Interplay of Modifiable and Non-Modifiable Risk Factors for Diabetes Mellitus in Saudi Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Sociodemographic of the Study Participants
3.2. Anthropometric, Lifestyle, and Genetic Risk Factors
3.3. Dietary Behaviors
3.4. Multivariate Predictors of Diabetes
4. Discussion
4.1. Age and Gender as Non-Modifiable Determinants
4.2. Education, Marital Status, and Income: Sociocultural Influences
4.3. Environmental and Genetic Risk Contributors
4.4. Lifestyle Factors: Obesity, Inactivity, Tobacco Use, and Diet
4.5. Implications
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2024. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Prevalence in Saudi Arabia. Available online: https://idf.org/our-network/regions-and-members/middle-east-and-north-africa/members/saudi-arabia (accessed on 2 May 2025).
- World Health Organization. Diabetes Country Profiles: Saudi Arabia; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Alqarni, S.S. A review of prevalence of obesity in Saudi Arabia. J. Obes. Eat. Disord. 2016, 2, 25. [Google Scholar] [CrossRef]
- Al-Hazzaa, H.M.; Abahussain, N.A.; Al-Sobayel, H.I.; Qahwaji, D.M.; Musaiger, A.O. Lifestyle behaviors and dietary habits among Saudi adolescents relative to age, gender and region. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 140. [Google Scholar] [CrossRef]
- Musaiger, A.O. Diet and prevention of non-communicable diseases in Arab countries: Similarities and differences. East Mediterr. Health J. 2016, 22, 684–693. [Google Scholar]
- Willi, C.; Bodenmann, P.; Ghali, W.A.; Faris, P.D.; Cornuz, J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2007, 298, 2654–2664. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Tobacco smoking and risk of type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 221–229. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Pearson, E.R. Type 2 diabetes: A multifaceted disease. Diabetologia 2019, 62, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Cupples, L.A.; Wilson, P.W. Parental transmission of type 2 diabetes: The Framingham Offspring Study. Diabetes 2000, 49, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Taskinen, M.R. Diabetic dyslipidemia. Atheroscler. Suppl. 2002, 3, 47–51. [Google Scholar] [CrossRef]
- El-Hazmi, M.A.; Al-Swailem, A.R.; Warsy, A.S.; Al-Swailem, A.M.; Sulaimani, R.; Al-Meshari, A.A. Consanguinity among the Saudi Arabian population. J. Med. Genet. 1995, 32, 623–626. [Google Scholar] [CrossRef]
- Corella, D.; Ordovas, J.M. Interactions between dietary components and genetic polymorphisms and their impact on lipid metabolism. Curr. Opin. Lipidol. 2005, 16, 66–71. [Google Scholar]
- Pearson, E.R. Translating T2DM genetic data into clinical practice. Nat. Rev. Endocrinol. 2019, 15, 577–588. [Google Scholar]
- Prasad, R.B.; Groop, L. Genetics of type 2 diabetes—Pitfalls and possibilities. Genes 2015, 6, 87–123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO STEPS Surveillance Manual: The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Helou, K.; El Helou, N.; Mahfouz, M.; Mahfouz, Y.; Salameh, P.; Harmouche-Karaki, M. Validity and reliability of an adapted Arabic version of the long International Physical Activity Questionnaire. BMC Public Health 2017, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- World Health Organization. BMI Classification. Global Database on Body Mass Index; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in Saudi Arabia: A comprehensive analysis. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Al-Manaa, H.A.; Khoja, T.A.; Ahmad, N.A.; Al-Sharqawi, A.H.; Siddiqui, K.; Alnaqeb, D.; Aburisheh, K.H.; Youssef, A.M.; Al-Batel, A.; et al. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. PLoS ONE 2015, 10, e0135059. [Google Scholar] [CrossRef]
- Alowayesh, M.S.; Aljunid, S.M.; Aladsani, A.; Alessa, T.; Alattar, A.; Alroudhan, D. Health-related quality of life of Kuwaiti adults living with diabetes. Front. Public Health 2023, 11, 1085928. [Google Scholar] [CrossRef]
- Al-Mawali, A.; Al-Harrasi, A.; Jayapal, S.K.; Morsi, M.; Pinto, A.D.; Al-Shekaili, W.; Al-Kharusi, H.; Al-Balush, Z.; Idikula , J. Prevalence and risk factors of diabetes in a large community-based study in the Sultanate of Oman: STEPS survey 2017. BMC Endocr. Disord. 2021, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, W.; Wang, L.; Gu, S.; Dong, S.; Chen, M.; Yin, H.; Zheng, J.; Wu, X.; Jin, J.; et al. Association of SLC30A8 gene polymorphism with diabetes mellitus in Arab populations: A meta-analysis. Endocrine 2022, 65, 521–533. [Google Scholar]
- Saudi Ministry of Health. Saudi Health Interview Survey: Results and Implications for Diabetes Prevention; Report No.: MOH-2019-0032; Ministry of Health: Riyadh, Saudi Arabia, 2019. [Google Scholar]
- Xie, J.; Zhang, X.; Shao, H.; Jing, S.; Shan, T.; Shi, Y.; Li, Y.; Liu, Y.; Liu, N. An affordable approach to classifying type 2 diabetes based on fasting plasma glucose, TyG index and BMI: A retrospective cohort study of NHANES Data from 1988 to 2014. Diabetol. Metab. Syndr. 2022, 14, 113. [Google Scholar] [CrossRef]
- Raghupathi, W.; Raghupathi, V. An empirical study of chronic diseases in the United States: A visual analytics approach. Int. J. Environ. Res. Public Health 2018, 15, 431. [Google Scholar] [CrossRef]
- Alqurashi, K.A.; Aljabri, K.S.; Bokhari, S.A. Prevalence of diabetes mellitus in a Saudi community. Ann. Saudi Med. 2011, 31, 19–23. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Alfayez, O.M.; Alsallum, A.A.; Aljabri, A.F.; Almutairi, F.S.; Al-Azzeh, O.; Almalki, O.S.; Al Yami, M.S.; Almohammed, O.A. The use of metformin for type 2 diabetes prevention: Observational multicenter study from Saudi Arabia. Front. Public Health 2022, 10, 989072. [Google Scholar] [CrossRef] [PubMed Central]
- Eastwood, S.V.; Mathur, R.; Atkinson, M.; Brophy, S.; Sudlow, C.; Flaig, R.; de Lusignan, S.; Allen, N.; Chaturvedi, N. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK Biobank. PLoS ONE 2016, 11, e0162388. [Google Scholar] [CrossRef]
- Bahijri, S.M.; Al-Raddadi, R.M.; Jambi, H.A.; Al-Raddadi, R.M.; Ferns, G.; Tuomilehto, J. Lifestyle habits in Saudi Arabia: Results of a large population-based survey in Jeddah. PLoS ONE 2016, 11, e0145835. [Google Scholar] [CrossRef]
- Alkathem, J.; Alawwad, H.; Alghamdi, T.; Majrashy, A.E.H.; Algarni, S.; Aljabari, L.; Al-Alwan, M.K.; Ahmed, R.A. Prevalence of metabolic syndrome amongst Saudi Arabian women with polycystic ovarian syndrome: A cross-sectional study. J. Adv. Trends Med. Res. 2024, 1, 290–295. [Google Scholar] [CrossRef]
- Bahrain Ministry of Health. National Non-Communicable Disease Survey 2021–2022; Ministry of Health: Manama, Bahrain, 2022. [Google Scholar]
- Qatar Ministry of Public Health. Qatar STEPwise Survey 2022: Diabetes and Metabolic Syndrome; Ministry of Public Health: Doha, Qatar, 2023. [Google Scholar]
- Bahijri, S.M.; Jambi, H.A.; Al-Raddadi, R.M.; Ferns, G.; Tuomilehto, J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia: A community-based survey. PLoS ONE 2016, 11, e0152559. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaim, A.A.; Al-Musharaf, S.; Al-Daghri, N.M. Impact of specialized PCOS clinics on early diabetes detection in Saudi Arabia: A prospective cohort study. J. Clin. Endocrinol. Metab. 2023, 108, e45–e52. [Google Scholar]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Beckles, G.L.; Chou, C.-F. Disparities in the prevalence of diagnosed diabetes—United States, 1999–2002 and 2011–2014. Morb. Mortal. Wkly. Rep. 2016, 65, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Al Mahroos, F.; Al-Roomi, K. Overweight and obesity in the Arabian Peninsula: An overview. J. R. Soc. Promot. Health 1999, 119, 251–253. [Google Scholar] [CrossRef]
- Alsaidan, A.A.; Alotaibi, S.F.; Thirunavukkarasu, A.; Alruwaili, B.F.; Alharbi, R.H.; Arnous, M.M.; Alsaidan, O.A.; Alduraywish, A.S.; Alwushayh, Y.A. Medication adherence and its associated factors among patients with type 2 diabetes mellitus attending primary health centers of Eastern Province, Saudi Arabia. Medicina 2023, 59, 989. [Google Scholar]
- Stringhini, S.; Carmeli, C.; Jokela, M.; Avendano, M.; Muennig, P.; Guida, F.; Ricceri, F.; D’Errico, A.; Barros, H.; Bochud, M.; et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1.7 million men and women. Lancet 2017, 389, 1229–1237. [Google Scholar] [CrossRef]
- Al-Asfour, A.; Lamb, M.M.; Barakat, M.T. Type 2 diabetes in Kuwait: The impact of marital status and socio-economic factors. Diabet. Med. 2011, 28, 607–612. [Google Scholar]
- Al-Lawati, J.A.; Al Riyami, A.M.; Mohammed, A.J.; Jousilahti, P. Increasing prevalence of diabetes mellitus in Oman. Diabet. Med. 2002, 19, 954–957. [Google Scholar] [CrossRef]
- Al-Senani, F.Y.; Al-Harbi, T.S.; Al-Zahrani, A.M.; Al-Ghamdi, S.M.; Al-Balawi, W.A. Polygamy and metabolic health: A cohort study of Saudi women. BMC Public Health 2021, 21, 1053. [Google Scholar] [CrossRef]
- Saudi General Authority for Statistics. Household Health and Lifestyle Survey 2021; Statistical Report No.: GASTAT-HHLS-2021; General Authority for Statistics: Riyadh, Saudi Arabia, 2022. [Google Scholar]
- Murad, M.A.; Abdulmageed, S.S.; Iftikhar, R.; Sagga, B.K. Assessment of the common risk factors associated with type 2 diabetes mellitus in Jeddah. Int. J. Endocrinol. 2014, 2014, 616145. [Google Scholar] [CrossRef]
- Al-Najjar, N.S.; Al-Mogbel, T.A.; Al-Shammari, H.A.; Al-Qahtani, F.A. Parity and diabetes risk in Saudi women: A case-control study. Diabetol. Metab. Syndr. 2023, 15, 12. [Google Scholar]
- Saudi Ministry of Health. Statistical Yearbook 2023; Ministry of Health: Riyadh, Saudi Arabia, 2023. Available online: https://www.moh.gov.sa/en/Ministry/Statistics/Pages/default.aspx (accessed on 20 July 2025).
- Saudi General Authority for Statistics. Household Income and Expenditure Survey 2022: Food Consumption Patterns; GASTAT: Riyadh, Saudi Arabia, 2023. [Google Scholar]
- Bener, A.; Zirie, M.; Janahi, I.M.; Al-Hamaq, A.O.; Musallam, M.; Wareham, N.J. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res. Clin. Pract. 2009, 84, 99–106. [Google Scholar]
- Al-Mazroui, A.M.; Al-Hamoudi, W.K.; Al-Rasadi, K.; Al-Zahrani, J.M.; Al-Otaibi, H.A. Impact of subsidized meal programs on cardiometabolic outcomes in the United Arab Emirates: A quasi-experimental study. Lancet Diabetes Endocrinol. 2023, 11, e12–e19. [Google Scholar]
- Kuwait Ministry of Commerce. Evaluation of Price Control Policies on Food Commodities 2019–2023; Ministry of Commerce: Kuwait City, Kuwait, 2023. [Google Scholar]
- Al-Haifi, A.R.; Al-Fayez, M.A.; Al-Athari, B.I.; Al-Ajmi, F.A.; Allafi, A.R.; Al-Hazzaa, H.M.; Musaiger, A.O. Relative contribution of physical activity, sedentary behaviors and dietary habits to the prevalence of obesity among Kuwaiti adolescents. Food Nutr. Bull. 2013, 34, 6–13. [Google Scholar] [CrossRef]
- Al-Lawati, J.A.; Mohammed, A.J.; Al-Hinai, H.Q. Diabetes mellitus in Oman: Prevalence and risk factors. Diabetes Res. Clin. Pract. 2002, 58, 267–274. [Google Scholar]
- Meigs, J.B.; Shrader, P.; Sullivan, L.M.; McAteer, J.B.; Fox, C.S.; Dupuis, J.; Manning, A.K.; Florez, J.C.; Wilson, P.W.; D’Agostino, R.B.S.; et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N. Engl. J. Med. 2008, 359, 2208–2219. [Google Scholar] [CrossRef] [PubMed]
- Almgren, P.; Lehtovirta, M.; Isomaa, B.; Sarelin, L.; Taskinen, M.R.; Lyssenko, V.; Tuomi, T.; Groop, L.; Botnia Study Group. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011, 54, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia. BMC Public Health 2014, 14, 1077. [Google Scholar]
- Al-Daghri, N.M.; Mohammed, A.K.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Draz, H.M.; Clerici, M.; Cagliani, R.; Sabico, S.; Chrousos, G.P. The association of E23K and S1369A polymorphisms in the KCNJ11 gene with type 2 diabetes mellitus in a Saudi population. Arch. Med. Res. 2012, 43, 387–393. [Google Scholar]
- Taylor, S.I.; Cherng, H.R.; Yazdi, Z.S.; Montasser, M.E.; Whitlatch, H.B.; Mitchell, B.D.; Shuldiner, A.R.; Streeten, E.A.; Beitelshees, A.L. Pharmacogenetics of SGLT2 inhibitors: Validation of a sex-agnostic pharmacodynamic biomarker. Diabetes Obes. Metab. 2023, 25, 3512–3520. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015, 219, 1–8. [Google Scholar]
- The InterAct Consortium. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: The EPIC-InterAct case-cohort study. PLoS Med. 2012, 9, e1001230. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Salem, V.; AlHusseini, N.; Abdul Razack, H.I.; Naoum, A.; Sims, O.T.; Alqahtani, S.A. Prevalence, risk factors, and interventions for obesity in Saudi Arabia: A systematic review. Obes. Rev. 2022, 23, e13448. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Mokhlesi, B. Obstructive sleep apnea and diabetes: A state of the art review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; WHO: Geneva, Switzerland, 2014; Available online: https://iris.who.int/items/188590ce-ad59-43df-9dd2-600de5a3eb75 (accessed on 2 June 2025).
- Al-Kaabi, J.; Al-Maskari, F.; Afandi, B.; Saadi, H.; Ganguly, S.S. Risk factors for diabetes mellitus and cardiovascular disease among Qatari adults. J. Clin. Hypertens. 2013, 15, 808–814. [Google Scholar]
- Teo, K.K.; Ounpuu, S.; Hawken, S.; Pandey, M.R.; Valentin, V.; Hunt, D.; Diaz, R.; Rashed, W.; Freeman, R.; Jiang, L.; et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: A case–control study. Lancet 2006, 368, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.K.; Kriska, A.M.; Venditti, E.M.; Miller, R.G.; Brooks, M.M.; Burke, L.E.; Siminerio, L.M.; Solano, F.X.; Orchard, T.J. Translating the Diabetes Prevention Program: A comprehensive model for prevention training and program delivery. Am. J. Prev. Med. 2009, 37, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, H.H. Measuring intake of fruit and vegetables among Saudi adults: Validation of the FAVVA tool. Nutrients 2019, 11, 1882. [Google Scholar]
| Variable | No | Yes | p Value * | ||
|---|---|---|---|---|---|
| n | Mean ± SD/Frequency (%) | n | Mean ± SD/Frequency (%) | ||
| Age | 2987 | 32 (13) | 424 | 48 (16) | p < 0.05 |
| Gender | 2987 | 424 | p < 0.05 | ||
| Female | 1504 | 50% | 172 | 41% | |
| Male | 1483 | 50% | 252 | 59% | |
| Education | 2987 | - | 424 | p < 0.05 | |
| Elementary | 66 | 2% | 30 | 7% | |
| Illiterate | 52 | 2% | 35 | 8% | |
| Intermediate | 92 | 3% | 27 | 6% | |
| Postgraduate | 106 | 4% | 16 | 4% | |
| Secondary | 1028 | 34% | 107 | 25% | |
| University | 1643 | 55% | 209 | 49% | |
| Social Status | 2987 | - | 424 | - | p < 0.05 |
| Divorced | 58 | 2% | 12 | 3% | |
| Married | 1282 | 43% | 309 | 73% | |
| Single | 1601 | 54% | 77 | 18% | |
| Widowed | 46 | 2% | 26 | 6% | |
| Income | 2987 | 424 | - | p < 0.05 | |
| <5000 SAR | 969 | 32% | 114 | 27% | |
| >5000 and <10,000 SAR | 566 | 19% | 73 | 17% | |
| >10,000 and <15,000 SAR | 635 | 21% | 112 | 26% | |
| >15,000 SAR | 817 | 27% | 125 | 29% | |
| Residence | 2987 | - | 424 | - | p > 0.05 |
| Rural | 1831 | 61% | 268 | 63% | |
| Urban | 1156 | 39% | 156 | 37% | |
| Housing | 2987 | - | 424 | p < 0.05 | |
| Owned-Apartment | 597 | 20% | 83 | 20% | |
| Owned-Traditional House | 619 | 21% | 117 | 28% | |
| Owned-Villa | 1375 | 46% | 192 | 45% | |
| Rented | 396 | 13% | 32 | 8% | |
| Family Size | 2987 | 6.9 (2.9) | 424 | 7.3 (2.9) | p < 0.05 |
| Variable | No | Yes | p Value * | ||
|---|---|---|---|---|---|
| n | Mean ± SD/ Frequency (%) | n | Mean ± SD/ Frequency (%) | ||
| Height | 2987 | 163 (9.2) | 424 | 164 (9.7) | p < 0.05 |
| Weight | 2987 | 66 (17) | 424 | 73 (16) | p < 0.05 |
| BMI | 2987 | 25 (5.5) | 424 | 27 (5.4) | p < 0.05 |
| Physical activity | 2987 | 424 | p > 0.05 | ||
| Moderate or vigorous activity for less than 30 min, 5 times per week | 1210 | 41% | 165 | 39% | |
| Moderate or vigorous activity for a minimum of 30 min, 5 times per week | 653 | 22% | 78 | 18% | |
| No physical activity is performed per week | 1124 | 38% | 181 | 43% | |
| Cigarettes | 2987 | 424 | p < 0.05 | ||
| No | 2747 | 92% | 369 | 87% | |
| Yes | 240 | 8% | 55 | 13% | |
| Shisha | 2987 | 424 | p < 0.05 | ||
| No | 2836 | 95% | 390 | 92% | |
| Yes | 151 | 5% | 34 | 8% | |
| Family history of DM | 2987 | 424 | p < 0.001 | ||
| No | 1582 | 53% | 59 | 14% | |
| Yes | 1405 | 47% | 365 | 86% | |
| Family history of Dyslipidemia | 2987 | 424 | p < 0.05 | ||
| No | 2829 | 95% | 390 | 92% | |
| Yes | 158 | 5% | 34 | 8% | |
| Variable | No | Yes | p Value * | ||
|---|---|---|---|---|---|
| n | Mean ± SD/ Frequency (%) | n | Mean ± SD/ Frequency (%) | ||
| Consumption of whole grain products | 2987 | 424 | p > 0.05 | ||
| No | 1564 | 52% | 225 | 53% | |
| Yes | 1423 | 48% | 199 | 47% | |
| Minimum of consuming 5 servings of fruits and vegetables per day | 2987 | 424 | p > 0.05 | ||
| No | 2327 | 78% | 313 | 74% | |
| Yes | 660 | 22% | 111 | 26% | |
| Choosing low fat meats | 2987 | 424 | p < 0.05 | ||
| No | 1516 | 51% | 169 | 40% | |
| Yes | 1471 | 49% | 255 | 60% | |
| Avoidance of high sugar food | 2987 | 424 | p < 0.05 | ||
| No | 1924 | 64% | 231 | 54% | |
| Yes | 1063 | 36% | 193 | 46% | |
| Choosing low fat products | 2987 | 424 | p < 0.05 | ||
| No | 2038 | 68% | 265 | 62% | |
| Yes | 949 | 32% | 159 | 38% | |
| Variable | Odds Ratios | CI | p Values |
|---|---|---|---|
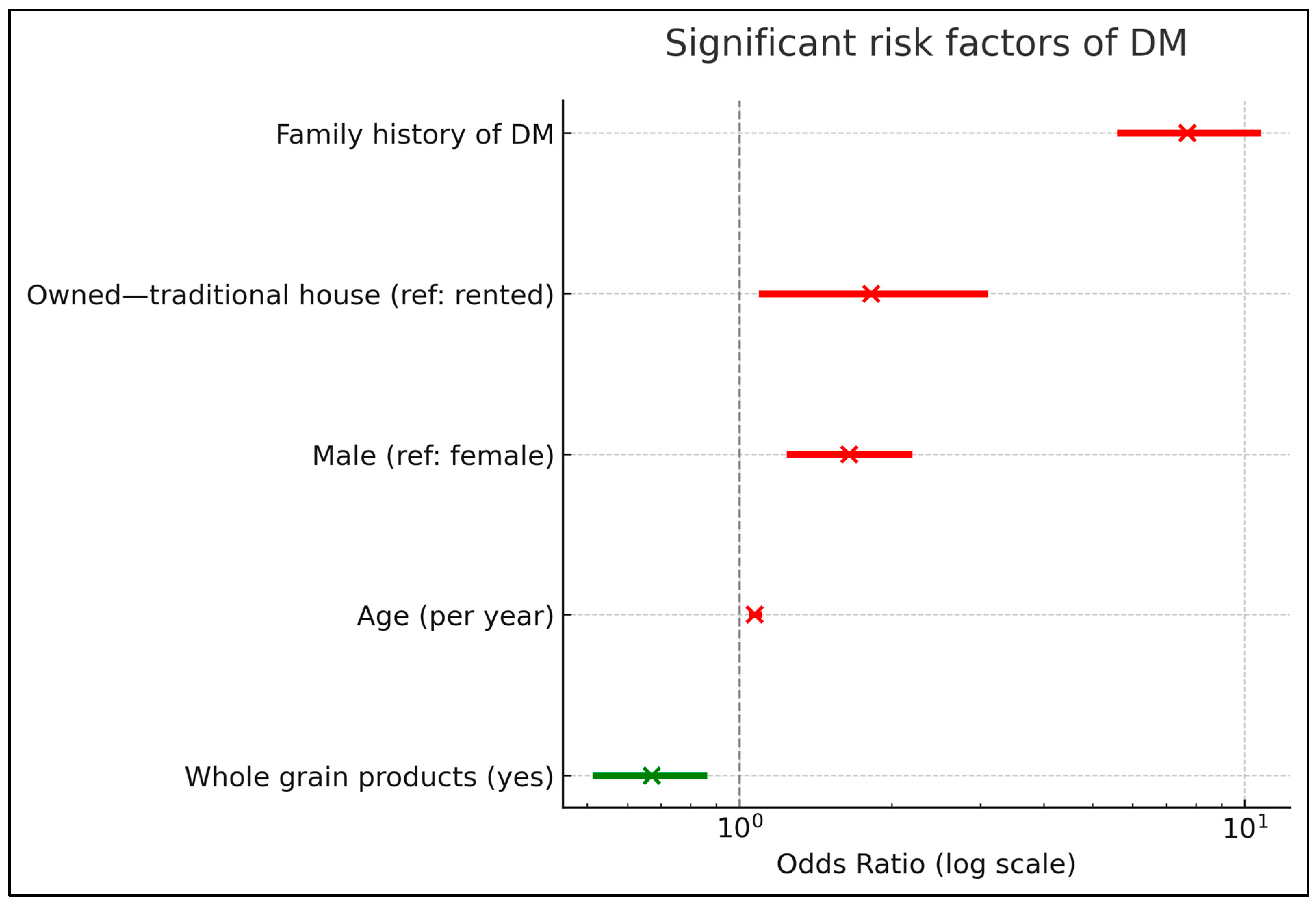
| Age | 1.07 | 1.06–1.09 | p < 0.001 |
| Gender | |||
| Male [Ref: Female] | 1.65 | 1.26–2.16 | p < 0.001 |
| Education [Ref: Illiterate] | |||
| Elementary | 1.20 | 0.58–2.49 | p > 0.05 |
| Intermediate | 1.56 | 0.72–3.36 | p > 0.05 |
| Postgraduate | 0.94 | 0.38–2.29 | p > 0.05 |
| Secondary | 1.22 | 0.63–2.38 | p > 0.05 |
| University | 0.93 | 0.49–1.79 | p > 0.05 |
| Social status [Ref: Single] | |||
| Divorced | 0.88 | 0.37–1.95 | p > 0.05 |
| Married | 1.10 | 0.72–1.69 | p > 0.05 |
| Widowed | 1.03 | 0.47–2.23 | p > 0.05 |
| Income [Ref: Less than 5000 SAR] | |||
| >5000 and <10,000 SAR | 0.69 | 0.47–1.01 | p > 0.05 |
| >10,000 and <15,000 SAR | 0.75 | 0.51–1.10 | p > 0.05 |
| >15,000 SAR | 0.98 | 0.67–1.42 | p > 0.05 |
| Residence [Ref: Rural] | |||
| Urban | 0.96 | 0.74–1.24 | p > 0.05 |
| Housing [Ref: Rented house] | |||
| Owned-apartment | 1.55 | 0.95–2.58 | p > 0.05 |
| Owned-traditional house | 1.82 | 1.11–3.05 | p < 0.05 |
| Owned-villa | 1.48 | 0.93–2.41 | p > 0.05 |
| Family size | 0.98 | 0.94–1.02 | p > 0.05 |
| BMI | 1.02 | 1.00–1.04 | p > 0.05 |
| Physical activity [Ref: No physical activity] | |||
| Moderate or vigorous activity for a less than 30 min, 5 times per week | 1.03 | 0.78–1.35 | p > 0.05 |
| Moderate or vigorous activity for a minimum of 30 min, 5 times per week | 1.10 | 0.78–1.54 | p > 0.05 |
| Reference [yes] | |||
| Smoking | 1.00 | 0.68–1.47 | p > 0.05 |
| Shisha | 1.08 | 0.67–1.70 | p > 0.05 |
| Sleep hours | 1.05 | 0.98–1.13 | p > 0.05 |
| Consumption of whole grain products | 0.67 | 0.52–0.85 | p < 0.05 |
| Minimum of consuming 5 serving of fruits and vegetables per day | 1.09 | 0.83–1.43 | p > 0.05 |
| Choosing low fat meats | 1.07 | 0.83–1.37 | p > 0.05 |
| Avoidance of food high in sugar | 1.13 | 0.88–1.44 | p > 0.05 |
| Choosing low fat products | 0.97 | 0.75–1.25 | p > 0.05 |
| Family history of DM | 7.68 | 5.67–10.57 | p < 0.05 |
| Family history of Dyslipidemia | 1.06 | 0.67–1.64 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jareebi, M.A.; Gosadi, I.M. Interplay of Modifiable and Non-Modifiable Risk Factors for Diabetes Mellitus in Saudi Adults. Diagnostics 2025, 15, 2451. https://doi.org/10.3390/diagnostics15192451
Jareebi MA, Gosadi IM. Interplay of Modifiable and Non-Modifiable Risk Factors for Diabetes Mellitus in Saudi Adults. Diagnostics. 2025; 15(19):2451. https://doi.org/10.3390/diagnostics15192451
Chicago/Turabian StyleJareebi, Mohammad A., and Ibrahim M. Gosadi. 2025. "Interplay of Modifiable and Non-Modifiable Risk Factors for Diabetes Mellitus in Saudi Adults" Diagnostics 15, no. 19: 2451. https://doi.org/10.3390/diagnostics15192451
APA StyleJareebi, M. A., & Gosadi, I. M. (2025). Interplay of Modifiable and Non-Modifiable Risk Factors for Diabetes Mellitus in Saudi Adults. Diagnostics, 15(19), 2451. https://doi.org/10.3390/diagnostics15192451







