Predictive Value of Arterial Enhancement Fraction Derived from Dual-Layer Spectral Computed Tomography for Thyroid Microcarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. DSCT Image Acquisition
2.3. Nodule Matching and Selection Criteria
2.4. Qualitative Image Analyses
2.5. Quantitative Measurements of Spectral Parameters
2.6. Quantitative Measurements of AEFD and AEFS
2.7. Statistical Analyses
3. Results
3.1. Comparative Analysis of Demographic and DSCT Parameters in Benign Versus Malignant Thyroid Micronodules
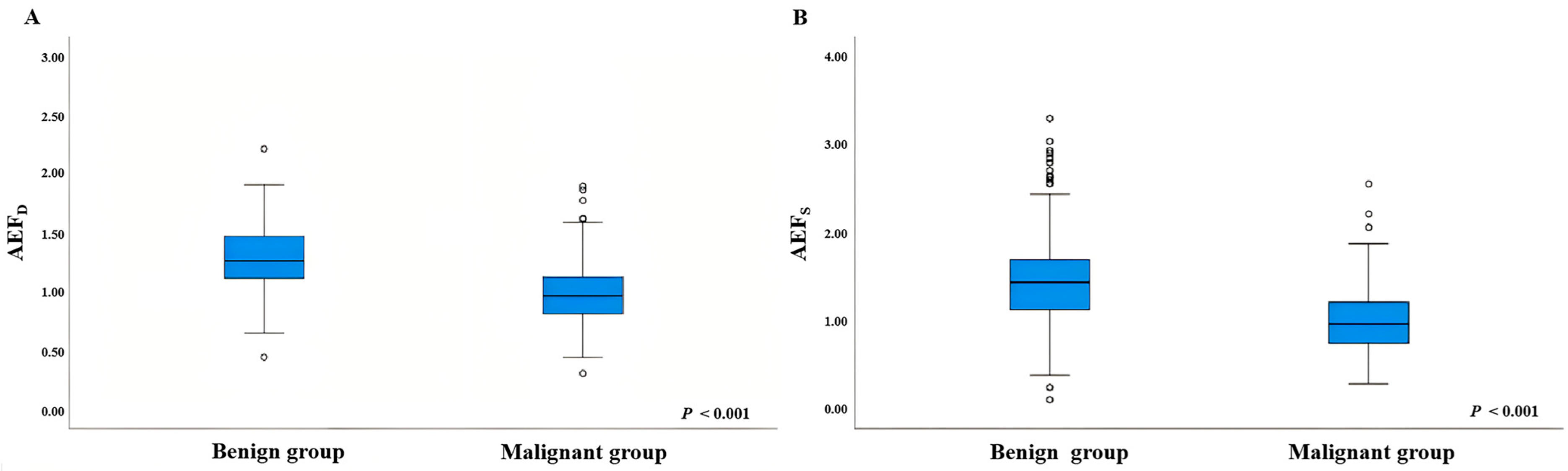
3.2. Comparison of AEF Values Between Benign and Malignant Thyroid Micronodules
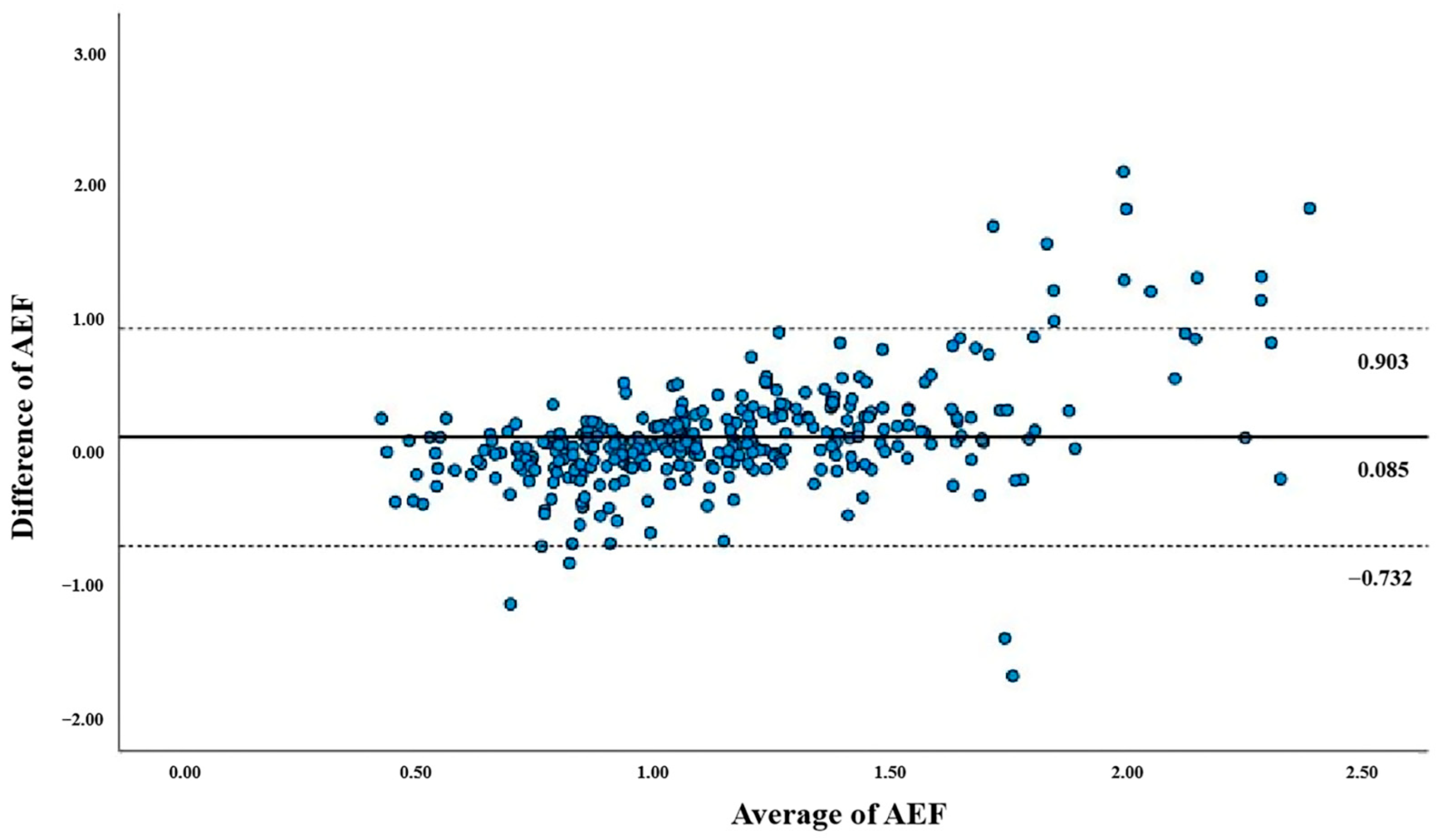
3.3. Correlation Between AEFD and AEFS
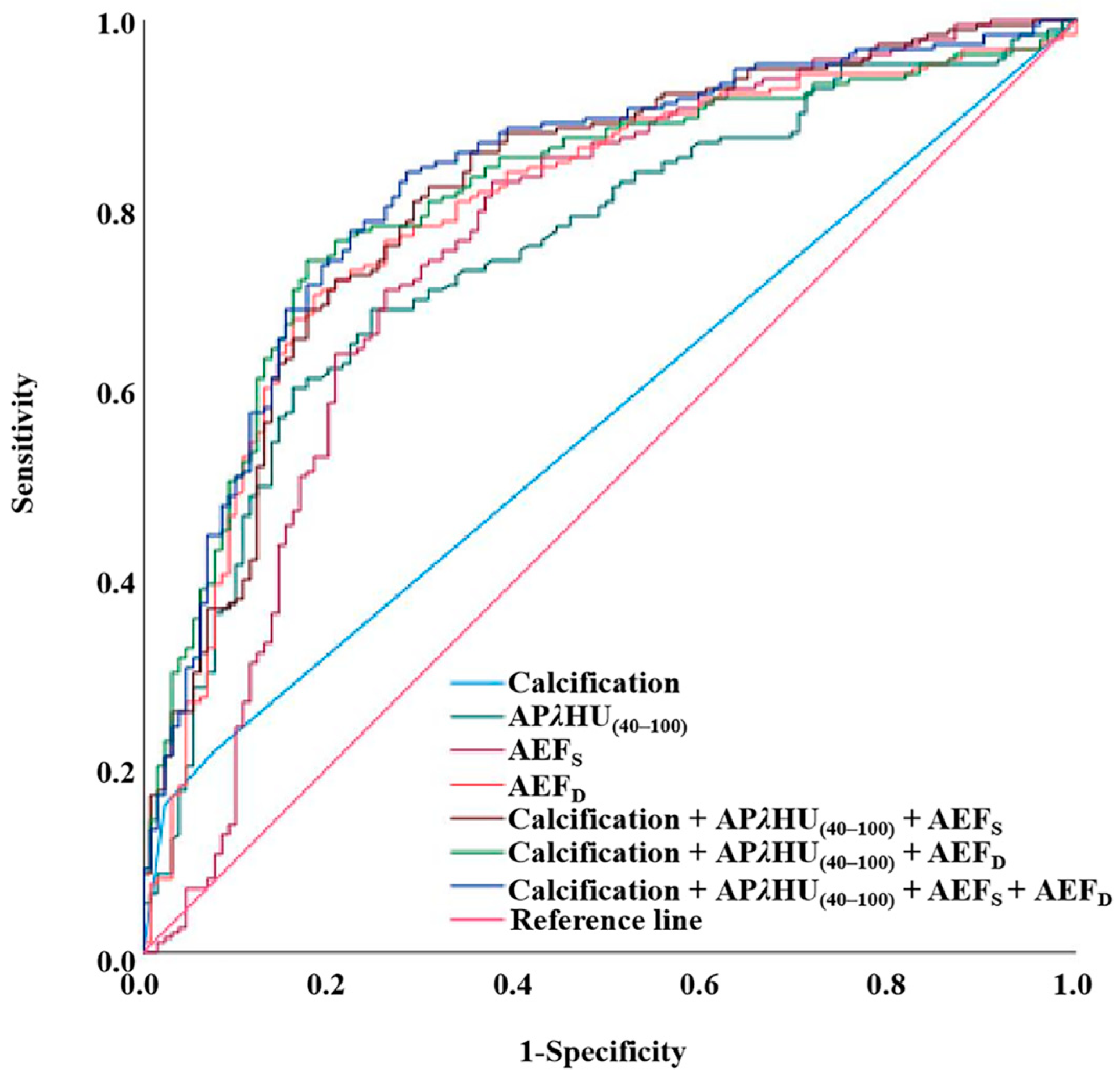
3.4. Diagnostic Efficiency of Spectral Parameters, AEF, and Conventional Image Feature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSCT | Dual-layer spectral computed tomography |
| AEF | Arterial enhancement fraction |
| NIC | Normalized iodine concentration |
| NZeff | Normalized effective atomic number |
| λHU | Slope of spectral Hounsfield unit curve |
| SECT | Single-energy computed tomography |
| AEFS | SECT-derived AEF |
| AEFD | DSCT-derived AEF |
| AUC | Area under the curve |
| ROC | Receiver operating characteristic |
| OR | Odds ratio |
| TMC | Thyroid microcarcinoma |
| FNAC | Fine needle aspiration cytology |
| F18-FDG-PET | 18-fludeoxyglucose positron emission tomography |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| FOV | Field of view |
| ROI | Region of interest |
| AP | Arterial phase |
| VP | Venous phase |
| IC | Iodine concentration |
| Zeff | Effective atomic number |
| HU | Hounsfield unit |
| IQR | Interquartile range |
| SD | Standard deviation |
| VIF | Variance inflation factor |
| CI | Confidence interval |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
References
- Kant, R.; Davis, A.; Verma, V. Thyroid Nodules: Advances in Evaluation and Management. Am. Fam. Physician 2020, 102, 298–304. [Google Scholar] [PubMed]
- Grani, G.; Sponziello, M.; Pecce, V.; Ramundo, V.; Durante, C. Contemporary Thyroid Nodule Evaluation and Management. J. Clin. Endocrinol. Metab. 2020, 105, 2869–2883. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.K.; Lim, H.K.; Lee, C.Y.; Sung, J.Y.; Yoon, J.H.; Han, S.Y.; Shin, J.H.; Kim, J.-H.; Jung, S.L.; et al. Standardized Ultrasound Evaluation for Active Surveillance of Low-Risk Thyroid Microcarcinoma in Adults: 2024 Korean Society of Thyroid Radiology Consensus Statement. Korean J. Radiol. 2024, 25, 942–958. [Google Scholar] [CrossRef]
- De Leo, S.; Brigante, G.; D’Elia, S.; Censi, S.; Madeo, B.; Morelli, S.; Nervo, A.; Repaci, A.; Sparano, C.; Stramazzo, I.; et al. Prospective Validation of ATA Risk Score for Papillary Thyroid Microcarcinoma: An ITCO Real-World Study. J. Clin. Endocrinol. Metab. 2025, dgaf190. [Google Scholar] [CrossRef] [PubMed]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Saxe, A.; Idris, M.; Gemechu, J. Does the Use of Intraoperative Neuromonitoring during Thyroid and Parathyroid Surgery Reduce the Incidence of Recurrent Laryngeal Nerve Injuries? A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 860. [Google Scholar] [CrossRef]
- Uppal, N.; Collins, R.; James, B. Thyroid Nodules: Global, Economic, and Personal Burdens. Front. Endocrinol. 2023, 14, 1113977. [Google Scholar] [CrossRef]
- Krajewska, J.; Kukulska, A.; Oczko-Wojciechowska, M.; Kotecka-Blicharz, A.; Drosik-Rutowicz, K.; Haras-Gil, M.; Jarzab, B.; Handkiewicz-Junak, D. Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival. Front. Endocrinol. 2020, 11, 571421. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Cheng, R. The Active Surveillance Management Approach for Patients with Low Risk Papillary Thyroid Microcarcinomas: Is China Ready? Cancer Biol. Med. 2021, 19, 619–634. [Google Scholar] [CrossRef]
- Liu, Q.; Song, M.; Zhang, H. Choice of Management Strategy for Papillary Thyroid. Microcarcinoma: Active Surveillance or Immediate Surgery? J. Cancer 2024, 15, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Evangelista, L.; Ravelli, I.; Magnani, F.; Iacobone, M.; Giraudo, C.; Camozzi, V.; Spimpolo, A.; Cecchin, D. 18F-Choline PET/CT and PET/MRI in Primary and Recurrent Hyperparathyroidism: A Systematic Review of the Literature. Ann. Nucl. Med. 2020, 34, 601–619. [Google Scholar] [CrossRef]
- Gąsiorowski, O.; Leszczyński, J.; Kaszczewska, J.; Stępkowski, K.; Kaszczewski, P.; Baryła, M.; Gałązka, Z. Comparison of Fine-Needle Aspiration Cytopathology with Histopathological Examination of the Thyroid Gland in Patients Undergoing Elective Thyroid Surgery: Do We Still Need Fine-Needle Aspiration Cytopathology? Diagnostics 2024, 14, 236. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.-N.; Jiang, L.; Yu, C.-X.; Chen, Y.-N.; Yu, X.-J.; Pan, M.-F. Conventional Ultrasonography and Elastosonography in Diagnosis of Malignant Thyroid Nodules: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 13, 1082881. [Google Scholar] [CrossRef]
- Eun, N.L.; Son, E.J.; Kim, J.-A.; Gweon, H.M.; Kang, J.-H.; Youk, J.H. Comparison of the Diagnostic Performances of Ultrasonography, CT and Fine Needle Aspiration Cytology for the Prediction of Lymph Node Metastasis in Patients with Lymph Node Dissection of Papillary Thyroid Carcinoma: A Retrospective Cohort Study. Int. J. Surg. 2018, 51, 145–150. [Google Scholar] [CrossRef]
- Montgomery, J.; Hendry, J.; Van der Horst, C.; Hunter, M.A.; MacKenzie, K.; Hilmi, O. Cytological Accuracy and Radiological Staging in Patients with Thyroid Cancer in Glasgow. Eur. Arch. Otorhinolaryngol. 2016, 273, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Grazhdani, H.; Tattaresu, G.; Pittari, A.; Foti, P.V.; Palmucci, S.; Spatola, C.; Lo Greco, M.C.; Inì, C.; Tiralongo, F.; et al. Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence. Biomedicines 2024, 12, 1676. [Google Scholar] [CrossRef]
- Bojunga, J.; Trimboli, P. Thyroid Ultrasound and Its Ancillary Techniques. Rev. Endocr. Metab. Disord. 2024, 25, 161–173. [Google Scholar] [CrossRef]
- Cantisani, V.; Grazhdani, H.; Drakonaki, E.; D’Andrea, V.; Di Segni, M.; Kaleshi, E.; Calliada, F.; Catalano, C.; Redler, A.; Brunese, L.; et al. Strain US Elastography for the Characterization of Thyroid Nodules: Advantages and Limitation. Int. J. Endocrinol. 2015, 2015, 908575. [Google Scholar] [CrossRef] [PubMed]
- Vriens, D.; Adang, E.M.M.; Netea-Maier, R.T.; Smit, J.W.A.; de Wilt, J.H.W.; Oyen, W.J.G.; de Geus-Oei, L.F. Cost-Effectiveness of FDG-PET/CT for Cytologically Indeterminate Thyroid Nodules: A Decision Analytic Approach. J. Clin. Endocrinol. Metab. 2014, 99, 3263–3274. [Google Scholar] [CrossRef]
- Iakovou, I.; Giannoula, E.; Sachpekidis, C. Imaging and Imaging-Based Management of Pediatric Thyroid Nodules. J. Clin. Med. 2020, 9, 384. [Google Scholar] [CrossRef]
- Uludag, M.; Unlu, M.T.; Kostek, M.; Aygun, N.; Caliskan, O.; Ozel, A.; Isgor, A. Management of Thyroid Nodules. Med. Bull. Sisli Etfal Hosp. 2023, 57, 287–304. [Google Scholar] [CrossRef]
- Lee, D.W.; Ji, Y.B.; Sung, E.S.; Park, J.S.; Lee, Y.J.; Park, D.W.; Tae, K. Roles of Ultrasonography and Computed Tomography in the Surgical Management of Cervical Lymph Node Metastases in Papillary Thyroid Carcinoma. Eur. J. Surg. Oncol. 2013, 39, 191–196. [Google Scholar] [CrossRef]
- Li, Q.; Song, Z.; Zhang, D.; Li, X.; Liu, Q.; Yu, J.; Wen, Y.; Zhang, J.; Ren, X.; Li, Z.; et al. Diagnostic Accuracy of Dual-Energy Computed Tomography-Based Nomogram for Differentiating Papillary Thyroid Microcarcinomas from Micronodular Goiters. Quant. Imaging Med. Surg. 2023, 13, 3428–3440. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT Imaging: Technical Principles of Dual-Energy CT and Multi-Energy Photon-Counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Q.; Zhang, D.; Li, X.; Yu, J.; Liu, Q.; Li, Z.; Huang, J.; Zhang, X.; Tang, Z. Nomogram Based on Spectral CT Quantitative Parameters and Typical Radiological Features for Distinguishing Benign from Malignant Thyroid Micro-Nodules. Cancer Imaging 2023, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.-K.; Geng, D.; Wang, J.-W.; Chen, X.-B.; Si, Y.; Shen, M.-P.; Su, G.-Y.; Xu, X.-Q.; Wu, F.-Y. Added Value of Arterial Enhancement Fraction Derived from Dual-Energy Computed Tomography for Preoperative Diagnosis of Cervical Lymph Node Metastasis in Papillary Thyroid Cancer: Initial Results. Eur. Radiol. 2024, 34, 1292–1301. [Google Scholar] [CrossRef]
- Li, F.; Huang, F.; Liu, C.; Pan, D.; Tang, X.; Wen, Y.; Chen, Z.; Qin, Y.; Chen, J. Parameters of Dual-Energy CT for the Differential Diagnosis of Thyroid Nodules and the Indirect Prediction of Lymph Node Metastasis in Thyroid Carcinoma: A Retrospective Diagnostic Study. Gland. Surg. 2022, 11, 913–926. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, Y.H.; Seo, H.S.; Lee, K.Y.; Suh, S.-I.; Ryoo, I.; You, S.-H.; Kim, B.; Yang, K.-S. Dual-Energy CT Iodine Quantification for Characterizing Focal Thyroid Lesions. Head Neck 2019, 41, 1024–1031. [Google Scholar] [CrossRef]
- Portulano, C.; Paroder-Belenitsky, M.; Carrasco, N. The Na+/I− Symporter (NIS): Mechanism and Medical Impact. Endocr. Rev. 2014, 35, 106–149. [Google Scholar] [CrossRef]
- Zhang, F.; Qiao, Y.; Zhang, H. Value of CT Features in the Diagnosis of Papillary Thyroid Tumors in Incidental Thyroid Nodules. Int. J. Endocrinol. 2020, 2020, 9342317. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Choi, Y.S.; Kwon, H.J.; Lee, J.S.; Heo, J.J.; Han, Y.J.; Park, Y.-H.; Kim, J.H. Relationship between Patterns of Calcification in Thyroid Nodules and Histopathologic Findings. Endocr. J. 2013, 60, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Caresio, C.; Caballo, M.; Deandrea, M.; Garberoglio, R.; Mormile, A.; Rossetto, R.; Limone, P.; Molinari, F. Quantitative Analysis of Thyroid Tumors Vascularity: A Comparison between 3-D Contrast-Enhanced Ultrasound and 3-D Power Doppler on Benign and Malignant Thyroid Nodules. Med. Phys. 2018, 45, 3173–3184. [Google Scholar] [CrossRef] [PubMed]

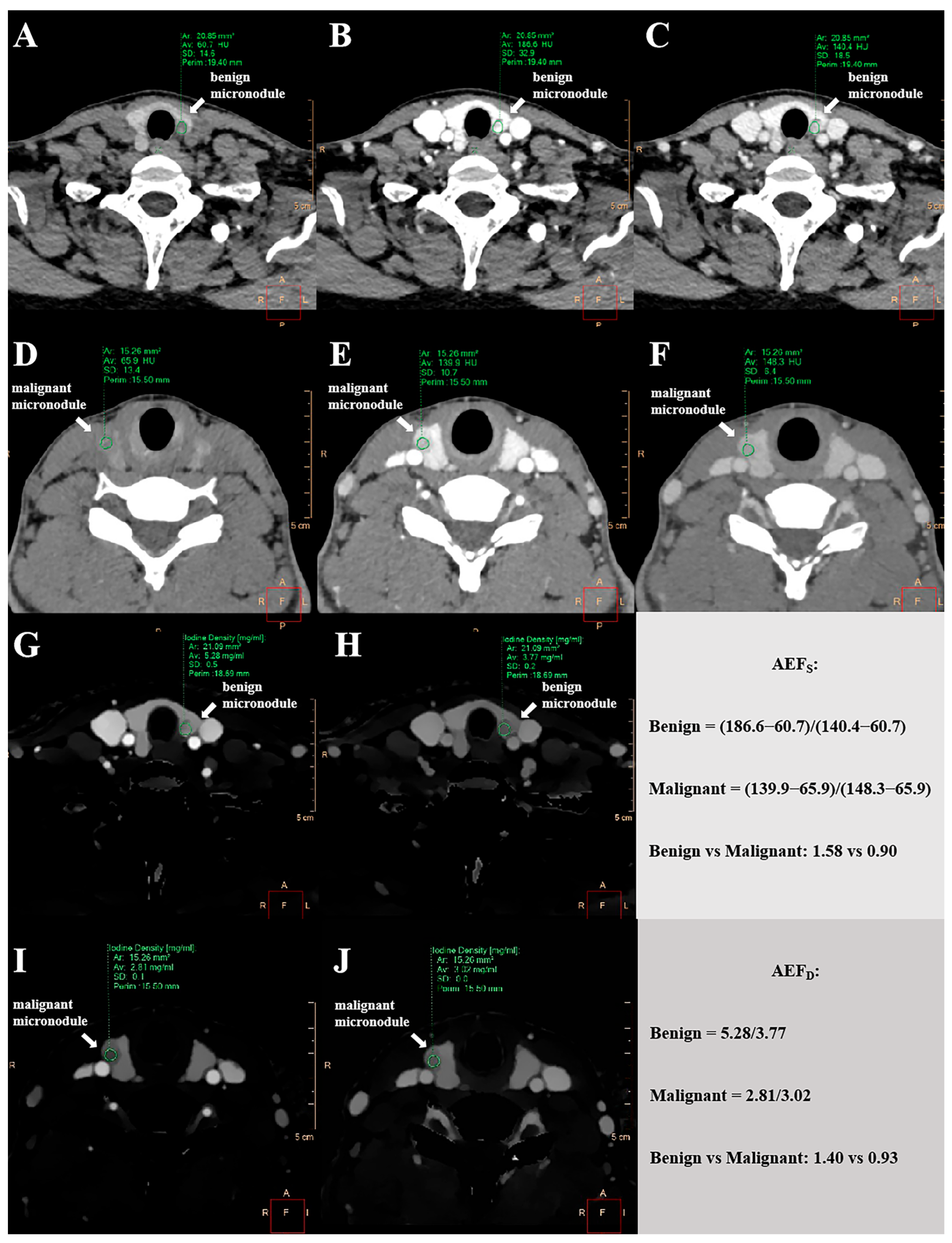

| Benign Micronodule Cohort (n = 131) | Malignant Micronodule Cohort (n = 190) | p Value | |
|---|---|---|---|
| Age, y (%) | <0.001 | ||
| <50 | 59 (55.1%) | 157 (85.8%) | |
| ≥50 | 48 (44.9%) | 26 (14.2%) | |
| Gender (%) | 0.070 | ||
| Female | 98 (91.6%) | 154 (84.2%) | |
| Male | 9 (8.4%) | 29 (15.8%) | |
| Maximum nodule size (mm) | 6 (5, 9) | 7 (6, 8) | 0.392 |
| Calcification (%) | <0.001 | ||
| Microcalcification | 3 (2.3%) | 30 (15.8%) | |
| Macrocalcification | 7 (5.3%) | 11 (5.8%) | |
| Absent calcification | 121 (92.4%) | 149 (78.4%) | |
| Enhanced blurring (%) | 0.722 | ||
| Yes | 45 (34.4%) | 124 (65.3%) | |
| No | 86 (65.6%) | 66 (34.7%) | |
| Arterial phase | |||
| λHU(40–100) | 4.453 (3.928, 5.250) | 3.329 (2.707, 4.227) | <0.001 |
| NIC | 0.372 (0.319, 0.415) | 0.285 (0.227, 0.339) | <0.001 |
| NZeff | 0.824 (0.797, 0.843) | 0.793 (0.766, 0.814) | <0.001 |
| Venous phase | |||
| λHU(40–100) | 3.457 (3.046, 4.098) | 3.481 (2.930, 4.135) | 0.942 |
| NIC | 0.680 (0.627, 0.771) | 0.720 (0.609, 0.842) | 0.194 |
| NZeff | 0.943 (0.929, 0.958) | 0.952 (0.927, 0.973) | 0.103 |
| Variables | Coefficient (β) | SD | Wald | Odds (95% CI) | p Value | VIF | F Statistics | Standardized R2 |
|---|---|---|---|---|---|---|---|---|
| 22.752 | 0.177 | |||||||
| APλHU(40–100) | 0.511 | 0.161 | 10.003 | 0.600 (0.437, 0.823) | 0.002 | 2.356 | ||
| APNIC | 4.589 | 3.126 | 2.154 | 0.010 (0.000, 4.658) | 0.142 | 3.933 | ||
| APNZeff | 0.883 | 4.755 | 0.034 | 0.414 (0.000, 4613.544) | 0.853 | 2.231 |
| AEF Value | Benign Micronodule Cohorts (n = 131) | Malignant Micronodule Cohorts (n = 190) | p Value |
|---|---|---|---|
| AEFS | 1.436 (1.126, 1.697) | 0.964 (0.747, 1.210) | <0.001 |
| AEFD | 1.259 (1.112, 1.469) | 0.958 (0.811, 1.123) | <0.001 |
| AUC (95% CI) | Unnecessary Biopsy Rate (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| Calcification | 0.573 (0.511, 0.636) | 7.6% (10/131) | 21.6% | 92.4% | 50.5% | 80.4% | 44.8% |
| APλHU(40–100) | 0.752 (0.698, 0.806) | 24.4% (32/131) | 68.9% | 75.6% | 71.7% | 80.4% | 62.7% |
| AEFS | 0.753 (0.695, 0.810) | 26.0% (34/131) | 71.1% | 74.0% | 72.3% | 79.9% | 63.8% |
| AEFD | 0.794 (0.743, 0.845) | 18.3% (24/131) | 70.5% | 81.7% | 75.1% | 84.8% | 65.6% |
| Calcification + APλHU(40–100) + AEFS | 0.811 (0.763, 0.860) | 30.5% (40/131) | 82.1% | 69.5% | 74.2% | 79.6% | 72.8% |
| Calcification + APλHU(40–100) + AEFD | 0.810 (0.762, 0.858) | 17.6% (23/131) | 74.2% | 82.4% | 78.4% | 86.0% | 68.8% |
| Calcification + APλHU(40–100) + AEFS + AEFD | 0.826 (0.779, 0.872) | 28.2% (37/131) | 83.7% | 71.8% | 78.8% | 81.1% | 75.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yu, J.; Lv, L.; Song, Z.; Huang, J.; Zhou, B.; Zou, X.; Zou, Y.; Zhang, D. Predictive Value of Arterial Enhancement Fraction Derived from Dual-Layer Spectral Computed Tomography for Thyroid Microcarcinoma. Diagnostics 2025, 15, 2427. https://doi.org/10.3390/diagnostics15192427
Chen Y, Yu J, Lv L, Song Z, Huang J, Zhou B, Zou X, Zou Y, Zhang D. Predictive Value of Arterial Enhancement Fraction Derived from Dual-Layer Spectral Computed Tomography for Thyroid Microcarcinoma. Diagnostics. 2025; 15(19):2427. https://doi.org/10.3390/diagnostics15192427
Chicago/Turabian StyleChen, Yuwei, Jiayi Yu, Liang Lv, Zuhua Song, Jie Huang, Bi Zhou, Xinghong Zou, Ya Zou, and Dan Zhang. 2025. "Predictive Value of Arterial Enhancement Fraction Derived from Dual-Layer Spectral Computed Tomography for Thyroid Microcarcinoma" Diagnostics 15, no. 19: 2427. https://doi.org/10.3390/diagnostics15192427
APA StyleChen, Y., Yu, J., Lv, L., Song, Z., Huang, J., Zhou, B., Zou, X., Zou, Y., & Zhang, D. (2025). Predictive Value of Arterial Enhancement Fraction Derived from Dual-Layer Spectral Computed Tomography for Thyroid Microcarcinoma. Diagnostics, 15(19), 2427. https://doi.org/10.3390/diagnostics15192427








