Tumor-Infiltrating Lymphocytes Predict Extranodal Extension and Prognosis in Regionally Advanced Oral Cavity Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| CI | Confidence interval |
| DFS | Disease-free survival |
| DOI | Depth of invasion |
| DSS | Disease-specific survival |
| ENE | Extranodal extension |
| HE | Hematoxylin–eosin |
| HR | Hazard ratio |
| iTIL (IM) | Intraepithelial tumor-infiltrating lymphocytes in the invasive margin |
| iTIL (TC) | Intraepithelial tumor-infiltrating lymphocytes in the tumor center |
| LNR | Lymph node ratio |
| LNY | Lymph node yield |
| LVI | Lymphovascular invasion |
| OCSCC | Oral cavity squamous cell carcinoma |
| OS | Overall survival |
| PNI | Perineural invasion |
| ROC | Receiver-operator characteristics |
| sTIL (IM) | Stromal tumor-infiltrating lymphocytes in the invasive margin |
| sTIL (TC) | Stromal tumor-infiltrating lymphocytes in the tumor center |
| TIL | Tumor-infiltrating lymphocytes |
| WHO | World Health Organization |
| WPOI-5 | Worst pattern of invasion-5 |
References
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef]
- Van Der Waal, I. Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e33–e37. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Nishimoto, I.N.; Califano, J.A.; Kowalski, L.P. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int. J. Cancer 2005, 114, 806–816. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Huang, T.Y.; Hsu, L.P.; Wen, Y.H.; Huang, T.T.; Chou, Y.F.; Lee, C.F.; Yang, M.C.; Chang, Y.K.; Chen, P.R. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010, 46, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Jardim, J.F.; Francisco, A.L.; Gondak, R.; Damascena, A.; Kowalski, L.P. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2015, 44, 23–28. [Google Scholar] [CrossRef]
- Tan, A.; Taskin, T. Tumor Budding Should Be in Oral Cavity Cancer Reporting: A Retrospective Cohort Study Based on Tumor Microenvironment. Cancers 2023, 15, 3905. [Google Scholar] [CrossRef]
- Ho, Y.; Wu, T.; Cheng, H.; Yang, C.; Wu, C. The significance of tumor budding in oral cancer survival and its relevance to the eighth edition of the American Joint Committee on Cancer staging system. Head Neck 2019, 41, 2991–3001. [Google Scholar] [CrossRef]
- Monroe, M.M.; Gross, N.D. Evidence-Based Practice. Otolaryngol. Clin. N. Am. 2012, 45, 1181–1193. [Google Scholar] [CrossRef]
- Greene, F.L.; Edge, S.; Schilsky, R.L.; Gaspar, L.E.; Washington, M.K.; Sullivan, D.C.; Brookland, R.K.; Amin, M.B. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2018; pp. 79–93. [Google Scholar]
- Henson, C.E.; Abou-Foul, A.K.; Morton, D.J.; McDowell, L.; Baliga, S.; Bates, J.; Lee, A.; Bonomo, P.; Szturz, P.; Nankivell, P.; et al. Diagnostic challenges and prognostic implications of extranodal extension in head and neck cancer: A state of the art review and gap analysis. Front. Oncol. 2023, 13, 1263347. [Google Scholar] [CrossRef]
- Mamic, M.; Lucijanic, M.; Manojlovic, L.; Muller, D.; Suton, P.; Luksic, I. Prognostic significance of extranodal extension in oral cavity squamous cell carcinoma with occult neck metastases. Int. J. Oral Maxillofac. Surg. 2021, 50, 309–315. [Google Scholar] [CrossRef]
- Noda, Y.; Ishida, M.; Ueno, Y.; Fujisawa, T.; Iwai, H.; Tsuta, K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: A retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer 2022, 22, 1291. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Pagès, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Angell, H.; Galon, J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Peled, M.; Onn, A.; Herbst, R.S. Tumor-Infiltrating Lymphocytes—Location for Prognostic Evaluation. Clin. Cancer Res. 2019, 25, 1449–1451. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Vandecasteele, K.; Rottey, S.; Ferdinande, L. TILs in Head. and Neck Cancer: Ready for Clinical Implementation and Why (Not)? Head Neck Pathol. 2017, 11, 354–363. [Google Scholar] [CrossRef]
- Wallis, S.P.; Stafford, N.D.; Greenman, J. Clinical relevance of immune parameters in the tumor microenvironment of head and neck cancers. Head Neck 2015, 37, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Englund, E.; Reitsma, B.; King, B.C.; Escudero-Esparza, A.; Owen, S.; Orimo, A.; Okroj, M.; Anagnostaki, L.; Jiang, W.G.; Jirström, K.; et al. The human complement inhibitor Sushi Domain-Containing Protein 4 (SUSD4) expression in tumor cells and infiltrating T cells is associated with better prognosis of breast cancer patients. BMC Cancer 2015, 15, 737. [Google Scholar] [CrossRef] [PubMed]
- Corredor, G.; Wang, X.; Zhou, Y.; Lu, C.; Fu, P.; Syrigos, K.; Rimm, D.L.; Yang, M.; Romero, E.; Schalper, K.A.; et al. Spatial Architecture and Arrangement of Tumor-Infiltrating Lymphocytes for Predicting Likelihood of Recurrence in Early-Stage Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, A.A.; Agaimy, A.; Almangush, A. Insight into Classification and Risk Stratification of Head and Neck Squamous Cell Carcinoma in Era of Emerging Biomarkers with Focus on Histopathologic Parameters. Cancers 2022, 14, 5514. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- De Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef]
- Heikkinen, I.; Bello, I.O.; Wahab, A.; Hagström, J.; Haglund, C.; Coletta, R.D.; Nieminen, P.; Mäkitie, A.A.; Salo, T.; Leivo, I.; et al. Assessment of Tumor-infiltrating Lymphocytes Predicts the Behavior of Early-stage Oral Tongue Cancer. Am. J. Surg. Pathol. 2019, 43, 1392–1396. [Google Scholar] [CrossRef]
- Almangush, A.; Leivo, I.; Mäkitie, A.A. Overall assessment of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma: Time to take notice. Acta Otolaryngol. 2020, 140, 246–248. [Google Scholar] [CrossRef]
- Abu Eid, R. Editorial: Advances in Head and Neck Cancer Immunology and Immunotherapy. Front. Oncol. 2019, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, U.; Muller, L.; Lechner, A.; Schlsser, H.A.; Beutner, D.; Lubli, H.; Zippelius, A.; Rothschild, S.I. Immunotherapy in head and neck cancer—Scientific rationale, current treatment options and future directions. Swiss Med. Wkly. 2018, 148, w14625. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M.; Wennhold, K.; Lehmann, J.; Garcia-Marquez, M.; Klein, S.; Kochen, E.; Lohneis, P.; Lechner, A.; Wagener-Ryczek, S.; Plum, P.S.; et al. Cancer-specific immune evasion and substantial heterogeneity within cancer types provide evidence for personalized immunotherapy. npj Precis. Oncol. 2021, 5, 52. [Google Scholar] [CrossRef] [PubMed]


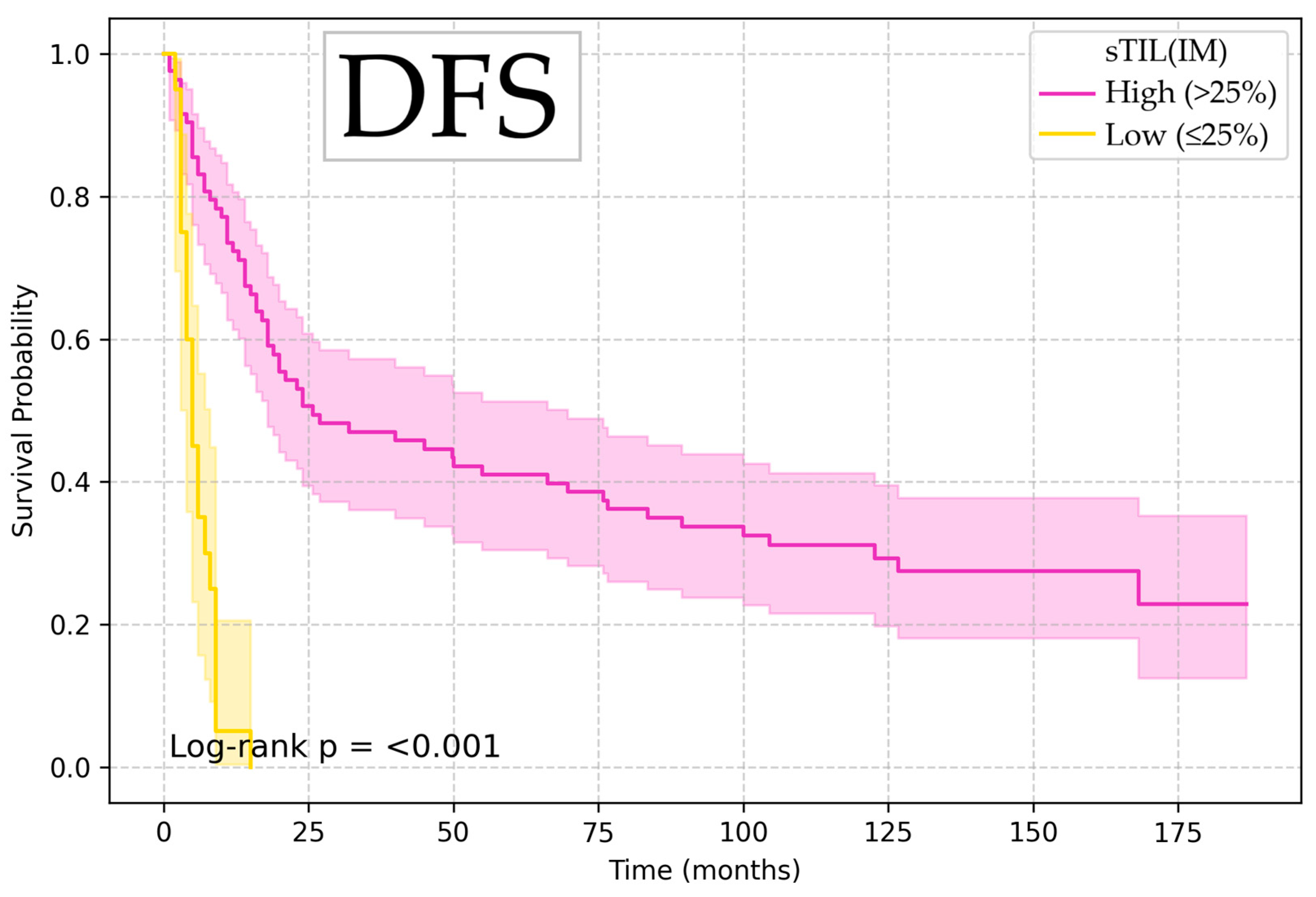
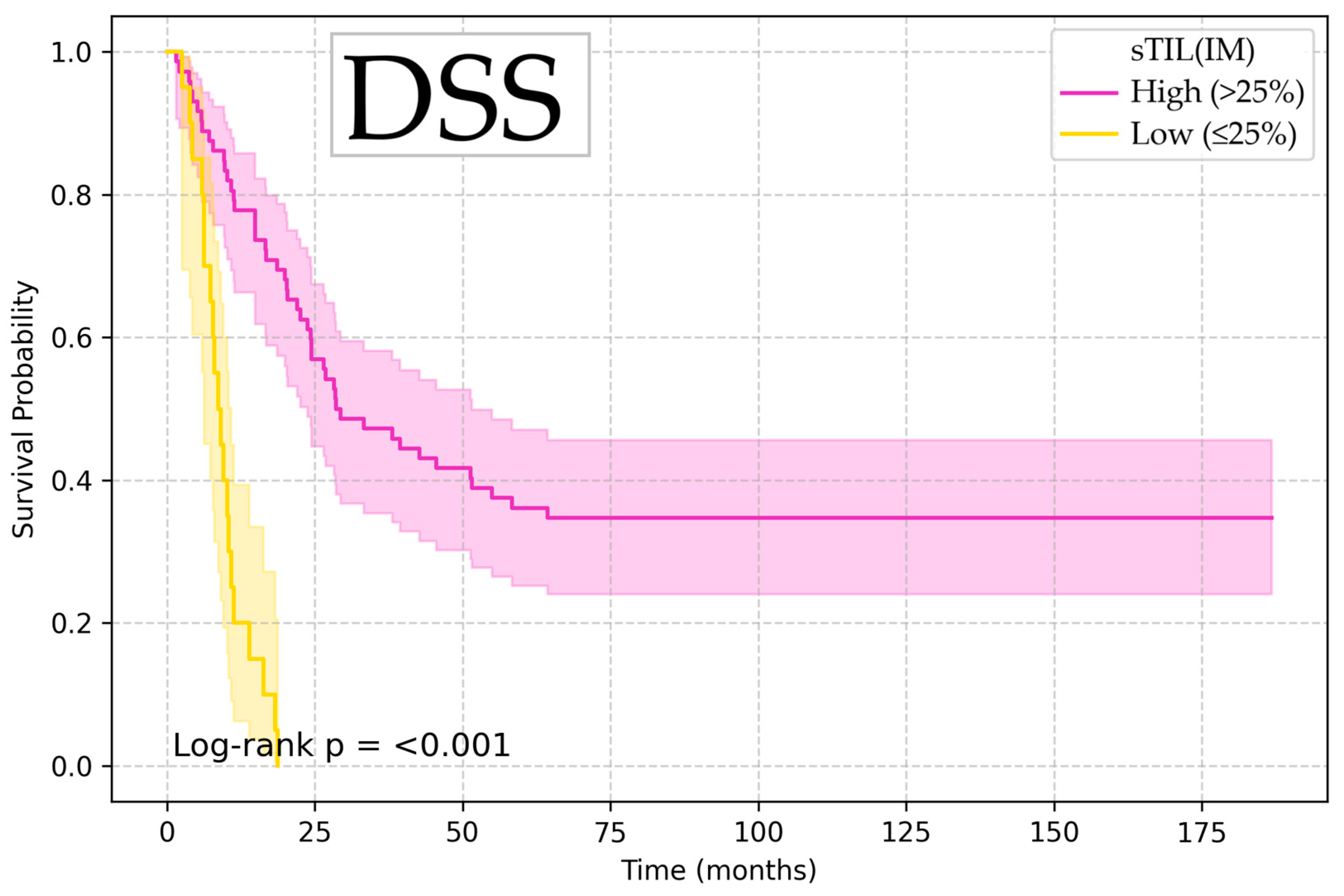
| Variable | Endpoint | Optimal Cutoff | p-Value |
|---|---|---|---|
| sTIL (TC) (%) | OS, DSS, DFS | 15% | <0.001 |
| sTIL (IM) (%) | OS, DSS, DFS | 25% | <0.001 |
| iTIL (TC) (%) | DSS | 10% | 0.042 |
| iTIL (IM) (%) | OS, DSS | 10% | 0.010/0.013 |
| iTIL (IM) (%) | DFS | 20% | 0.027 |
| DOI (mm) | OS, DSS, DFS | 9 mm | <0.001 |
| Resection margin (mm) | OS, DSS, DFS | 2.5 mm | 0.012/0.015/0.009 |
| Lymph node ratio (LNR) | OS, DSS, DFS | 0.15 | <0.001 |
| Extranodal extension (mm) | OS, DSS, DFS | 2 mm | <0.001 |
| Variables | Total n = 103 | High sTIL (IM) (>25%) | Low sTIL (IM) (≤25%) | p-Value |
|---|---|---|---|---|
| Age (y) | 0.576 | |||
| ≤60 | 66 (64.1%) | 53 (80.3%) | 13 (19.7%) | |
| 60 | 37 (35.9%) | 30 (81.1%) | 7 (18.9%) | |
| Sex | 0.442 | |||
| Male | 90 (87.4%) | 71 (78.9%) | 19 (21.1%) | |
| Female | 13 (12.6%) | 12 (92.3%) | 1 (7.7%) | |
| Tumor site | 0.391 | |||
| Oral tongue | 39 (37.9%) | 31 (79.5%) | 8 (20.5%) | |
| Floor of the mouth | 34 (33.0%) | 30 (88.2%) | 4 (11.8%) | |
| Lower alveolar ridge | 13 (12.6%) | 8 (61.5%) | 5 (38.5%) | |
| Retromolar trigone | 12 (11.7%) | 10 (83.3%) | 2 (16.7%) | |
| Upper alveolar ridge | 2 (1.9%) | 2 (100%) | 0 (0.0%) | |
| Buccal mucosa | 2 (1.9%) | 1 (50.0%) | 1 (50.0%) | |
| Mucosal lip | 1 (1.0%) | 1 (100%) | 0 (0.0%) | |
| Stage (AJCC) 1 | 0.288 | |||
| III | 10 (9.7%) | 9 (90.0%) | 1 (10.0%) | |
| IV.A | 47 (45.6%) | 40 (85.1%) | 7 (14.9%) | |
| IV.B | 46 (44.7%) | 34 (73.9%) | 12 (26.1%) | |
| Grade | 0.427 | |||
| I | 43 (41.7%) | 37 (86.0%) | 6 (14.0%) | |
| II | 44 (42.7%) | 33 (75.0%) | 11 (25.0%) | |
| III | 16 (15.5%) | 13 (81.2%) | 3 (18.8%) | |
| PNI | 0.287 | |||
| Absent | 39 (37.9%) | 34 (87.2%) | 5 (12.8%) | |
| Present | 64 (62.1%) | 49 (76.6%) | 15 (23.4%) | |
| LVI | 0.273 | |||
| Absent | 65 (63.1%) | 55 (84.6%) | 10 (15.4%) | |
| Present | 38 (36.9%) | 28 (73.7%) | 10 (26.3%) | |
| WPOI-5 | 0.104 | |||
| Absent | 45 (43.7%) | 40 (88.9%) | 5 (11.1%) | |
| Present | 58 (56.3%) | 43 (74.1%) | 15 (25.9%) | |
| ENE | 0.142 | |||
| Absent | 52 (50.5%) | 45 (86.5%) | 7 (13.5%) | |
| Present | 51 (49.5%) | 38 (74.5%) | 13 (25.5%) |
| Variables | OS HR (95% CI) | DSS HR (95% CI) | DFS HR (95% CI) |
|---|---|---|---|
| LVI | |||
| Absent | 1 | 1 | 1 |
| Present | 1.67 (1.06–2.63) | 1.66 (1.03–2.70) | 1.58 (1.01–2.47) |
| p | 0.027 | 0.039 | 0.046 |
| WPOI-5 | |||
| Absent | 1 | 1 | 1 |
| Present | 1.67 (1.06–2.65) | 1.67 (1.00–2.79) | 1.75 (1.11–2.76) |
| p | 0.028 | 0.048 | 0.016 |
| iTIL (IM) | |||
| Low | 1 | 1 | 1 |
| High | 0.52 (0.31–0.86) | 0.49 (0.27–0.87) | 0.44 (0.21–0.93) |
| p | 0.011 | 0.015 | 0.031 |
| sTIL (TC) | |||
| Low | 1 | 1 | 1 |
| High | 0.28 (0.17–0.47) | 0.32 (0.19–0.55) | 0.30 (0.18–0.50) |
| p | <0.001 | <0.001 | <0.001 |
| sTIL (IM) | |||
| Low | 1 | 1 | 1 |
| High | 0.12 (0.06–0.23) | 0.14 (0.08–0.28) | 0.13 (0.07–0.25) |
| p | <0.001 | <0.001 | <0.001 |
| DOI | |||
| Low (≤9 mm) | 1 | 1 | 1 |
| High (>9 mm) | 2.95 (1.55–5.60) | 3.73 (1.70–8.18) | 3.20 (1.69–6.08) |
| p | <0.001 | 0.001 | <0.001 |
| Resection margin | |||
| >2.5 mm | 1 | 1 | 1 |
| ≤2.5 mm | 1.91 (1.14–3.18) | 1.83 (1.12–2.99) | 1.95 (1.18–3.22) |
| p | 0.014 | 0.016 | 0.009 |
| LNR | |||
| Low (≤0.15) | 1 | 1 | 1 |
| High (>0.15) | 2.42 (1.48–3.94) | 2.78 (1.64–4.72) | 2.28 (1.41–3.71) |
| p | <0.001 | <0.001 | <0.001 |
| ENE | |||
| Micro (≤2 mm) | 1 | 1 | 1 |
| Macro (>2 mm) | 3.41 (2.07–5.62) | 3.14 (1.88–5.25) | 3.43 (2.08–5.64) |
| p | <0.001 | <0.001 | <0.001 |
| Variable | OS HR (95% CI) | DSS HR (95% CI) | DFS HR (95% CI) |
|---|---|---|---|
| sTIL (IM) | |||
| High (>25%) | 1 | 1 | 1 |
| Low (≤25%) | 4.53 (1.57–13.10) | 4.49 (1.48–13.58) | 3.42 (1.17–9.99) |
| p | 0.005 | 0.008 | 0.025 |
| DOI (depth of invasion) | |||
| Low (≤9 mm) | 0.42 (0.21–0.85) | 0.31 (0.13–0.73) | 0.38 (0.19–0.78) |
| High (>9 mm) | 1 | 1 | 1 |
| p | 0.015 | 0.007 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorencin Bulic, M.; Jurlina, M.; Müller, D.; Lijovic, L.; Mamic, M.; Luksic, I. Tumor-Infiltrating Lymphocytes Predict Extranodal Extension and Prognosis in Regionally Advanced Oral Cavity Cancer. Diagnostics 2025, 15, 2431. https://doi.org/10.3390/diagnostics15192431
Lorencin Bulic M, Jurlina M, Müller D, Lijovic L, Mamic M, Luksic I. Tumor-Infiltrating Lymphocytes Predict Extranodal Extension and Prognosis in Regionally Advanced Oral Cavity Cancer. Diagnostics. 2025; 15(19):2431. https://doi.org/10.3390/diagnostics15192431
Chicago/Turabian StyleLorencin Bulic, Mia, Martin Jurlina, Danko Müller, Lada Lijovic, Matija Mamic, and Ivica Luksic. 2025. "Tumor-Infiltrating Lymphocytes Predict Extranodal Extension and Prognosis in Regionally Advanced Oral Cavity Cancer" Diagnostics 15, no. 19: 2431. https://doi.org/10.3390/diagnostics15192431
APA StyleLorencin Bulic, M., Jurlina, M., Müller, D., Lijovic, L., Mamic, M., & Luksic, I. (2025). Tumor-Infiltrating Lymphocytes Predict Extranodal Extension and Prognosis in Regionally Advanced Oral Cavity Cancer. Diagnostics, 15(19), 2431. https://doi.org/10.3390/diagnostics15192431







