Abstract
Background: The 99th-percentile upper reference limit (99% URL) is essential for high-sensitivity cardiac troponin I (hs-cTnI) assays to diagnose acute coronary syndromes. We derived the gender-specific 99% URLs for the new Snibe hs-cTnI assay and verified its high-sensitivity performance. Methods: The Snibe hs-cTnI assay has a claimed limit of blank/detection/quantitation of 0.5/1.0/2.0 ng/L, a precision of 5.67% (@ 9.83 ng/L), 4.65% (@ 21.0 ng/L) and 3.77% (@ 174 ng/L), an analytical measuring range of 1.00–500,000 ng/L, and a claimed 99% URL of 20.1/11.8/17.5 ng/L (males/females/overall). Assay precision was evaluated using kit control materials. A total of 846 (M 444, F 402) anonymized leftover samples from healthy individuals were assessed for the derivation of 99% URLs. Results: The inter-assay CV was 6.2/4.4% (@ 10.1/20.6 ng/L). The 10/20% CV corresponded to a concentration of 5.2/3.0 ng/L. The assay recorded detectable hs-cTnI values in 64% of women and 70% of men. Hs-cTnI values were significantly lower in females than males (females vs. males median 2.6 vs. 3.2 ng/L, Mann–Whitney p = 0.005). The derived 99% URL for the population was 7.7 ng/L (90% CI 6.93–22.8 ng/L) for women and 13.7 ng/L (90% CI 10.6–41.4 ng/L) for men. When confined to subjects ≥40 years old only, males had a 99% URL of 14.8 ng/L (n = 412, 90% CI 10.6–41.4 ng/L) and 8.1 ng/L (n = 377, 90% CI 6.95–22.8 ng/L) for females, respectively. Conclusions: The Snibe hs-cTnI assay fulfills the requirements of a hs-cTn assay. Gender-specific 99% URLs are derived for this assay and they increased with age.
1. Introduction
The 99th-percentile upper reference limit (99% URL) of troponin is recommended as a cut-off to diagnose acute coronary syndromes by the fourth universal definition of myocardial infarction [1]. The 99% URL remains the recommended diagnostic threshold for myocardial injury and myocardial infarction by both the International Federation of Clinical Chemistry [2] and cardiology specialty societies [3]. In addition, it is increasingly recognized that the troponin 99% URL may serve other functions, for example, in the prognostication of patients with myocardial infarction, where nearly 50% of patients with 0 h troponins above the 99% URLs may experience poorer outcomes (rehospitalization, death, non-fatal myocardial infarction or revascularization) compared to 70% in patients who did not exceed that threshold [4]. Even in asymptomatic individuals, hs-cTnI has been shown to effectively predict cardiovascular disease, for example, in the BiomarCARE project, where a threshold of 6 ng/L had a hazard ratio of 1.63 for all cause mortality, and a hazard ratio of 2.6 for cardiovascular death in community living subjects between the age of 20–99 years (mean age 52.2 years) [5].
New high-sensitivity cardiac troponin (hs-cTn) I and T assays have a better negative predictive than older assays, and facilitate the rapid rule—out of myocardial injury/infarction [6]. Algorithms based on these newer troponin assays have been shown to effectively decrease the length of stay in some emergency departments by up to 2.2 h [7], with a single hs-cTn measurement ruling out 24.1% of chest pain patients, and increasing to 63.8% after the second measurement 1 h later. Indeed, less than 15% of patients required hospital transfer after clinic assessment, which greatly reduced the burden of care in the emergency department. These algorithms have also proven levels of safety. In patients discharged from the emergency department using a 0/1 h algorithm, only 0.45% experienced morbidity/mortality within 30 days, compared to 0.61% in control hospitals [8]. Indeed, when the diagnostic performance of a new hs-cTnI assay (Hybiome hs-cTnI assay, correlation with Beckman hs-cTnI r = 0.926) was assessed using four different methods (limit of detection cut-off, 99% URL cut-off, 0/1 h algorithm, 0/2 h algorithm for the diagnosis of NSTEMI), the single 99% URL cut-off had a negative predictive value/accuracy of 98.48/85.4%, whereas the 0/2 h algorithm provided slightly better performance with negative predictive value/accuracy of 98.5/88.6% [9].
However, there remains substantial variability in the thresholds used for the 99% URL. In fact, in a study of 276 participating institutions, only a third followed the guidelines to use the 99% URL decision levels in the diagnosis of myocardial infarction [10], among 21 unique troponin assays from 9 manufacturers. The picture becomes even further muddied with the introduction of newer point-of-care troponin testing, especially with the recent introduction of point-of-care troponin assays that can fulfill the criteria for high-sensitivity. For example, the Siemens Atellica hs-cTnI point-of-care assay demonstrated excellent sensitivity (98.9%) and negative predictive values (99.5%) when compared to an Abbott hs-cTnI assay [11], and also when compared to a Beckman hs-cTnI assay: Siemens/Beckman ROC areas were 0.94/0.97. These point-of-care tests will also require the use of specific 99% URL diagnostic thresholds. Already efforts are being made to try to determine cut-offs for their use in rapid rule-out algorithms [12].
The 99% URL should be derived from a reference cohort of healthy individuals, using a minimum of 800 subjects (400 subjects per gender) to ensure statistical power [2]. There are some advantages in the use of hs-cTnI, as it is less affected by skeletal muscle disorders. In one study of patients with muscular disease (including myotonic dystrophy, dermatomyositis, myasthenic syndromes) [13], hs-cTnT assay concentrations were much higher in patients with skeletal muscle disorders (median 16 ng/L vs. 5 ng/L in controls), compared to hs-cTnI (2.5 ng/L vs. 2.9 ng/L in controls), with up to 55% of muscular disease patients having a higher hs-cTnT than the upper limit of normal. In another study of 18 patients with myopathy, 17 had increased cTnT values with no increase in cTnI [14]. A similar trend was found in subjects with statin induced myopathy [13,15], with median cTnT increasing to 0.19 ug/L on presentation despite no increase in cTnI. This can cause confusion when using hs-cTnT in the clinical setting, as up to 15.3% of patients with myopathy would be mistakenly ruled-in for myocardial infarction based on European Society of Cardiology algorithms [16]. However, both troponins T and I are increased in chronic kidney disease (CKD), but less so with troponin I [17]. In a study of 119 patients with a wide range of estimated glomerular filtration rates (eGFR), hs-cTnT demonstrated a strong relationship with decreasing eGFR (R2 = 0.625), whereas no significant relationship with hs-cTnI was found (R2 = 0.013) [18]. The effect of CKD on the accuracy of a single hs-cTnI at presentation can be quite stark: hs-cTnI <5 ng/L at presentation identified only 17% of patients with CKD as low risk for myocardial events, as opposed to 56% without renal impairment, with positive predictive values and specificity at the 99% URL lower in renal impairment (50% and 70.9% vs. 62.4% and 92.1% in non-CKD subjects) [19]. Indeed, the use of serial changes in cardiac troponin concentration is essential in the assessment of myocardial disease in patients with chronic kidney disease [20].
Snibe has recently introduced its latest generation of hs-cTnI assay for its automated chemiluminescence immunoassay system, with reportedly greater selectivity and less interference. As the 99% URL is essential for the use of any troponin result, we sought to derive gender-specific 99% URLs for this new hs-cTnI assay.
2. Materials and Methods
2.1. The Snibe hs-cTnI Assay
The Snibe hs-cTnI assay (Shenzhen New Industries Biomedical Engineering Co., Ltd. (Snibe), Shenzhen, Guangdong, China) is a sandwich immunoassay, with a claimed limit of blank/detection/quantitation of 0.5/1.0/2.0 ng/L, a claimed precision of 5.67% (9.83 ng/L), 4.65% (21.0 ng/L) and 3.77% (174 ng/L), and a linear range of 2.00–50,000 ng/L, an analytical measuring range of 1.00–500,000 ng/L (see Table 1). It has a throughput of 180 tests/hour, and an assay time of 21 min. The sample, magnetic microbeads coated with cTnI monoclonal antibody, N-(-4-aminobutyl)-N-ethylisoluminol (ABEI) labeled with another cTnI monoclonal antibody and buffer are mixed thoroughly. Following incubation, the reactants form sandwich complexes. In designing the assay, coating/labeling cTnI antibody pairs were selected with the highest signal-to-noise ratio. A three-step strategy of signal-to-noise ratio screening, interference assessment (TNNI1, TNNI2 and heparin), and clinical sample validation was adopted for 12 antibodies covering four epitopes of cTnI (24–40, 41–49, 87–91, 171–191 aar). The optimal antibody combination was finally determined: the capture antibody targeting 41–49 aar, and the detection antibody targeting 24–40 aar and 87–91 aar. The optimal reaction system parameters were determined through single-factor optimization: magnetic bead dilution ratio of 1.0 mg/mL, ABEI-labeled antibody dilution ratio of 1:500, 50 μL sample loading volume, and 10 min of incubation time. After precipitation in a magnetic field, the supernatant is decanted followed by a wash cycle. Subsequently, a starter solution is added to initiate a chemiluminescent reaction. A composite blocking system was also employed during the coating process of the magnetic particles, integrating macromolecular and small-molecule blockers to reduce non-specific binding. A 100 μg/mL heterophilic antibody blocker is also added to inhibit human anti-mouse antibody and rheumatoid factor interference (interference rate <5%). In addition, this detection system adopted an improved ABEI label, uniform-sized magnetic microspheres, and a composite blocking system, combined with a dual-labeled antibody strategy. This has enhanced the reagent’s anti-interference ability while ensuring detection sensitivity, providing a reliable solution for rapid and accurate clinical hs-cTnI detection. It has a claimed 99% URL of 11.8 ng/L for females, and 20.1 ng/L for males, derived from 617 apparently healthy individuals in China.

Table 1.
Comparison of previous and new generation of the Snibe hs-cTnI assay.
2.2. Assay Precision Analysis and Derivation of the 99% URL
Assay precision was evaluated according to CLSI EP5-A2 protocols [21] using the assay’s internal quality control material.
2.3. Derivation of the 99% URL
We analyzed anonymized leftover samples from a population of 866 healthy individuals (no history of diabetes, renal impairment, or cardiovascular disease), with 846 subjects (M = 444, F = 402) in the final population after excluding outliers (Tukey method). All subjects had normal renal function (eGFR > 90 mL/min) and no history of heart disease. Visibly hemolyzed or turbid samples were excluded. We subsequently derived the 99% URL (99% right sided interval, non-parametric, following CLSI guidelines [22] for percentiles and their CIs) for males and female hs-cTnI.
2.4. Statistical Analysis
We used MedCalc Statistical Software (version 20.008, MedCalc Software Ltd., Ostend, Belgium) for statistical analyses.
As this investigation was part of routine clinical laboratory service evaluation/population surveillance, national/hospital regulations exempt such testing from institutional board review.
3. Results
3.1. Assay Performance
The inter-assay CV of internal quality control materials was 6.2% (@ 10.1 ng/L) and 4.4% (@ 20.6 ng/L). From the inter-assay precision profile, the 10/20% CV corresponded to a concentration of 5.2/3.0 ng/L (see Figure 1). The assay recorded detectable hs-cTnI values more than the limit of detection in 64% of women and 70% of men. In a repeat analysis at another center, CV% was noted to be 32.4/11.7/6.6/5.6/2.4/2.7% (@1.1/2.1/3.3/4.6/5/5/6.6 ng/L), with a 10% CV at 2.46 ng/L and a 20% CV at 1.53 ng/L. It is recommended that hs-cTn assays should not report values below the 20% CV limit of quantitation, and as such, results <3.0 ng/L on this assay would be reported as <3.0 ng/L.
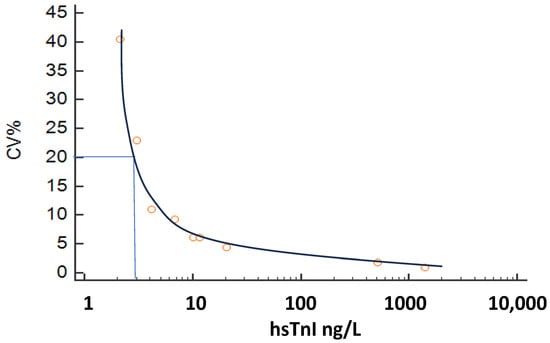
Figure 1.
Imprecision profile of the Snibe hs-cTnI. 20% CV at 3.0 ng/L.
3.2. Population hs-cTnI 99% URL
The age of subjects ranged from 18 to 94 years (mean 60.1 ± 12.7 years). Males were from 18 to 94 years (mean 59.7 ± 12.8 years), and females were 20–90 years (mean 60.5 ± 12.6 years) (see Figure 2).
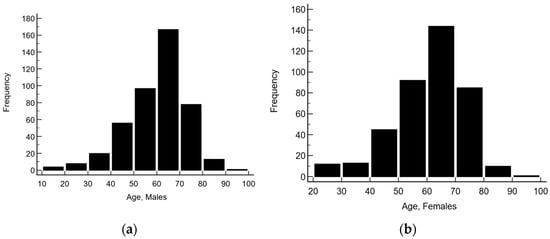
Figure 2.
Age distribution of (a) males and (b) females.
Median hs-cTnI of the study population was 2.9 ng/L (SD 2.6 ng/L) (Kolmogorov–Smirnov test D = 0.24, normality of distribution rejected). For females, the hs-TnI values were significantly lower than males (median: females vs. males 2.6 vs. 3.2 ng/L, Mann–Whitney p = 0.005) (see Figure 3). The derived 99% URL for the population was 7.7 ng/L (90% CI 6.93–22.8 ng/L) for women and 13.7 ng/L (90% CI 10.6–41.4 ng/L) for men. When we restricted analysis to only subjects ≥40 years old, males had a 99% URL of 14.8 ng/L (n = 412, 90% CI 10.6–41.4 ng/L) and females had a 99% URL of 8.1 ng/L (n = 377, 90% CI 6.95–22.8 ng/L).
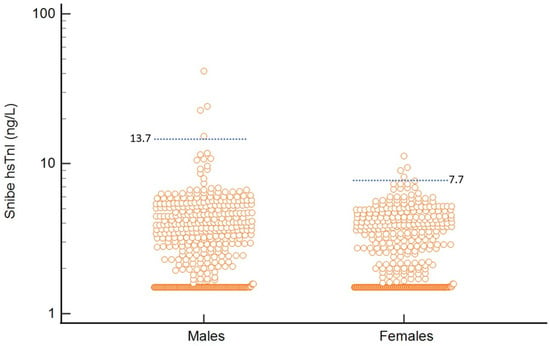
Figure 3.
Distribution and 99% URL of Snibe hs-cTnI in females vs. males in the total population.
4. Discussion
The Snibe hs-cTnI assay demonstrated excellent performance and fulfilled the necessary criteria to confirm its status as a high-sensitivity assay (detectable hs-cTnI values > LOD in 64% of women and 70% of men, with a CV of 10% at 5.2 ng/L). Although our 99% URLs differ from the manufacturer’s values (males 13.7 vs. 20.1 ng/L, females 7.7 vs. 11.8 ng/L), these may be attributed to demographic/geographical differences between the reference populations: the manufacturer information stated that their 99% URLs were derived from 617 apparently healthy individuals in China; however, we have no information on the eGFR, age and distribution of said subjects (all our subjects had eGFR >90 mL/min). In addition, we do not have information on assay lot differences, calibration methods or statistical methods used to derive the manufacturer’s proposed 99% URLs, and it is possible that these may have further contributed to the above differences (our study involved one lot of reagents). This highlights the importance of verifying the applicability of manufacturer 99% URLs prior to usage, and it is highly recommended that population specific cut-offs are derived from healthy local populations prior to hs-cTn assay use and moreover, inclusion of a younger cohort of healthy individuals or subjects with eGFRs of only >90 mL/min would further decrease the 99% URL. In addition, our derived 99% URLs using this assay were higher in males and older subjects, which is similar to findings from other studies reporting 99% URLs for hs-cTnI assays [23].
Defining the 99% URL from a healthy cohort is a contentious issue, as the definition of a healthy cohort is not standardized, and can be affected by the age and renal function of the selected population. Naturally, reference cohorts need to exclude major comorbidities such as known cardiovascular disease, treatment for hyperlipidemia/hypertension, diabetes, chronic renal disease, abnormal BMI, smoking and any other chronic condition that could affect the myocardium. However, by incorporating subjects of all ages (usually 18 years old and above), results can be skewed towards the younger population that tends to be healthier with less comorbidities than older individuals. Furthermore, it has been shown that troponin levels increase in a step-wise fashion with age [24,25]. From small countries like Singapore, to larger nations like the UK, cardiovascular disease occurs overwhelmingly in older patients > 40 years old [26,27]. In Singapore, the overall median age of onset of ACS was 71.4 years old in 2022, and only <2% of reported cases were <40 years old. This effect would make a 99% URL derived from a skewed younger cohort much more likely to cause a larger number of false positives. Indeed, some studies [28] do show that the influence of age can be more pronounced in males, increasing by a greater degree in men aged 40–44 years old (male/female median cTnI 2.7/2.1) vs. 60–65 years old (male/female median cTnI 3.4/2.7). This is also seen in our study, where the 99% URLs increased in males to a greater degree when we restricted our analysis to subjects ≥ 40 years old (males 13.7 to 14.8 ng/L, females 7.7 to 8.1 ng/L). As such, a reasonable age distribution should still be sought in studies that attempt to derive the 99% URL for hs-cTns, with special effort to include sufficient numbers of older subjects. Indeed, age also seems to affect the prognostic values of cTnI, with predictive ability of cTnI dropping after 50 years old from an odds ratio for myocardial infarction decreasing from 9.4 when <30 years old to only 3–5 after 50 years old [29]. One strength of our study was the inclusion of more older subjects to generate the 99% URL; the mean age of our overall population was 60 years old, with a good balance of males (53%) and females. Another strength is that we have used a sufficiently large population (>800 subjects) with >400 subjects in each gender for sufficient statistical power [2].
Undoubtedly, gender-specific hs-cTn 99% URLs should be determined and reported. The fact that females have generally lower hs-cTn values is well established for both hs-cTnI and hs-cTnT [30,31,32]. Indeed, in a recent systemic review [33], 19 studies deriving gender-specific 99% URLs for 11 different hs-cTnI assays found lower female 99% URLs compared to male and overall 99% URLs. This is expected given that women have smaller cardiac mass than men [17]. In addition, studies have also shown that hs-cTnI can be even more strongly associated with ACS in females, with increased hazard ratios at a hs-cTnI of 10ng/L in females than males (9.7 vs. 5.6 in females vs. males) in 19,501 subjects [30]. In the light of these caveats to the 99% URL, some studies have suggested the use of alternative strategies to rule-in ACS, including combined tools incorporating age, sex and paired cTn measurements to generate risk scores, or other novel algorithms [34]. However, these modifications have yet to be more widely validated and adopted. Further efforts would be required to encourage the establishment and usage of gender-specific 99% URLs to rule-out myocardial infarction.
In addition to age and gender, the other most common factor that affects cTn is renal function [20]. This is an important issue, as even minor levels of renal impairment can significantly affect cTn levels, and subsequently the derivation of the 99% URL: in one Danish study [35], 99% URLs were lower with an eGFR cut-off of ≥90 mL/min/1.73 m2 than if a cut-off of 60 mL/min/1.73 m2 was used in the reference population, and this was consistent across three analyzers (Siemens/Abbott/Roche 99% URL was 46/14/10 ng/L if eGFR ≥ 90, 70/18/13 if eGFR 60). This is supported by other studies [25,31] that show a consistent step-wise increase in cTns with decreasing renal function even with a drop from eGFR 90 to 60. This in turn will interfere the diagnostic performance of 0/2 h algorithms for ruling in myocardial injury. Compared to patients with CKD, positive predictive values at hs-cTnT thresholds at >200/300 ng/L was 73/80%, compared to 59/54% in patients with CKD [36]. Thus, we only included subjects with an eGFR ≥ 90 mL/min/1.73 m2 in our reference population in order to account for these effects.
One limitation of our study is that our reference population was from a single geographical area (Southeast Asia) and as such, results may not be generalizable to other populations. In addition, we have yet to compare the performance of the Snibe assay to other platforms, and further studies would be required. However, it must still be noted that hs-cTnI assays are not standardized or harmonized [37], in part because of the extremely varied capture/detection antibodies between manufacturers. For example, detection methods using antibodies targeting the cTnI carboxyl-terminal region can exhibit altered reactivity with cTnI fragments [38], leading to falsely elevated values. In a recent study of 9 hs-cTnI assays [32] there was extensive variation between assays even with gender-specific 99% URLs, with hs-cTnI 99% URLs ranging from 8 to 68 ng/L in men and 3–40 ng/L in women. When diagnostic power is compared between the 99% URLs of different assays [4], variation was also noted in performance, with ROC curve areas varying from 0.874 to 0.897, with varying sensitivities ranging from 87.3 to 98.6% (when using a unisex 99% URL). This variation becomes even more problematic in patients with severe CKD, in part due to decreased renal clearance of troponins, chronic inflammation in CKD, and uremic toxins like homocysteine that contribute to atherosclerosis and myocardial damage [39]. As such, when in doubt, serial measurements of hs-cTn are strongly recommended, especially in patients with CKD, as the deltas based on the same assay would be the most helpful in ruling out myocardial injury [40].
While the investigation of biological interference for the Snibe hs-cTnI assay was beyond the scope of the current study, we did exclude outliers with high hs-cTnI values. Hs-cTnI assays are also more prone to the effects of macrotroponin, resulting in falsely elevated troponin results in the absence of myocardial injury [41]. Notably, the prevalence of macrotroponin I can be quite high, with reported values of between 5 and 52% in community studies, especially in patients with hs-cTnI concentrations above the 99% URL, with macrotroponin I affecting results across platforms [42,43]. Indeed, diagnostic performance was comparatively better in patients without macrotroponin than those with macrotroponin (in the Beckman hs-cTnI assay, ROC in patients with macrotroponin vs. without was 0.576 vs. 0.8338, p = 0.0006) [43]. Furthermore, the effects of macrotroponin on the 99% URLs can potentially be serious. In one study of healthy individuals who had previously been used to generate 99% URLs, macrotroponin I was found to be present in 76% of high hs-cTnI samples, and after exclusion of these samples, 99th URLs decreased from 117 ng/L to 22 ng/L in men, and 37 ng/L to 9 ng/L in women for the Siemens Atellica assay. Indeed, the hs-cTnT comparator in this study had no cases of macrotroponin [44]. Thus, macrotroponin can potentially severely affect the classification rates of myocardial events. However, a limitation of our study is that we did not investigate for macrotroponin in our population. The prevalence of macrotroponin in the Snibe assay in our cohort is unknown, which may potentially affect final results. Although we excluded outliers (Tukey), further investigation on the effects of macrotroponin (complexes formed between auto-antibodies and troponin I) will need to be undertaken in the future for the Snibe assay in our population.
5. Conclusions
In conclusion, we have successfully derived gender-specific 99% URLs for the new Snibe hs-cTnI assay, and established the high-sensitivity status of the assay. Further studies would be required to determine rule-out/in cut-offs for this new assay, as well as the clinical performance of the assay in the diagnosis of myocardial infarction.
Author Contributions
Conceptualization, Y.Z., K.Y. and T.C.A.; methodology, Y.Z., K.Y. and T.C.A.; formal analysis, T.C.A., Y.L.L. and C.S.L.; investigation, S.K.P. and Y.L.L.; resources, Y.Z. and K.Y.; data curation, T.C.A. and C.S.L.; writing—original draft preparation, T.C.A. and C.S.L.; writing—review and approval, all; supervision, T.C.A.; project administration, S.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
As this was part of routine clinical laboratory method evaluation/population surveillance using anonymized leftover sera, national regulations exempts such investigation from IRB review.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy issues and national laws but are available from the corresponding author on reasonable request under the provision that data may not leave the hospital/center premises.
Acknowledgments
We thank Snibe for the generous supply of reagents for this study. Snibe had no role in the design and conduct of this study as well as the preparation of this manuscript.
Conflicts of Interest
Y.Z. and K.Y. are employees of Shenzhen New Industries Biomedical Engineering Co., Ltd. (Snibe). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 99% URL | 99th-percentile upper reference limit |
| Hs-cTnI/T | High-sensitivity cardiac troponin I/T |
| eGFR | Estimated glomerular filtration rate |
| CKD | Chronic kidney disease |
| ABEI | N-(-4-Aminobutyl)-N-ethylisoluminol |
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Aakre, K.M.; Saenger, A.K.; Body, R.; Collinson, P.; Hammarsten, O.; Jaffe, A.S.; Kavsak, P.; Omland, T.; Ordonez-Lianos, J.; Apple, F.S. Analytical Considerations in Deriving 99th Percentile Upper Reference Limits for High-Sensitivity Cardiac Troponin Assays: Educational Recommendations from the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin. Chem. 2022, 68, 1022–1030. [Google Scholar] [CrossRef]
- Sandoval, Y.; Apple, F.S.; Mahler, S.A.; Body, R.; Collinson, P.O.; Jaffe, A.S. International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers High-Sensitivity Cardiac Troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guidelines for the Evaluation and Diagnosis of Acute Chest Pain. Circulation 2022, 146, 569–581. [Google Scholar] [CrossRef]
- Lehmacher, J.; Sörensen, N.A.; Twerenbold, R.; Goßling, A.; Haller, P.M.; Hartikainen, T.S.; Schock, A.; Toprak, B.; Zeller, T.; Westermann, D.; et al. Diagnostic and Prognostic Value of the Sex-Specific 99th Percentile of Four High-Sensitivity Cardiac Troponin Assays in Patients with Suspected Myocardial Infarction. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 3–12. [Google Scholar] [CrossRef]
- Blankenberg, S.; Salomaa, V.; Makarova, N.; Ojeda, F.; Wild, P.; Lackner, K.J.; Jørgensen, T.; Thorand, B.; Peters, A.; Nauck, M.; et al. Troponin I and Cardiovascular Risk Prediction in the General Population: The BiomarCaRE Consortium. Eur. Heart J. 2016, 37, 2428–2437. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, T.R.; Ruud, S.E.; Larstorp, A.C.K.; Atar, D.; Halvorsen, S.; Nilsen, B.; Vallersnes, O.M. Rapid Rule-out of Acute Myocardial Infarction Using the 0/1-Hour Algorithm for Cardiac Troponins in Emergency Primary Care: The OUT-ACS Implementation Study. BMC Prim. Care 2025, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, A.; Forberg, J.L.; Sandgren, J.; Hård Af Segerstad, C.; Ellehuus, C.; Ekström, U.; Björk, J.; Lindahl, B.; Khoshnood, A.; Ekelund, U. Effectiveness and Safety of the ESC-TROP (European Society of Cardiology 0h/1h Troponin Rule-Out Protocol) Trial. J. Am. Heart Assoc. 2024, 13, e036307. [Google Scholar] [CrossRef]
- Wu, J.; Hua, Y.; Ge, Y.; Chen, K.; Chen, S.; Yang, J.; Yuan, H. Clinical Performance Validation and Four Diagnostic Strategy Assessments of High-Sensitivity Troponin I Assays. Sci. Rep. 2025, 15, 14442. [Google Scholar] [CrossRef] [PubMed]
- Bagai, A.; Alexander, K.P.; Berger, J.S.; Senior, R.; Sajeev, C.; Pracon, R.; Mavromatis, K.; Lopez-Sendón, J.L.; Gosselin, G.; Diaz, A.; et al. Use of Troponin Assay 99th Percentile as the Decision Level for Myocardial Infarction Diagnosis. Am. Heart J. 2017, 190, 135–139. [Google Scholar] [CrossRef]
- Apple, F.S.; Smith, S.W.; Greenslade, J.H.; Sandoval, Y.; Parsonage, W.; Ranasinghe, I.; Gaikwad, N.; Schulz, K.; Stephensen, L.; Schmidt, C.W.; et al. Single High-Sensitivity Point-of-Care Whole-Blood Cardiac Troponin I Measurement to Rule Out Acute Myocardial Infarction at Low Risk. Circulation 2022, 146, 1918–1929. [Google Scholar] [CrossRef]
- Cullen, L.; Greenslade, J.; Parsonage, W.; Stephensen, L.; Smith, S.W.; Sandoval, Y.; Ranasinghe, I.; Gaikwad, N.; Khorramshahi Bayat, M.; Mahmoodi, E.; et al. Point-of-Care High-Sensitivity Cardiac Troponin in Suspected Acute Myocardial Infarction Assessed at Baseline and 2 h. Eur. Heart J. 2024, 45, 2508–2515. [Google Scholar] [CrossRef]
- du Fay de Lavallaz, J.; Prepoudis, A.; Wendebourg, M.J.; Kesenheimer, E.; Kyburz, D.; Daikeler, T.; Haaf, P.; Wanschitz, J.; Löscher, W.N.; Schreiner, B.; et al. Skeletal Muscle Disorders: A Noncardiac Source of Cardiac Troponin T. Circulation 2022, 145, 1764–1779. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Vasile, V.C.; Milone, M.; Saenger, A.K.; Olson, K.N.; Apple, F.S. Diseased Skeletal Muscle: A Noncardiac Source of Increased Circulating Concentrations of Cardiac Troponin T. J. Am. Coll. Cardiol. 2011, 58, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Rittoo, D.; Jones, A.; Lecky, B.; Neithercut, D. Elevation of Cardiac Troponin T, but Not Cardiac Troponin I, in Patients with Neuromuscular Diseases: Implications for the Diagnosis of Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Liesinger, L.; Birner-Gruenberger, R.; Stojakovic, T.; Scharnagl, H.; Dieplinger, B.; Asslaber, M.; Radl, R.; Beer, M.; Polacin, M.; et al. Elevated Cardiac Troponin T in Patients with Skeletal Myopathies. J. Am. Coll. Cardiol. 2018, 71, 1540–1549. [Google Scholar] [CrossRef]
- Braghieri, L.; Badwan, O.Z.; Skoza, W.; Fares, M.; Menon, V. Evaluating Troponin Elevation in Patients with Chronic Kidney Disease and Suspected Acute Coronary Syndrome. Clevel. Clin. J. Med. 2023, 90, 483–489. [Google Scholar] [CrossRef]
- Guclu, T.; Bolat, S.; Şenes, M.; Yucel, D. Relationship between High Sensitivity Troponins and Estimated Glomerular Filtration Rate. Clin. Biochem. 2016, 49, 467–471. [Google Scholar] [CrossRef]
- Miller-Hodges, E.; Anand, A.; Shah, A.S.V.; Chapman, A.R.; Gallacher, P.; Lee, K.K.; Farrah, T.; Halbesma, N.; Blackmur, J.P.; Newby, D.E.; et al. High-Sensitivity Cardiac Troponin and the Risk Stratification of Patients with Renal Impairment Presenting with Suspected Acute Coronary Syndrome. Circulation 2018, 137, 425–435. [Google Scholar] [CrossRef]
- Lau, C.S.; Aw, T.C. Application of High-Sensitivity Troponin in Suspected Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2482. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Precision of Quantitative Measurement Procedures, 3rd ed.; CLSI: Wayne, PA, USA, 2019; Volume CLSI EP05-A3. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, 3rd ed.; CLSI: Wayne, PA, USA, 2020; Volume CLSI EP28-A3c. [Google Scholar]
- He, B.; Wang, K.; Xu, P.; Zhou, Q.; Xu, J. Determination of Age- and Sex-Specific 99th Percentile Upper Reference Limits for High-Sensitivity Cardiac Troponin I in Healthy Chinese Adults. Cardiology 2022, 147, 261–270. [Google Scholar] [CrossRef]
- Lee, D.J.W.; Aw, T.-C. Population Differences and High-Sensitivity Troponin Values: A Narrative Review. J. Lab. Precis. Med. 2023, 8, 14. [Google Scholar] [CrossRef]
- Aw, T.-C.; Huang, W.; Le, T.-T.; Pua, C.-J.; Ang, B.; Phua, S.-K.; Yeo, K.-K.; Cook, S.A.; Chin, C.W.L. High-Sensitivity Cardiac Troponins in Cardio-Healthy Subjects: A Cardiovascular Magnetic Resonance Imaging Study. Sci. Rep. 2018, 8, 15409. [Google Scholar] [CrossRef] [PubMed]
- Singapore Acute Myocardial Infarction (AMI)—National Registry of Diseases Office. Available online: https://www.nrdo.gov.sg/publications/ami (accessed on 15 August 2025).
- Conrad, N.; Molenberghs, G.; Verbeke, G.; Zaccardi, F.; Lawson, C.; Friday, J.M.; Su, H.; Jhund, P.S.; Sattar, N.; Rahimi, K.; et al. Trends in Cardiovascular Disease Incidence among 22 Million People in the UK over 20 Years: Population Based Study. BMJ 2024, 385, e078523. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.-C.; Phua, S.-K.; Tan, S.-P. Measurement of Cardiac Troponin I in Serum with a New High-Sensitivity Assay in a Large Multi-Ethnic Asian Cohort and the Impact of Gender. Clin. Chim. Acta 2013, 422, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, R.B.; Schytz, P.A.; Schultz, M.; Sindet-Pedersen, C.; Kristensen, J.H.; Strandkjær, N.; Knudsen, S.S.; Pries-Heje, M.; Pareek, M.; Kragholm, K.H.; et al. Implications of Age for the Diagnostic and Prognostic Value of Cardiac Troponin T and I. Clin. Chem. 2024, 70, 1231–1240. [Google Scholar] [CrossRef]
- Kimenai, D.M.; Shah, A.S.V.; McAllister, D.A.; Lee, K.K.; Tsanas, A.; Meex, S.J.R.; Porteous, D.J.; Hayward, C.; Campbell, A.; Sattar, N.; et al. Sex Differences in Cardiac Troponin I and T and the Prediction of Cardiovascular Events in the General Population. Clin. Chem. 2021, 67, 1351–1360. [Google Scholar] [CrossRef]
- Lau, C.S.; Asavapuriyothin, N.; Low, C.H.; Phua, S.K.; Liang, Y.; Aw, T.C. Analytical Performance of the New Sysmex High-Sensitivity Troponin T Assay. Diagnostics 2025, 15, 1838. [Google Scholar] [CrossRef]
- Apple, F.S.; Wu, A.H.B.; Sandoval, Y.; Sexter, A.; Love, S.A.; Myers, G.; Schulz, K.; Duh, S.-H.; Christenson, R.H. Sex-Specific 99th Percentile Upper Reference Limits for High Sensitivity Cardiac Troponin Assays Derived Using a Universal Sample Bank. Clin. Chem. 2020, 66, 434–444. [Google Scholar] [CrossRef]
- Cao, M.; Pierce, A.E.; Norman, M.S.; Thakur, B.; Diercks, K.; Hale, C.; Issioui, Y.; Diercks, D.B. Systematic Review of Sex-Specific High Sensitivity Cardiac Troponin I and T Thresholds. Clin. Ther. 2024, 46, 988–994. [Google Scholar] [CrossRef]
- Hickman, P.E.; Potter, J.M.; Cullen, L.; Eggers, K.M.; Than, M.; Pickering, J.W.; Parsonage, W.; Doust, J. Evidence-Based Medicine and the Cardiac Troponin 99th Percentile for the Diagnosis of Acute Myocardial Infarction. Eur. Heart J. Acute Cardiovasc. Care 2025, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, R.B.; Jørgensen, N.; Kristensen, J.; Strandkjær, N.; Kock, T.O.; Lange, T.; Ostrowski, S.R.; Nissen, J.; Larsen, M.H.; Pedersen, O.B.V.; et al. Sex and Population-Specific 99th Percentiles of Troponin for Myocardial Infarction in the Danish Population (DANSPOT). J. Appl. Lab. Med. 2024, 9, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.D.; Ola, O.; De Michieli, L.; Akula, A.; Mehta, R.A.; Dworak, M.; Crockford, E.; Lobo, R.; Slusser, J.; Rastas, N.; et al. Diagnosis of Acute Myocardial Infarction in Patients with Renal Failure Using High-Sensitivity Cardiac Troponin T. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 546–558. [Google Scholar] [CrossRef]
- Lazar, D.R.; Lazar, F.-L.; Homorodean, C.; Cainap, C.; Focsan, M.; Cainap, S.; Olinic, D.M. High-Sensitivity Troponin: A Review on Characteristics, Assessment, and Clinical Implications. Dis. Markers 2022, 2022, 9713326. [Google Scholar] [CrossRef]
- Lackner, K.J. Cardiac Troponins—A Paradigm for Diagnostic Biomarker Identification and Development. Clin. Chem. Lab. Med. 2023, 61, 795–800. [Google Scholar] [CrossRef]
- Zhu, Y.; Lai, Y.; Hu, Y.; Fu, Y.; Zhang, Z.; Lin, N.; Huang, W.; Zheng, L. The Mechanisms Underlying Acute Myocardial Infarction in Chronic Kidney Disease Patients Undergoing Hemodialysis. Biomed. Pharmacother. 2024, 177, 117050. [Google Scholar] [CrossRef]
- Geladari, E.V.; Vallianou, N.G.; Evangelopoulos, A.; Koufopoulos, P.; Panagopoulos, F.; Margellou, E.; Dalamaga, M.; Sevastianos, V.; Geladari, C.V. Cardiac Troponin Levels in Patients with Chronic Kidney Disease: “Markers of High Risk or Just Noise”? Diagnostics 2024, 14, 2316. [Google Scholar] [CrossRef]
- Nardi-Agmon, I.; Di Meo, A.; Lam, L.; Kyle, C.; Abdel-Qadir, H.; Amir, E.; Thavendiranathan, P. Circulating Macrotroponin Complexes and Their Impact on Cardiac Troponin Measurements: Potential Implications for Cardio-Oncology. JACC CardioOncol. 2024, 6, 608–611. [Google Scholar] [CrossRef]
- Warner, J.V.; Marshall, G.A. High Incidence of Macrotroponin I with a High-Sensitivity Troponin I Assay. Clin. Chem. Lab. Med. 2016, 54, 1821–1829. [Google Scholar] [CrossRef]
- Lam, L.; Tse, R.; Gladding, P.; Kyle, C. Effect of Macrotroponin in a Cohort of Community Patients with Elevated Cardiac Troponin. Clin. Chem. 2022, 68, 1261–1271. [Google Scholar] [CrossRef]
- Strandkjær, N.; Hansen, M.B.; Afzal, S.; Hasselbalch, R.B.; Knudsen, S.S.; Kristensen, J.H.; Kronborg, A.H.; Kamstrup, P.R.; Kobylecki, C.J.; Hilsted, L.M.; et al. Influence of Macrotroponin on the 99th Percentile Threshold in 2 High-Sensitivity Cardiac Troponin Assays. Clin. Chem. 2025, 71, 884–895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).