Intersecting Pathologies: COL1A1-Related Syndrome in the Setting of Childhood-Onset Hypopituitarism: Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
2.1. Initial Presentation
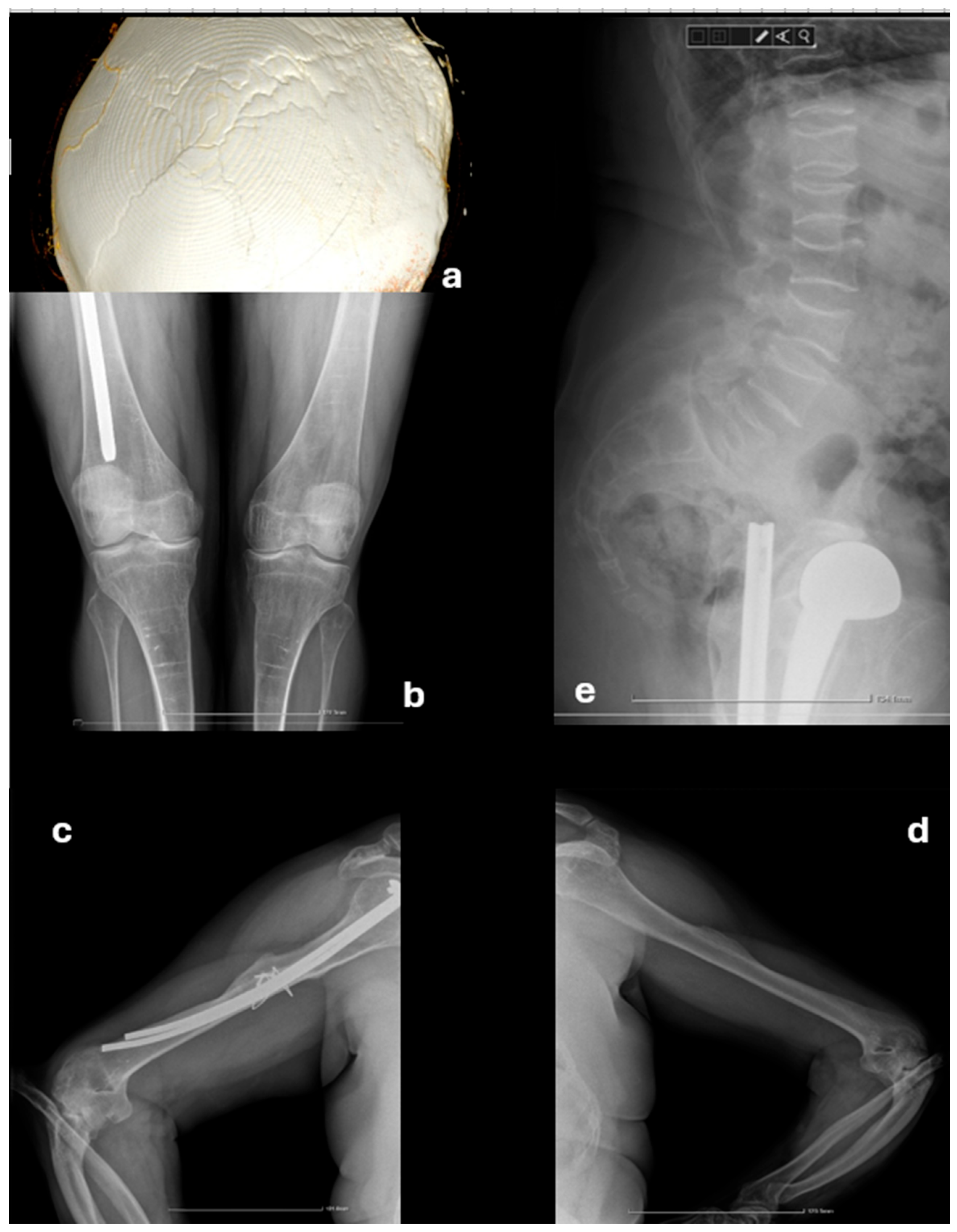
2.2. Further Investigations
2.3. Management and Follow-Up
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25OH vitaminD | 25-hydroxyvitamin D |
| a-EDS | Arthrocalasia Ehlers–Danlos syndrome |
| Anti-TPO Ab | Anti-thyroperioxydase antibodies |
| BMD | Bone mineral density |
| C1ROD | COL1-related overlap disorder |
| c-EDS | Classic Ehlers–Danlos syndrome |
| CT | Computed tomography |
| cv-EDS | Cardiac-valvular Ehlers–Danlos syndrome |
| dB | Decibels |
| EDS | Ehlers–Danlos syndrome |
| FSH | Follicular stimulating hormone |
| FT3 | Free triiodothyronine |
| FT4 | Free thyroxine |
| GH | Growth hormone |
| IGF-1 | Insulin-like growth factor-1 |
| iPTH | Intact parathyroid hormone |
| LH | Luteinizing hormone |
| MRI | Magnetic resonance Imaging |
| OI | Osteogenesis imperfecta |
| TSH | Thyroid-stimulating hormone |
| v-EDS | Vascular Ehlers–Danlos syndrome |
Appendix A
| Case No. | Author | Gender | Joint Hypermobility | Joint Dislocation | Skin Hyperextensibility | Easy Bruising | Atrophic Scars | Fractures | Osteopenia | Blue Sclerae | Short Stature | Dental Abnormalities | Facial Abnormalities | Variant | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Byers [19] | F | + | + | + | - | - | - | - | + | - | - | + | Intron 5 | |
| 2 | Symoens [20] | F | +(8/9) | - | + | - | - | + | + | + | - | + | - | c.3790A > G p.Met1264Val | Exon 49 |
| 3 | Cabral [10] | M | + | - | - | - | - | - | - | + | + | - | - | c.588 + 4A > T | skip Exon 7 |
| 4 | F | +(9/9) | - | - | - | - | - | + | + | - | - | - | c.572G > A p.Gly191Asp | Exon 7 | |
| 5 | F | +(7/9) | - | - | - | - | - | - | + | + | - | - | c.609G > A p.Gly203Val | Exon 8 | |
| 6 | F | +(7/9) | - | - | - | - | - | + | + | + | - | - | c.609G > A p.Gly203Val | Exon 8 | |
| 7 | M | - | - | - | - | - | - | - | - | - | - | - | c.634G > C p.Gly212Arg | Exon 8 | |
| 8 | F | +(5/9) | - | - | - | - | - | + | + | + | - | - | c.761G > A p.Gly254Glu | Exon 11 | |
| 9 | F | +(7/9) | - | - | - | - | - | + | + | + | - | - | c.796G > A p.Gly266Glu | Exon 11 | |
| 10 | Cabral [21] | M | +(2/9) | - | - | - | - | + | + | + | - | - | - | c.3196C > T p.Arg1066Cys | Exon 44 |
| 11 | M | +(4/9) | - | - | - | - | + | - | + | - | - | - | c.3196C > T p.Arg1066Cys | Exon 44 | |
| 12 | M | +(2/9) | - | - | - | - | + | + | + | - | - | - | c.3196C > T p.Arg1066Cys | Exon 44 | |
| 13 | M | - | - | - | - | - | - | - | + | - | - | - | c.3196C > T p.Arg1066Cys | Exon 44 | |
| 14 | Lund [22] | M | +(9/9) | - | - | + | - | + | - | + | - | + | - | c.3106C > T p.Arg1036Cys | Exon 44 |
| 15 | F | +(7/9) | - | - | + | - | + | - | - | - | - | - | c.3106C > T p.Arg1036Cys | Exon 44 | |
| 16 | F | +(7/9) | - | - | + | + | + | - | + | - | + | - | c.3106C > T p.Arg1036Cys | Exon 44 | |
| 17 | Malfait [3] | M | +(8/9) | + | + | + | + | + | + | + | + | - | - | c.563G > A p.Gly188Asp | Exon 7 |
| 18 | M | +(9/9) | + | + | + | + | + | + | + | + | - | - | c.607G > T p.Gly203Cys | Exon 8 | |
| 19 | Vandersteen [23] | M | +(2/9) | - | - | - | - | + | - | + | + | + | - | c.3567delT | Exon 49 |
| 20 | F | +(7/9) | - | - | - | - | - | - | + | - | - | - | c.643G > A p.Gly215Ser | Exon 9 | |
| 21 | F | + | + | - | - | + | - | - | + | - | - | - | c.643G > A p.Gly215Ser | Exon 9 | |
| 22 | M | - | - | - | - | + | + | - | + | - | - | - | Deletion Exon 11-20 | ||
| 23 | Shi [9] | M | - | - | - | - | - | - | - | + | - | - | - | c.3521C > T p.Ala1174Val | Exon 48 |
| 24 | F | +(3/9) | + | - | + | - | + | + | + | + | - | - | c.3521C > T p.Ala1174Val | Exon 48 | |
| 25 | M | +(5/9) | + | - | + | - | + | - | + | - | + | - | c.3521C > T p.Ala1174Val | Exon 48 | |
| 26 | M | +(5/9) | + | - | - | - | + | - | + | + | - | - | c.3521C > T p.Ala1174Val | Exon 48 | |
| 27 | M | + | + | - | - | - | + | - | + | - | - | + | c.3521C > T p.Ala1174Val | Exon 48 | |
| 28 | F | - | - | - | - | - | - | - | - | - | - | - | c.3521C > T p.Ala1174Val | Exon 48 | |
| 29 | Ackermann [24] | M | - | - | - | - | - | - | - | - | - | - | - | c.3196C > T p.Pro814Leufs * 266 | Exon 44 |
| 30 | F | - | - | - | - | - | + | + | + | - | - | - | c.2522delC p.Arg1066Cys | Exon 37 | |
| 31 | F | + | - | - | - | - | + | + | + | - | - | - | c.3196C > T p.Pro814Leufs * 266; c.2522delC p.Arg1066Cys | Exon 37,44 | |
| 32 | M | + | - | - | - | - | + | + | + | - | - | - | c.3196C > T p.Pro814Leufs * 266; c.2522delC p.Arg1066Cys | Exon 37,44 | |
| 33 | M | - | - | - | - | - | + | - | + | - | - | - | c.3196C > T p.Pro814Leufs * 266; c.2522delC p.Arg1066Cys | Exon 37,44 | |
| 34 | Mackenroth [14] | F * | +(5/9) | - | - | - | - | + | + | - | - | - | - | c4009-1G > A | Intron 49 |
| 35 | M | +(6/9) | - | - | - | - | + | + | - | - | - | - | c.4009-1G-A | Intron 49 | |
| 36 | Symoens [25] | F | +(3/9) | + | - | + | + | + | - | - | - | - | - | c.3150_3158del p.Ala1053_Gly1055del | Exon 44 |
| 37 | Lu [26] | M | +(6/9) | + | + | + | - | + | - | + | + | + | + | c.671G > A p.Gly224Asp | Exon 9 |
| 38 | Lin [2] | F ** | + | + | - | + | + | + | - | + | - | - | + | c.2010delT p.Gly671Alafs * 95 | Exon 30 |
| 39 | F ** | + | - | - | + | + | + | - | + | - | - | + | c.2010delT p.Gly671Alafs * 95 | Exon 30 | |
| 40 | M | - | - | - | - | - | + | - | + | - | - | + | c.2010delT p.Gly671Alafs * 95 | Exon 30 | |
| 41 | Morlino [11] | M | +(8/9) | + | + | - | + | + | - | + | - | - | - | c.326G > A p.Gly109Asp | Exon 3 |
| 42 | M | +(5/9) | + | + | - | - | - | + | + | - | - | - | c.581G > C p.Gly194Ala | Exon 7 | |
| 43 | M | +(5/9) | + | - | - | + | + | - | + | - | - | - | c.2073delT p.Gly692Valfs * 74 | Exon 31 | |
| 44 | F | +(6/9) | - | - | - | - | - | - | + | - | - | - | c.2073delT p.Gly692Valfs * 74 | Exon 31 | |
| 45 | F | +(7/9) | - | - | - | - | + | + | + | - | - | - | c.1243C > T p.Arg415 * | Exon 19 | |
| 46 | M | + (4/9) | - | + | + | + | + | - | + | - | - | - | c.670G > A p.Gly224Ser | Exon 9 | |
| 47 | Duong [16] | F | +(9/9) | + | + | - | + | - | - | - | - | - | - | c.934C > T p.Arg312Cys | Exon 14 |
| 48 | F | + | - | + | - | + | + | - | - | - | + | - | c.934C > T p.Arg312Cys | Exon 14 | |
| 49 | Foy [13] | F | +(6/9) | + | + | + | - | + | + | + | - | + | - | c.935G > T p.Arg312Leu | Exon 14 |
| 50 | F | + (7/9) | + | - | + | - | + | + | + | - | - | - | c.935G > T p.Arg312Leu | Exon 14 | |
| 51 | F | +(7/9) | + | + | + | - | + | - | + | - | - | - | c.935G > T p.Arg312Leu | Exon 14 | |
| 52 | Morabito [8] | F | +(7/9) | - | - | - | - | - | + | + | + | + | + | c.3235G > A p.Gly1079Ser | Exon 44 |
| 53 | Takeda [27] | M | + | - | + | - | - | + | - | + | - | - | - | c.658C > T p.Arg220 * | Exon 9 |
| 54 | F | + | + | - | + | - | + | + | + | + | - | - | c.571G > T p.Gly191Cys | Exon 7 | |
| 55 | Venable [4] | M | + | + | - | - | - | + | - | + | + | - | - | c.423_433delinsT p.Gly142Aspfs * 120 | Exon 5 |
| 56 | M | - | - | - | - | - | + | - | + | - | - | - | c.1128del p.Gly377Alafs * 164 | Exon 17 | |
| 57 | F | +(4/9) | - | - | + | - | + | + | + | - | - | - | c.1587del p.Gly530Valfs * 11 | Exon 23 | |
| 58 | F | +(7/9) | + | - | + | + | - | - | - | - | - | - | c.2197C > T p.Arg733Cys | Exon32 | |
| 59 | M | - | - | - | + | + | + | + | + | - | - | - | c.3421C > T p.Arg1141 * | Exon 46 | |
| 60 | Hassib [28] | F | +(3/9) | - | - | - | - | - | + | + | + | + | + | c.4340T > G p.Val1447Gly | Exon 51 |
| 61 | F | +(3/9) | - | - | - | - | - | + | + | + | + | + | c.1678G > A p.Gly560Ser | Exon 25 | |
| 62 | Nunez-Ordonez [29] | F | + | - | + | - | - | - | - | + | - | - | + | c.572G > A p.Gly191Arg | Exon 7 |
| Total (feature present/absent) | 51/11 | 21/41 | 14/48 | 19/43 | 15/47 | 40/22 | 26/36 | 52/10 | 17/45 | 12/50 | 11/51 | ||||
References
- Gnoli, M.; Brizola, E.; Tremosini, M.; Pedrini, E.; Maioli, M.; Mosca, M.; Bassotti, A.; Castronovo, P.; Giunta, C.; Sangiorgi, L. COL1-Related Disorders: Case Report and Review of Overlapping Syndromes. Front. Genet. 2021, 12, 640558. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, J.; Wang, X. Compound Phenotype of Osteogenesis Imperfecta and Ehlers-Danlos Syndrome Caused by Combined Mutations in COL1A1 and COL5A1. Biosci. Rep. 2019, 39, BSR20181409. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Symoens, S.; Goemans, N.; Gyftodimou, Y.; Holmberg, E.; López-González, V.; Mortier, G.; Nampoothiri, S.; Petersen, M.B.; De Paepe, A. Helical Mutations in Type i Collagen That Affect the Processing of the Amino-Propeptide Result in an Osteogenesis Imperfecta/Ehlers-Danlos Syndrome Overlap Syndrome. Orphanet J. Rare Dis. 2013, 8, 78. [Google Scholar] [CrossRef]
- Venable, E.; Knight, D.R.T.; Thoreson, E.K.; Baudhuin, L.M. COL1A1 and COL1A2 Variants in Ehlers-Danlos Syndrome Phenotypes and COL1-Related Overlap Disorder. Am. J. Med. Genet. Part C Semin. Med. Genet. 2023, 193, 147–159. [Google Scholar] [CrossRef]
- Scheres, L.J.J.; van Dijk, F.S.; Harsevoort, A.J.; van Dijk, A.T.H.; Dommisse, A.M.; Janus, G.J.M.; Franken, A.A.M. Adults with Osteogenesis Imperfecta: Clinical Characteristics of 151 Patients with a Focus on Bisphosphonate Use and Bone Density Measurements. Bone Rep. 2018, 8, 168–172. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 International Classification of the Ehlers–Danlos Syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Cole, W.G.; Chan, D.; Chambers, G.W.; Walker, I.D.; Bateman, J.F. Deletion of 24 Amino Acids from the Pro-A1(I) Chain of Type I Procollagen in a Patient with the Ehlers-Danlos Syndrome Type VII. J. Biol. Chem. 1986, 261, 5496–5503. [Google Scholar] [CrossRef]
- Morabito, L.A.; Allegri, A.E.M.; Capra, A.P.; Capasso, M.; Capra, V.; Garaventa, A.; Maghnie, M.; Briuglia, S.; Wasniewska, M.G. Osteogenesis Imperfecta/Ehlers–Danlos Overlap Syndrome and Neuroblastoma—Case Report and Review of Literature. Genes 2022, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lu, Y.; Wang, Y.; Zhang, Y.A.; Teng, Y.; Han, W.; Han, Z.; Li, T.; Chen, M.; Liu, J.; et al. Heterozygous Mutation of c.3521C>T in COL1A1 May Cause Mild Osteogenesis Imperfecta/Ehlers-Danlos Syndrome in a Chinese Family. Intractable Rare Dis. Res. 2015, 4, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Cabral, W.A.; Makareeva, E.; Colige, A.; Letocha, A.D.; Ty, J.M.; Yeowell, H.N.; Pals, G.; Leikin, S.; Marini, J.C. Mutations near Amino End of A1(I) Collagen Cause Combined Osteogenesis Imperfecta/Ehlers-Danlos Syndrome by Interference with N-Propeptide Processing. J. Biol. Chem. 2005, 280, 19259–19269. [Google Scholar] [CrossRef]
- Morlino, S.; Micale, L.; Ritelli, M.; Rohrbach, M.; Zoppi, N.; Vandersteen, A.; Mackay, S.; Agolini, E.; Cocciadiferro, D.; Sasaki, E.; et al. COL1-Related Overlap Disorder: A Novel Connective Tissue Disorder Incorporating the Osteogenesis Imperfecta/Ehlers-Danlos Syndrome Overlap. Clin. Genet. 2020, 97, 396–406. [Google Scholar] [CrossRef]
- Thiele, F.; Cohrs, C.M.; Flor, A.; Lisse, T.S.; Przemeck, G.K.H.; Horsch, M.; Schrewe, A.; Gailus-Durner, V.; Ivandic, B.; Katus, H.A.; et al. Cardiopulmonary Dysfunction in the Osteogenesis Imperfecta Mouse Model Aga2 and Human Patients Are Caused by Bone-Independent Mechanisms. Hum. Mol. Genet. 2012, 21, 3535–3545. [Google Scholar] [CrossRef]
- Foy, M.; De Mazancourt, P.; Métay, C.; Carlier, R.; Allamand, V.; Gartioux, C.; Gillas, F.; Miri, N.; Jobic, V.; Mekki, A.; et al. A Novel COL1A1 Variant in a Family with Clinical Features of Hypermobile Ehlers-Danlos Syndrome That Proved to Be a COL1-Related Overlap Disorder. Clin. Case Rep. 2021, 9, e04128. [Google Scholar] [CrossRef] [PubMed]
- Mackenroth, L.; Fischer-Zirnsak, B.; Egerer, J.; Hecht, J.; Kallinich, T.; Stenzel, W.; Spors, B.; von Moers, A.; Mundlos, S.; Kornak, U.; et al. An Overlapping Phenotype of Osteogenesis Imperfecta and Ehlers-Danlos Syndrome Due to a Heterozygous Mutation in COL1A1 and Biallelic Missense Variants in TNXB Identified by Whole Exome Sequencing. Am. J. Med. Genet. Part A 2016, 170, 1080–1085. [Google Scholar] [CrossRef]
- Marini, J.C.; Forlino, A.; Cabral, W.A.; Barnes, A.M.; San Antonio, J.D.; Milgrom, S.; Hyland, J.C.; Körkkö, J.; Prockop, D.J.; De Paepe, A.; et al. Consortium for Osteogenesis Imperfecta Mutations in the Helical Domain of Type I Collagen: Regions Rich in Lethal Mutations Align with Collagen Binding Sites for Integrins and Proteoglycans. Hum. Mutat. 2007, 28, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Duong, J.; Rideout, A.; MacKay, S.; Beis, J.; Parkash, S.; Schwarze, U.; Horne, S.G.; Vandersteen, A. A Family with Classical Ehlers-Danlos Syndrome (CEDS), Mild Bone Fragility and without Vascular Complications, Caused by the p.Arg312Cys Mutation in COL1A1. Eur. J. Med. Genet. 2020, 63, 103730. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Ishihara, Y.; Kusakabe, T.; Tsuiki, M.; Nanba, K.; Hiroshima-Hamanaka, K.; Nomura, T.; Satoh-Asahara, N.; Yasoda, A.; Tagami, T. A Case of Osteogenesis Imperfecta Caused by a COL1A1 Variant, Coexisting with Pituitary Stalk Interruption Syndrome. Endocr. J. 2023, 70, 839–846. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Guan, H.; Wang, X. Osteogenesis Imperfecta Due to Combined Heterozygous Mutations in Both COL1A1 and COL1A2, Coexisting with Pituitary Stalk Interruption Syndrome. Front. Endocrinol. 2019, 10, 193. [Google Scholar] [CrossRef]
- Byers, P.H.; Duvic, M.; Atkinson, M.; Robinow, M.; Smith, L.T.; Krane, S.M.; Greally, M.T.; Ludman, M.; Matalon, R.; Pauker, S.; et al. Ehlers-Danlos Syndrome Type VIIA and VIIB Result From Splice-Junction Mutations or Genomic Deletions That Involve Exon 6 in the COL1A1 and COL1A2 Genes of Type I Collagen. Am. J. Med. Genet. 1997, 72, 94–105. [Google Scholar] [CrossRef]
- Symoens, S.; Nuytinck, L.; Legius, E.; Malfait, F.; Coucke, P.J.; De Paepe, A. Met>Val Substitution in a Highly Conserved Region of the Pro-Alpha1(I) Collagen C-Propeptide Domain Causes Alternative Splicing and a Mild EDS/OI Phenotype. J. Med. Genet. 2004, 41, e96. [Google Scholar] [CrossRef]
- Cabral, W.A.; Makareeva, E.; Letocha, A.D.; Scribanu, N.; Fertala, A.; Steplewski, A.; Keene, D.R.; Persikov, A.V.; Leikin, S.; Marini, J.C. Y-Position Cysteine Substitution in Type I Collagen (A1(I) R888C/p.R1066C) Is Associated with Osteogenesis Imperfecta/Ehlers-Danlos Syndrome Phenotype. Hum. Mutat. 2007, 28, 396–405. [Google Scholar] [CrossRef]
- Lund, A.M.; Joensen, F.; Christensen, E.; Dunø, M.; Skovby, F.; Schwartz, M. A Novel Arginine-to-Cysteine Substitution in the Triple Helical Region of the A1(I) Collagen Chain in a Family with an Osteogenesis Imperfecta/Ehlers-Danlos Phenotype. Clin. Genet. 2008, 73, 97–101. [Google Scholar] [CrossRef]
- Vandersteen, A.M.; Lund, A.M.; Ferguson, D.J.P.; Sawle, P.; Pollitt, R.C.; Holder, S.E.; Wakeling, E.; Moat, N.; Pope, F.M. Four Patients with Sillence Type I Osteogenesis Imperfecta and Mild Bone Fragility, Complicated by Left Ventricular Cardiac Valvular Disease and Cardiac Tissue Fragility Caused by Type I Collagen Mutations. Am. J. Med. Genet. Part A 2014, 164, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, A.M.; Levine, M.A. Compound Heterozygous Mutations in COL1A1 Associated with an Atypical Form of Type I Osteogenesis Imperfecta. Am. J. Med. Genet. Part A 2017, 173, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Symoens, S.; Steyaert, W.; Demuynck, L.; De Paepe, A.; Diderich, K.E.M.; Malfait, F.; Coucke, P.J. Tissue-Specific Mosaicism for a Lethal Osteogenesis Imperfecta COL1A1 Mutation Causes Mild OI/EDS Overlap Syndrome. Am. J. Med. Genet. Part A 2017, 173, 1047–1050. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Rauch, F.; Li, H.; Zhang, Y.; Zhai, N.; Zhang, J.; Ren, X.; Han, J. Osteogenesis Imperfecta Type III/Ehlers-Danlos Overlap Syndrome in a Chinese Man. Intractable Rare Dis. Res. 2018, 7, 37–41. [Google Scholar] [CrossRef]
- Takeda, R.; Yamaguchi, T.; Hayashi, S.; Sano, S.; Kawame, H.; Kanki, S.; Taketani, T.; Yoshimura, H.; Nakamura, Y.; Kosho, T. Clinical and Molecular Features of Patients with COL1-Related Disorders: Implications for the Wider Spectrum and the Risk of Vascular Complications. Am. J. Med. Genet. Part A 2022, 188, 2560–2575. [Google Scholar] [CrossRef]
- Hassib, N.F.; Elhossini, R.M.; Sayed, I.S.M.; Aglan, M.S.; Abdel-Hamid, M.S. COL1-Related Overlap Disorder: An Emerging Phenotype Linked to Mono- and Bi-Allelic COL1A1/2 Variants. Arch. Oral Biol. 2025, 178, 106344. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Ordonez, N.; Amado-Olivares, A.F.; Jimenez-Ordonez, A.F.; Obando, C.; Chalela, T.; Senosiain, J.; Sandoval, N.; Camacho-Mackenzie, J.; Villa-Hincapié, C. Case Report: A Usual Procedure in an Unusual Situation: A Patient with a Rare Ehlers Danlos/Osteogenesis Imperfecta Overlap Undergoing Aortic Valve Replacement. Front. Cardiovasc. Med. 2025, 12, 1480363. [Google Scholar] [CrossRef]

| Measurement | Value |
|---|---|
| Height (cm) | 155 |
| Weight (kg) | 75 |
| Head circumference (cm) | 60.5 |
| Armspan (cm) | 153 |
| Wingspan-to-height ratio | 0.98 |
| Torso length (cm) | 50 |
| Left lower limb length (cm) | 99 |
| Right lower limb length (cm) | 99 |
| Left upper limb length (cm) | 69.5 |
| Right upper limb length (cm) | 66 |
| Investigation | Value | Reference Range |
|---|---|---|
| GH (ng/mL) | <0.05 | 0.07–2.47 |
| IGF-1 (ng/mL) | 18.26 | 53–215 |
| TSH (mUI/L) | 7.26 | 0.55–4.78 |
| Free T4 (pmol/L) | 5.03 | 11.48–22.70 |
| Free T3 (pmol/L) | 3.60 | 3.54–6.47 |
| Cortisol (mcg/dL) | 2.94 | 5.27–22.45 |
| FSH (mIU/mL) | 0.06 | 1.4–18.1 |
| LH (mIU/mL) | 0.02 | 1.5–9.3 |
| Testosterone (ng/dL) | <7.00 | 187.7–684.1 |
| Prolactin (ng/mL) | 15.92 | 3.7–17.9 |
| Total serum calcium (mg/dL) | 10.9 | 8.4–10.2 |
| Serum phosphate (mg/dL) | 5.1 | 2.5–4.5 |
| Alkaline phosphatase (U/L) | 51 | 38–126 |
| iPTH (pg/mL) | 32.8 | 18.5–88 |
| 25OH Vitamin D (ng/mL) | 7.10 | >30 |
| Anti-TPO Ab (U/L) | 493 | 0–60 |
| At Diagnosis | Present | |||
|---|---|---|---|---|
| Parameter | T-Score (SD) | BMD (g/) | T-Score (SD) | BMD (g/) |
| Total score lumbar spine | −4.4 | 0.60 | −3.0 | 0.76 |
| Score L1 | −4.5 | 0.51 | −2.8 | 0.70 |
| Score L2 | −4.5 | 0.59 | −2.6 | 0.80 |
| Score L3 | −4.4 | 0.61 | −3.0 | 0.77 |
| Score L4 | −4.1 | 0.69 | −3.2 | 0.79 |
| Total score hip | −5.3 | 0.23 | ||
| Score femoral neck | −4.5 | 0.31 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelineagră, O.-E.; Golu, I.; Chiriţă-Emandi, A.; Balaş, M.; Andreescu, N.I.; Munteanu, C.V.; Amzăr, D.-G.; Plotuna, I.; Aruncutean, D.; Vlad, M. Intersecting Pathologies: COL1A1-Related Syndrome in the Setting of Childhood-Onset Hypopituitarism: Case Report and Literature Review. Diagnostics 2025, 15, 2453. https://doi.org/10.3390/diagnostics15192453
Pelineagră O-E, Golu I, Chiriţă-Emandi A, Balaş M, Andreescu NI, Munteanu CV, Amzăr D-G, Plotuna I, Aruncutean D, Vlad M. Intersecting Pathologies: COL1A1-Related Syndrome in the Setting of Childhood-Onset Hypopituitarism: Case Report and Literature Review. Diagnostics. 2025; 15(19):2453. https://doi.org/10.3390/diagnostics15192453
Chicago/Turabian StylePelineagră, Oriana-Eliana, Ioana Golu, Adela Chiriţă-Emandi, Melania Balaş, Nicoleta Ioana Andreescu, Cătălin Vasile Munteanu, Daniela-Georgiana Amzăr, Iulia Plotuna, Diana Aruncutean, and Mihaela Vlad. 2025. "Intersecting Pathologies: COL1A1-Related Syndrome in the Setting of Childhood-Onset Hypopituitarism: Case Report and Literature Review" Diagnostics 15, no. 19: 2453. https://doi.org/10.3390/diagnostics15192453
APA StylePelineagră, O.-E., Golu, I., Chiriţă-Emandi, A., Balaş, M., Andreescu, N. I., Munteanu, C. V., Amzăr, D.-G., Plotuna, I., Aruncutean, D., & Vlad, M. (2025). Intersecting Pathologies: COL1A1-Related Syndrome in the Setting of Childhood-Onset Hypopituitarism: Case Report and Literature Review. Diagnostics, 15(19), 2453. https://doi.org/10.3390/diagnostics15192453






