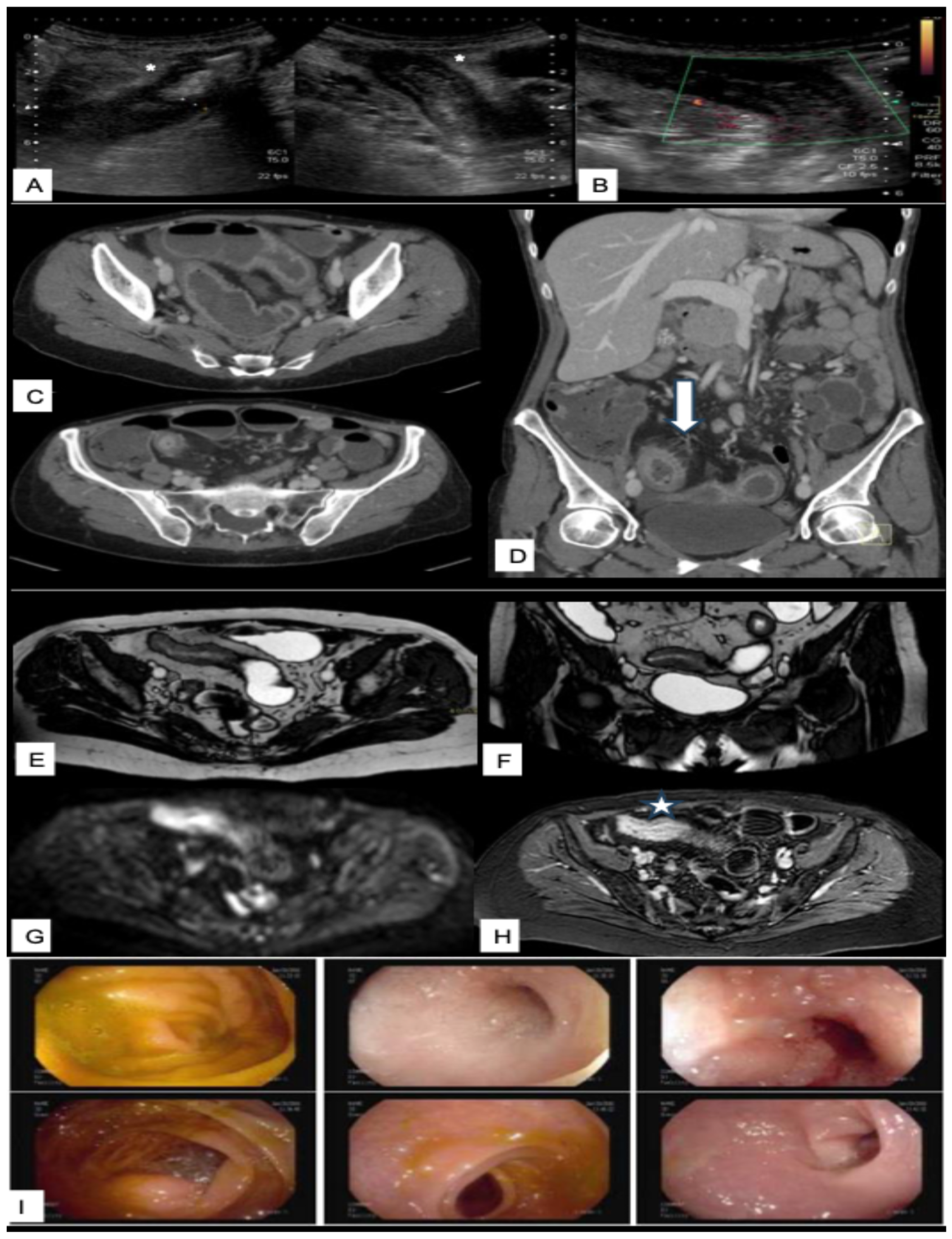
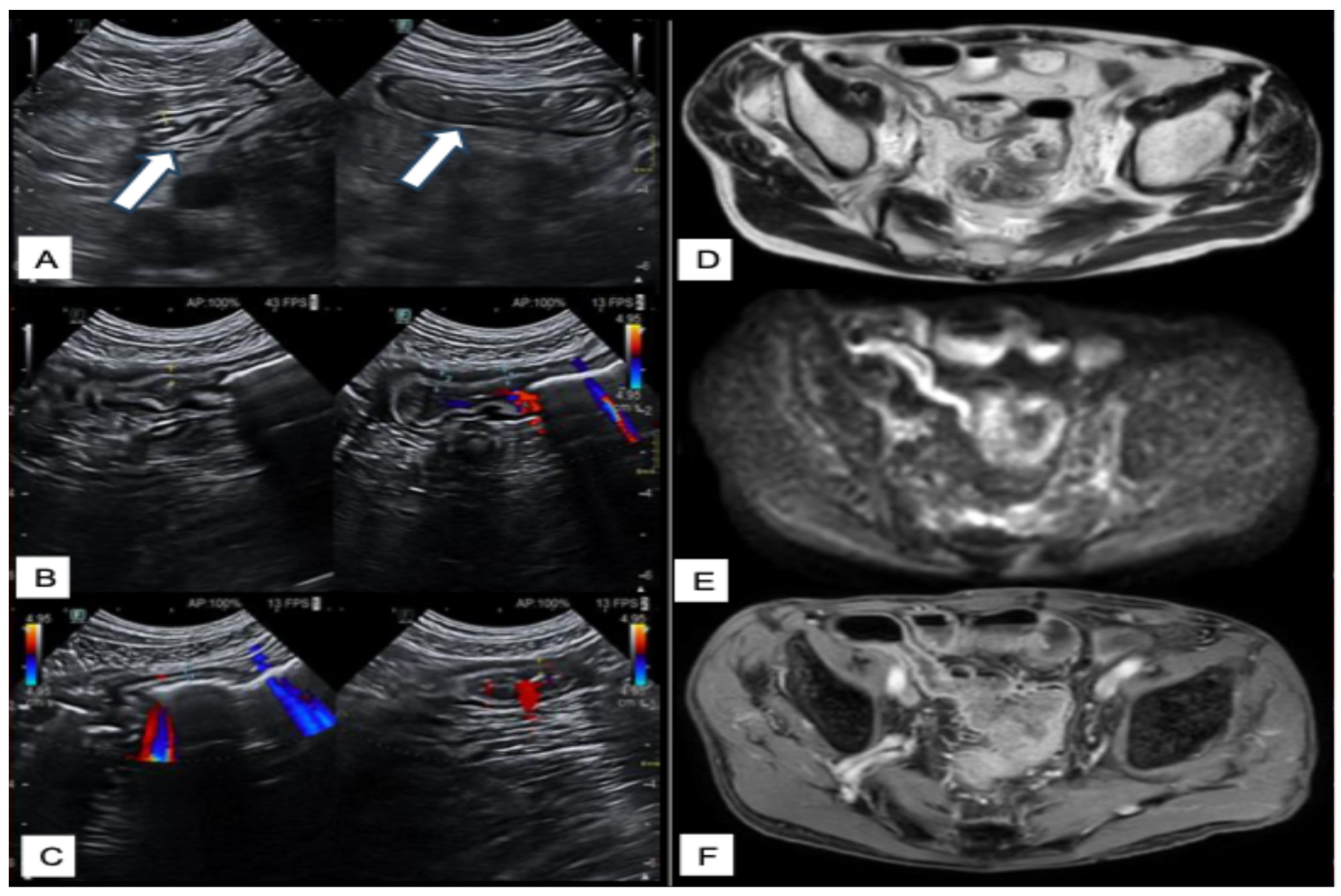
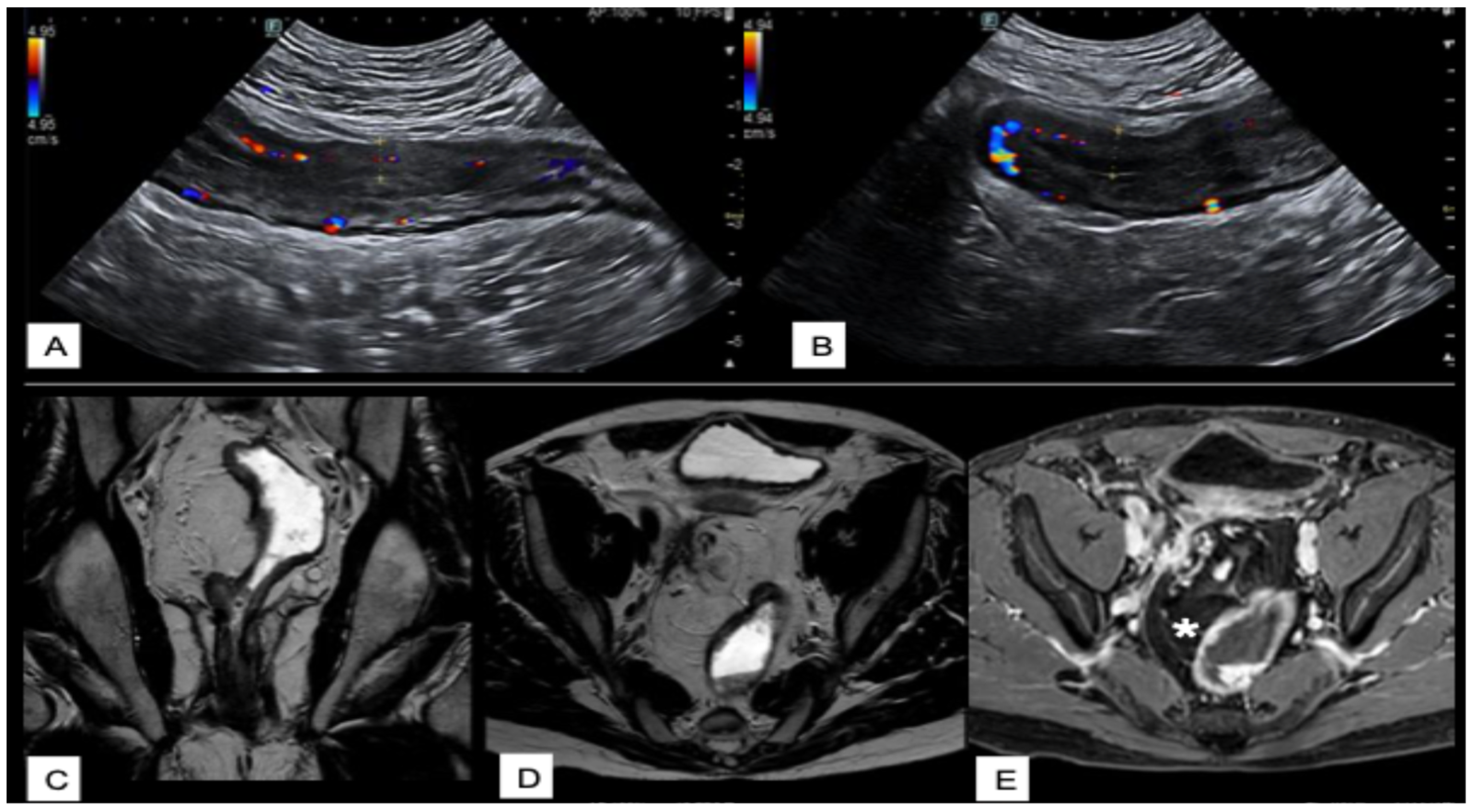

| Score Name | Disease | Type | Description |
|---|---|---|---|
| Truelove and Witts Severity Index [11] | UC | Clinical | Classifies UC severity into mild, moderate, or severe based on stool frequency, blood in stool, temperature, heart rate, hemoglobin, and ESR (or CRP). |
| Mayo Score (Full Mayo Score) [12] | UC | Clinical + Endoscopic | Composite index evaluating stool frequency, rectal bleeding, endoscopic findings, and physician’s global assessment; total score ranges from 0 to 12. |
| Simple Clinical Colitis Activity Index (SCCAI) [13] | UC | Clinical | Assesses disease activity based on stool frequency, urgency, blood in stool, general well-being, and extracolonic features; scores range from 0 to 19. |
| Ulcerative Colitis Disease Activity Index (UCDAI) [14] | UC | Clinical + Endoscopic | Includes stool frequency, rectal bleeding, mucosal appearance, and physician’s assessment; total score ranges from 0 to 12. |
| Ulcerative Colitis Endoscopic Index of Severity (UCEIS) [18] | UC | Endoscopic | Evaluates vascular pattern, bleeding, and erosions/ulcers during endoscopy; scores range from 0 to 8. |
| Crohn’s Disease Activity Index (CDAI) [15] | CD | Clinical | Composite index assessing stool frequency, abdominal pain, general well-being, complications, use of antidiarrheal agents, hematocrit, and body weight; scores < 150 indicate remission. |
| Harvey–Bradshaw Index (HBI) [16] | CD | Clinical | Simplified version of CDAI evaluating general well-being, abdominal pain, number of liquid stools, abdominal mass, and complications; scores ≤ 4 suggest remission. |
| Perianal Disease Activity Index (PDAI) [17] | CD | Clinical | Assesses perianal disease severity based on discharge, pain, sexual activity restriction, type of perianal disease, and degree of induration; scores > 4 suggest active disease. |
| Crohn’s Disease Endoscopic Index of Severity (CDEIS) [19] | CD | Endoscopic | Evaluates the presence and extent of ulcerations, stenosis, and mucosal lesions across different bowel segments; scores > 5 indicate active disease. |
| Simple Endoscopic Score for Crohn’s Disease (SES-CD) [20] | CD | Endoscopic | Assesses ulcer size, ulcerated surface, affected surface, and presence of narrowings in five bowel segments; scores range from 0 to 56. |
| Rutgeerts Score [21] | CD | Endoscopic | Used postoperatively to assess recurrence in the neoterminal ileum; scores range from i0 (no lesions) to i4 (diffuse inflammation with large ulcers and/or nodules/cobble and/or narrowing/stenosis). |
| Pediatric Ulcerative Colitis Activity Index (PUCAI) [22] | UC (Pediatric) | Clinical | Non-invasive index assessing abdominal pain, rectal bleeding, stool consistency, number of stools, nocturnal stools, and activity level; scores < 10 indicate remission. |
| Pediatric Crohn’s Disease Activity Index (PCDAI) [23] | CD (Pediatric) | Clinical | Evaluates abdominal pain, stool frequency, general well-being, weight, height, and laboratory markers; scores < 10 suggest remission. |
| Scoring System | Disease | Key Parameters | Imaging Findings | Clinical Utility |
|---|---|---|---|---|
| SUS-CD (Simple Ultrasound Score for Crohn’s Disease) [65] | CD | Bowel Wall Thickness (BWT), Color Doppler Signal (CDS) | Increased BWT, hyperemia | Assesses disease activity; correlates with endoscopic indices |
| BUSS (Bowel Ultrasound Score) | CD | BWT, CDS | BWT > 3 mm, increased vascularity | Evaluates treatment response; correlates with endoscopic findings |
| IBUS-SAS (International Bowel Ultrasound Segmental Activity Score) [47] | CD | BWT, Bowel Wall Stratification (BWS), CDS, Inflammatory Fat | Loss of stratification, increased vascularity, hyperechoic mesenteric fat | Standardized assessment across centers; high interobserver agreement |
| UCS (Ultrasound Consolidated Score) | CD | BWT, Symmetry, Peribowel Fat Echogenicity, CDS, BWS, Bowel Wall Echogenicity | Asymmetrical thickening, hyperechoic fat, increased vascularity | Comprehensive assessment; correlates with endoscopic scores |
| MUC (Milan Ultrasound Criteria) [25] | UC | BWT, Colonic Wall Flow | BWT > 3 mm, increased vascularity | Differentiates active from inactive disease; validated against endoscopy; evaluates treatment response; predicts risk of colectomy |
| UC-IUS (Ulcerative Colitis Intestinal Ultrasound Score) [68] | UC | BWT, CDS, Haustration Patterns, Fat Wrapping | Loss of haustration, increased vascularity, hyperechoic fat | Correlates with Mayo Endoscopic Sub-score; monitors disease activity |
| SPAUSS (Simple Pediatric Activity Ultrasound Score) | Pediatric UC | BWT, CDS across colonic segments | Segmental BWT increase, hyperemia | Assesses disease activity in pediatric patients; correlates with clinical indices |
| Civitelli Index [68] | UC | BWT, CDS | Increased BWT, enhanced vascularity | Quantitative measure of disease activity; applicable in adults and children |
| Parameter | Details |
|---|---|
| Fasting | Patients should fast for at least 4–6 h prior to the examination to minimize residual gastric contents and reduce motion artifacts. |
| Oral Contrast Agent | Ingest 1.5–2 L of a neutral or low-density oral contrast agent (e.g., water, polyethylene glycol solution, low-concentration barium) over 45–60 min before scanning to achieve adequate small bowel distension. |
| Patient Positioning | The patient is positioned supine on the CT table. Scanning is typically performed in the supine position, but prone positioning may be used to redistribute bowel loops and improve visualization. |
| Intravenous Contrast | Administer 100–120 mL of iodinated contrast material intravenously at a rate of 3–5 mL/s. Scanning is performed during the enteric phase, approximately 60–70 s after injection, to optimize visualization of the bowel wall and mesenteric vasculature. |
| Scan Range | Acquire images from the diaphragm to the symphysis pubis to encompass the entire small bowel and adjacent structures. |
| Slice Thickness | Utilize thin-slice acquisition (0.625–1.25 mm) with multiplanar reconstructions (axial, coronal, and sagittal) for detailed assessment of the bowel wall and surrounding tissues. |
| Use of Antispasmodics | Consider administration of antispasmodic agents (e.g., glucagon or hyoscine butylbromide) to reduce bowel peristalsis and motion artifacts, enhancing image quality. |
| Post-Processing | Perform multiplanar reconstructions and, if necessary, 3D volume rendering to evaluate the extent of disease, identify complications such as fistulas or abscesses, and assist in surgical planning. |
| MRI Sequence | Technical Principle | Imaging Appearance | Clinical Relevance in IBD |
|---|---|---|---|
| T1-weighted (pre/post-contrast) | Short TR/TE; fat appears bright, fluid dark. After gadolinium, enhances vascularized tissues. | Inflamed bowel wall shows post-contrast enhancement, often layered (mucosa/submucosa). | Detects mural hyperenhancement, ulcers, and stratification; useful for assessing active inflammation and differentiating fibrotic vs. inflammatory changes. |
| T2-weighted (with fat suppression) | Long TR/TE; fluid appears bright, fat suppressed for better contrast. | Bowel wall edema and ulcers appear as hyperintense areas; lumen fluid is also bright. | Highlights mural edema and inflammatory changes; sensitive for acute disease activity. |
| DWI | Sensitive to restriction of water molecule motion; high signal on high b-value images. | Inflamed segments show restricted diffusion (bright signal, low ADC values). | Identifies active inflammation even without contrast; useful for treatment monitoring. |
| Balanced SSFP | Gradient echo sequence with steady-state free precession; high signal from fluids. | Provides bright depiction of intraluminal fluid and bowel wall in real time. | Useful for assessing bowel motility, luminal narrowing, strictures, and overall loop anatomy. |
| Technical Parameter | Recommended Specification |
|---|---|
| Patient Preparation | |
| Fasting | 4–6 h prior to the exam |
| Oral Contrast | Approximately 1000–1500 mL hyperosmolar oral contrast solution (e.g., Mannitol 2.5%, PEG), administered 45–60 min before imaging |
| Antiperistaltic Medication | Hyoscine Butylbromide, IV 20 mg, or Glucagon 1 mg IV (unless contraindicated) |
| Intravenous Contrast | Gadolinium-based contrast agent (0.1 mmol/kg) |
| Coil | Multichannel phased-array torso/body coil |
| Magnetic Field Strength | 1.5 Tesla or 3 Tesla |
| Slice Thickness | 3–5 mm |
| Slice Gap | 0–1 mm |
| Imaging Planes | Coronal and Axial mandatory; Sagittal optional |
| Acquisition Technique | Breath-hold or free-breathing with respiratory triggering |
| Total Scan Duration | Approximately 25–35 min |
| MRI Sequences and Parameters | |
| Cor T2-w (HASTE/SSFSE) | |
| Purpose | Overview, bowel distension, fluid detection |
| TR/TE | >1000 ms/80–120 ms |
| Matrix | 256–320 × 256–320 |
| FOV | 350–400 mm |
| Slice Thickness | 3–5 mm |
| Ax and Cor T2-w (Fat-Suppressed) | |
| Purpose | Detection of bowel wall edema/inflammation |
| TR/TE | >1000 ms/80–120 ms |
| Matrix | 256–320 × 256–320 |
| FOV | 300–400 mm |
| Slice Thickness | 3–4 mm |
| Balanced SSFP (TrueFISP/FIESTA) | |
| Purpose | Identification of bowel motility, strictures, and fistulas |
| TR/TE | Shortest possible (<5 ms) |
| Flip angle | 45–90° |
| Matrix | 256 × 256 |
| FOV | 300–400 mm |
| Slice Thickness | 4–6 mm |
| Diffusion-Weighted Imaging (DWI) | |
| Purpose | Identification of active inflammation |
| b-values | Typically 50, 600, and 800 s/mm2 |
| Matrix | 128–192 × 128–192 |
| Slice Thickness | 4–5 mm |
| Acquisition | Single-shot EPI |
| Pre and post-contrast 3D T1-weighted Gradient Echo | |
| Purpose | Evaluate enhancement pattern, mural stratification, ulcers |
| TR/TE | <5 ms/1–2 ms |
| Flip angle | 10–15° |
| Matrix | 256–320 × 192–256 |
| FOV | 350–400 mm |
| Slice Thickness | 3 mm (with interpolation) |
| Phase | Pre-contrast, arterial (20–25 s), enteric (~45 s), and portal venous (~70 s) phases |
| Clinical Scenario | Preferred Imaging Modality | Key Strengths | Limitations |
|---|---|---|---|
| Assessment of strictures (fibrotic vs. inflammatory) | MRE ± CEUS, CTE if urgent | MRE: superior for mural edema, stratification, fibrosis vs. inflammation; CEUS quantifies vascularity | CTE uses radiation; MRI limited availability |
| Detection of fistulae (entero-enteric, entero-vesical, entero-cutaneous) | MRE, pelvic MRI | High soft-tissue contrast; maps fistula tracts; defines complexity | Requires expertise; limited in acute emergencies |
| Perianal fistulae and abscesses | Pelvic MRI | Gold standard; precise classification (Parks); detection of abscesses | Cost, exam duration |
| Detection of abscesses (intra-abdominal) | MRE or CTE | Excellent sensitivity for fluid collections and inflammatory masses | MRI availability; CT involves radiation |
| Postoperative recurrence (Crohn’s disease) | IUS and MRE | Non-invasive monitoring; Rutgeerts score correlation; detects anastomotic recurrence | May miss subtle mucosal lesions |
| Disease monitoring in UC | IUS, CEUS, MRI | Non-invasive follow-up; correlates with endoscopic activity | Mild superficial disease may escape detection |
| Pediatric patients | IUS and MRE (no radiation) | Safe for repeated follow-up; good correlation with endoscopy | Requires cooperation and expertise |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavalle, S.; Vitello, A.; Masiello, E.; Dell’Anna, G.; Romeo, P.; Montana, A.; Privitera, G.; Cosenza, M.; Santangelo, D.; Russo, T.; et al. The Role of Imaging in Inflammatory Bowel Diseases: From Diagnosis to Individualized Therapy. Diagnostics 2025, 15, 2457. https://doi.org/10.3390/diagnostics15192457
Lavalle S, Vitello A, Masiello E, Dell’Anna G, Romeo P, Montana A, Privitera G, Cosenza M, Santangelo D, Russo T, et al. The Role of Imaging in Inflammatory Bowel Diseases: From Diagnosis to Individualized Therapy. Diagnostics. 2025; 15(19):2457. https://doi.org/10.3390/diagnostics15192457
Chicago/Turabian StyleLavalle, Salvatore, Alessandro Vitello, Edoardo Masiello, Giuseppe Dell’Anna, Placido Romeo, Angelo Montana, Giambattista Privitera, Michele Cosenza, Domenico Santangelo, Tommaso Russo, and et al. 2025. "The Role of Imaging in Inflammatory Bowel Diseases: From Diagnosis to Individualized Therapy" Diagnostics 15, no. 19: 2457. https://doi.org/10.3390/diagnostics15192457
APA StyleLavalle, S., Vitello, A., Masiello, E., Dell’Anna, G., Romeo, P., Montana, A., Privitera, G., Cosenza, M., Santangelo, D., Russo, T., Bonomo, F., Sinagra, E., Pal, P., Facciorusso, A., Macaluso, F. S., Orlando, A., & Maida, M. (2025). The Role of Imaging in Inflammatory Bowel Diseases: From Diagnosis to Individualized Therapy. Diagnostics, 15(19), 2457. https://doi.org/10.3390/diagnostics15192457










