Dual-Energy Computed Tomography (DECT) for Diagnosing Contrast-Induced Encephalopathy (CIE) Mimicking Intracranial Hemorrhage (ICH): A Rare Case
Abstract
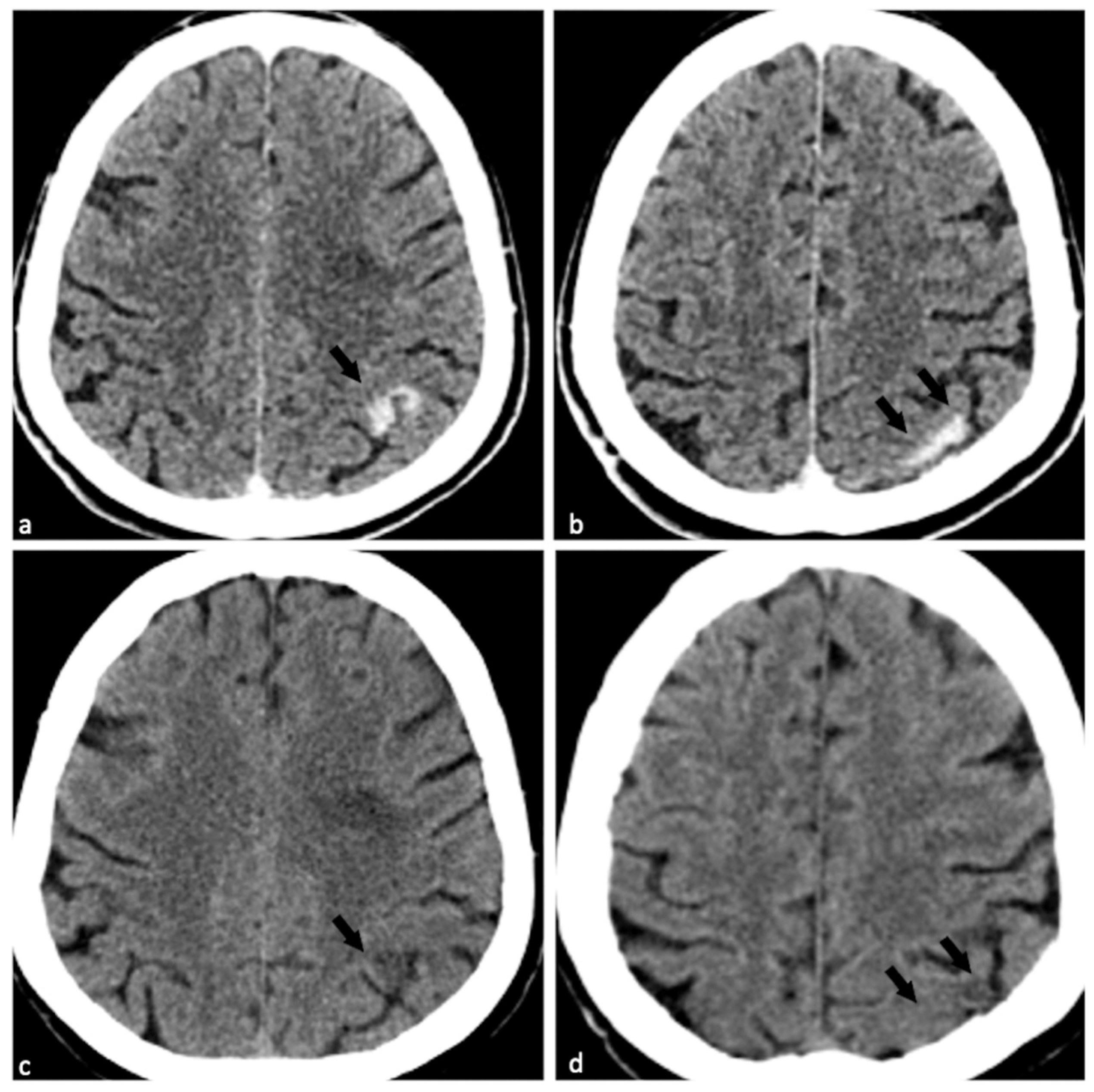
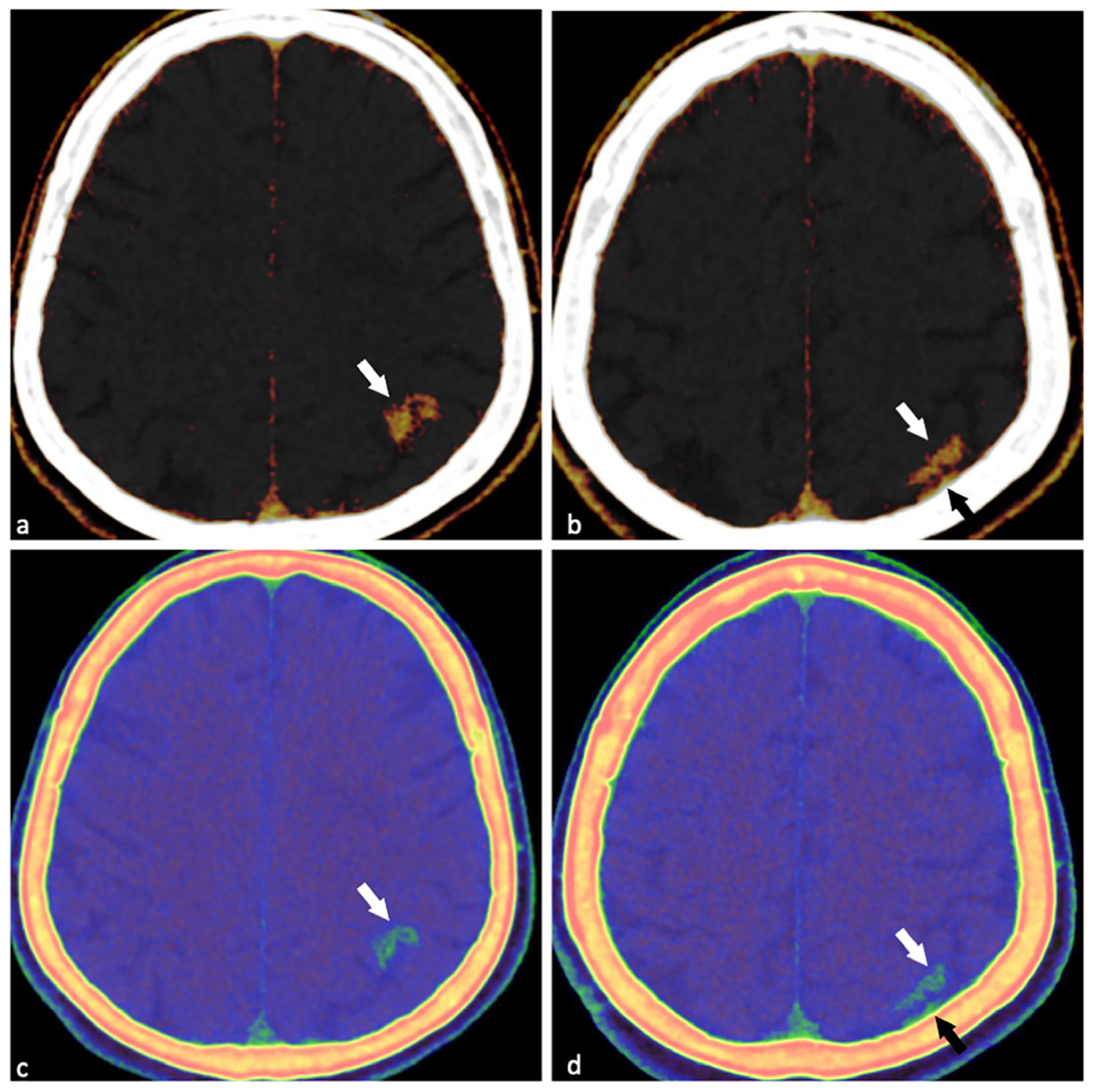
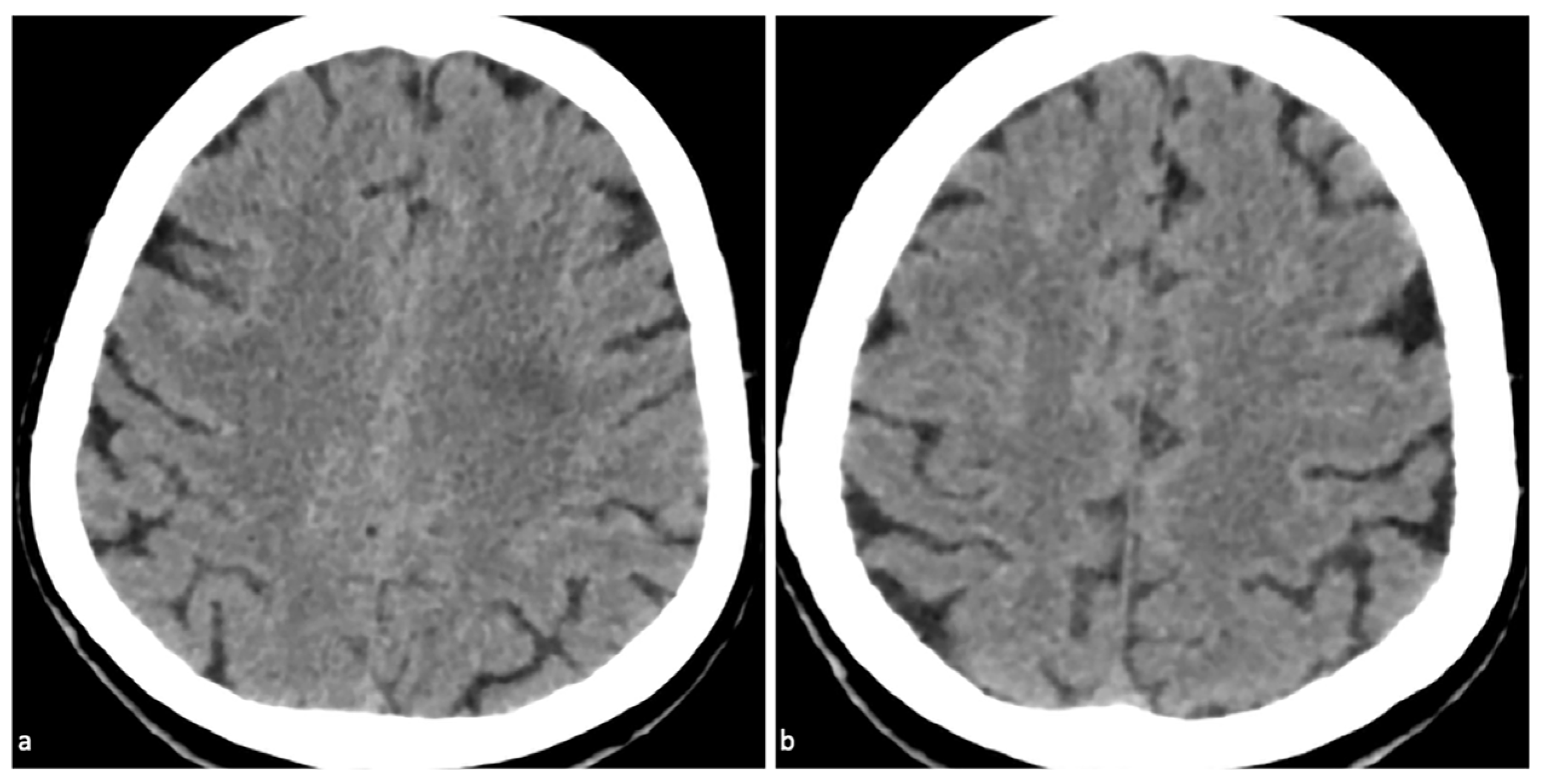
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| CIE | contrast-induced encephalopathy |
| CM | contrast medium |
| CT | computed tomography |
| DAPT | dual antiplatelet therapy |
| DE | dual-energy |
| HU | Hounsfield unit |
| IC | iodine concentration |
| ICH | intracranial hemorrhage |
| PCI | percutaneous coronary intervention |
| VNC | virtual non-contrast |
| Zeff | effective atomic number |
References
- Koo, A.B.; Zhou, L.; Hameed, I.; Rivier, C.A.; Clocchiatti-Tuozzo, S.; Kamel, H.; Falcone, G.J.; Ney, J.; Sharma, R.; Matouk, C.; et al. Acute Ischemic Stroke Risk Following Cardiac Interventions in the United States From 2016 to 2021. Neurology 2025, 105, e213766. [Google Scholar] [CrossRef] [PubMed]
- Spina, R.; Simon, N.; Markus, R.; Muller, D.W.; Kathir, K. Contrast-induced encephalopathy following cardiac catheterization. Catheter. Cardiovasc. Interv. 2017, 90, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Pašara, V.; Momčilović, M.; Bujan Kovač, A.; Šulentić, V.; Perković, R.; Lučev, L.; Narančić, M.; Lovrić, D. Contrast medium-induced encephalopathy following coronary angiography and evidence for reversal using intravenous levetiracetam in a heart transplant patient with cardiac allograft vasculopathy: A case report. Int. J. Clin. Pharmacol. Ther. 2025, 63, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, Y.; Shao, M.; Wang, Y.; Du, J.; Li, X.; Yang, P.; Fu, D.; Dong, Z.; Liu, M. An unusual contrast-induced encephalopathy following percutaneous coronary intervention in patients with cerebrovascular abnormalities: A case report. Front. Cardiovasc. Med. 2022, 9, 957779. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Jeong, H.W.; Heo, Y.J.; Yun, S.; Kang, M.; Kim, B.; Kim, E.J.; Lim, S.M.; Lee, B. Comparison of Safety and Diagnostic Efficacy of Iohexol 240 mgI/mL, Iopamidol 250 mgI/mL, and Iodixanol 270 mgI/mL in Cerebral Angiography: A Prospective, Multicenter Study. Neurointervention 2023, 19, 82–91. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Wang, X.; Hao, L.; Zhang, J.; Zhang, X.; Sheng, Z.; Liu, K. The diagnostic value of quantitative parameters on dual-layer detector-based spectral CT in identifying ischemic stroke. Front. Neurol. 2023, 14, 1056941. [Google Scholar]
- Mariajoseph, F.P.; Lai, L.T.; Praeger, A.; Moore, J.; Chandra, R.V.; Asadi, H.; Fawzy, P.; de Villiers, L.; Goldschlager, T.; Gan, C.; et al. Nationwide multicenter experience of contrast-induced encephalopathy following neurointervention: Clinical course and outcomes. J. NeuroInterv. Surg. 2025, jnis-2025-023533. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Ye, T. Dual-Energy Computed Tomography (DECT) for Diagnosing Contrast-Induced Encephalopathy (CIE) Mimicking Intracranial Hemorrhage (ICH): A Rare Case. Diagnostics 2025, 15, 2426. https://doi.org/10.3390/diagnostics15192426
Shen Y, Ye T. Dual-Energy Computed Tomography (DECT) for Diagnosing Contrast-Induced Encephalopathy (CIE) Mimicking Intracranial Hemorrhage (ICH): A Rare Case. Diagnostics. 2025; 15(19):2426. https://doi.org/10.3390/diagnostics15192426
Chicago/Turabian StyleShen, Yuhong, and Tianhe Ye. 2025. "Dual-Energy Computed Tomography (DECT) for Diagnosing Contrast-Induced Encephalopathy (CIE) Mimicking Intracranial Hemorrhage (ICH): A Rare Case" Diagnostics 15, no. 19: 2426. https://doi.org/10.3390/diagnostics15192426
APA StyleShen, Y., & Ye, T. (2025). Dual-Energy Computed Tomography (DECT) for Diagnosing Contrast-Induced Encephalopathy (CIE) Mimicking Intracranial Hemorrhage (ICH): A Rare Case. Diagnostics, 15(19), 2426. https://doi.org/10.3390/diagnostics15192426




