Ultrasonographic Median Nerve Cross-Sectional Area and Clinical, Electrodiagnostic, and Laboratory Biomarkers in Electrodiagnostically Confirmed Carpal Tunnel Syndrome: A Single-Center Correlational Study
Abstract
1. Introduction
2. Materials and Methods
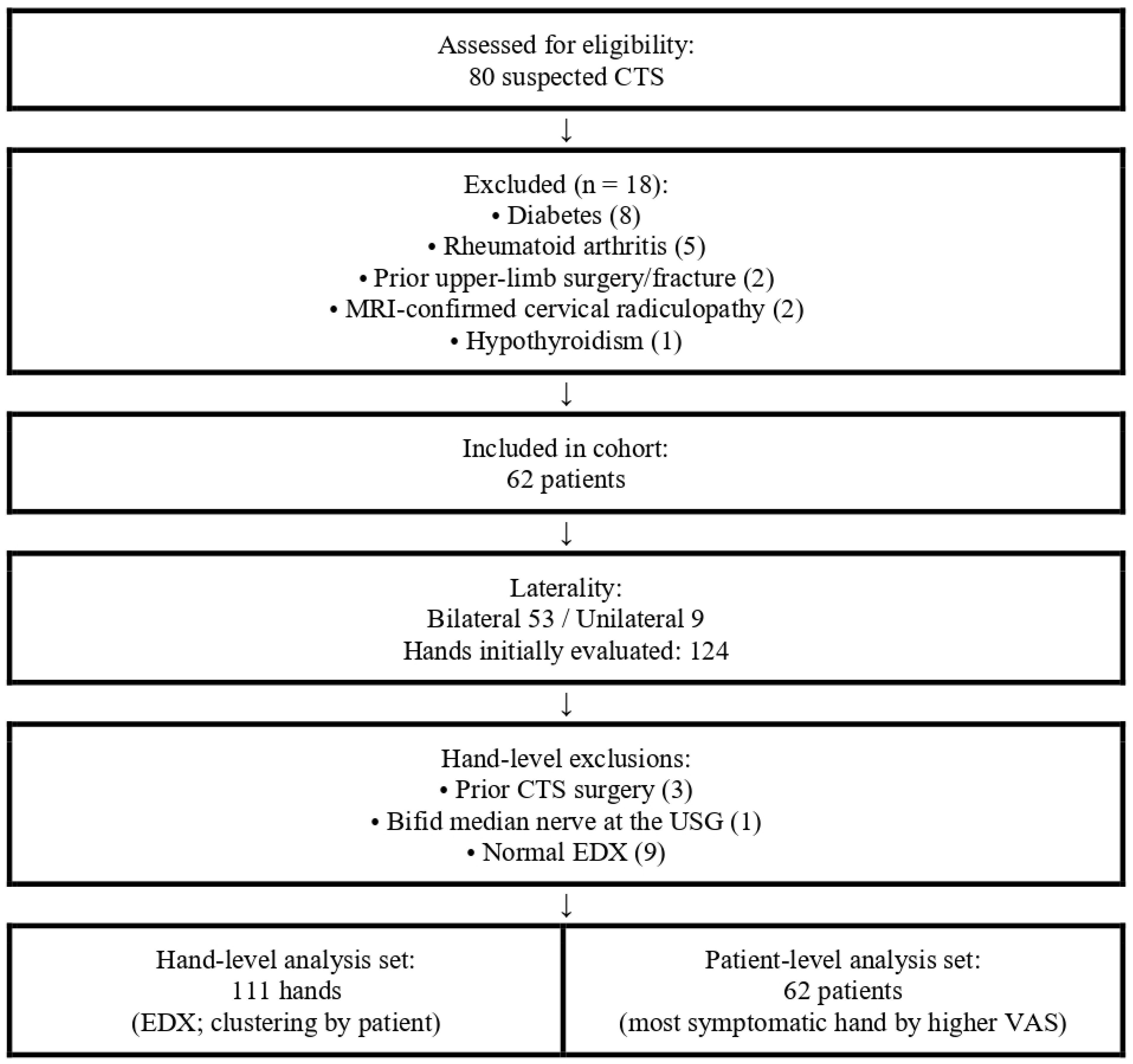
2.1. Study Design and Participants
2.2. Clinical Evaluation
- Symptom Severity Scale (SSS): This section assesses the intensity and frequency of symptoms, including pain, numbness, and tingling in the hand and wrist.
- Functional Status Scale (FSS): This section assesses how carpal tunnel syndrome affects a person’s ability to perform daily activities, such as writing, buttoning clothes, or holding objects.
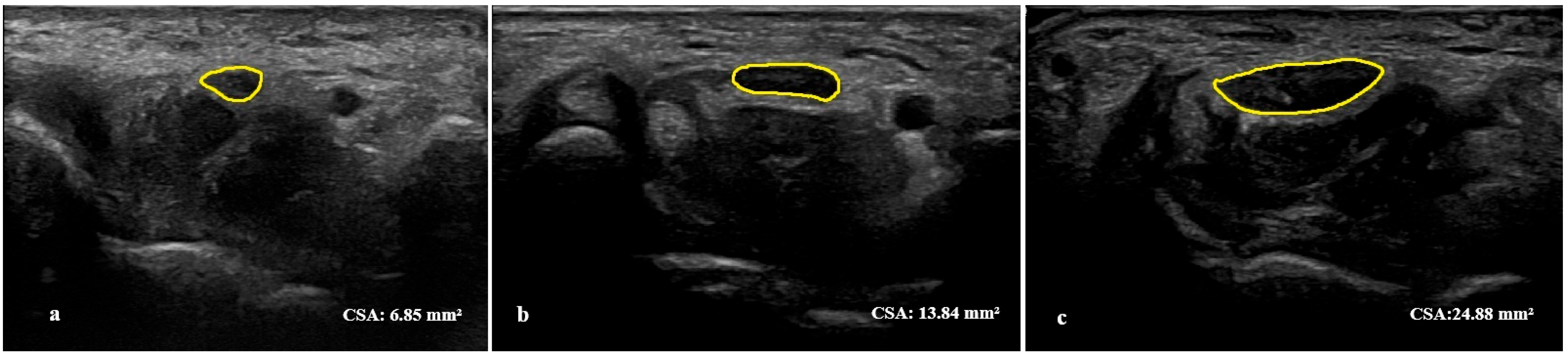
2.3. Ultrasonographic Assessment
2.4. Electrodiagnostic Studies
- Negative: All standard median studies within normal limits.
- Mild: Abnormal sensory conduction across the wrist (prolonged sensory latency/decreased velocity), DML within normal limits.
- Moderate: Abnormal sensory conduction and prolonged DML.
- Severe: Absent SNAP, low-amplitude or absent CMAP (DML prolonged).
2.5. Laboratory Assessments
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Correlation Between Median Nerve CSA and Clinical Parameters
3.3. Correlation Between Median Nerve CSA and Laboratory Parameters
3.4. Correlation Between Median Nerve CSA and EDX Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mooar, P.A.; Doherty, W.J.; Murray, J.N.; Pezold, R.; Sevarino, K.S. Management of carpal tunnel syndrome. JAAOS-J. Am. Acad. Orthop. Surg. 2018, 26, e128–e130. [Google Scholar] [CrossRef]
- Zamborsky, R.; Kokavec, M.; Simko, L.; Bohac, M. Carpal tunnel syndrome: Symptoms, causes and treatment options. Literature reviev. Ortop. Traumatol. Rehabil. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Foley, M.; Silverstein, B.; Polissar, N. The economic burden of carpal tunnel syndrome: Long-term earnings of CTS claimants in Washington State. Am. J. Ind. Med. 2007, 50, 155–172. [Google Scholar] [CrossRef]
- Rotaru-Zavaleanu, A.-D.; Lungulescu, C.V.; Bunescu, M.G.; Vasile, R.C.; Gheorman, V.; Gresita, A.; Dinescu, V.C. Occupational Carpal Tunnel Syndrome: A scoping review of causes, mechanisms, diagnosis, and intervention strategies. Front. Public Health 2024, 12, 1407302. [Google Scholar] [CrossRef]
- Shapiro, L.M.; Kamal, R.N.; Management of Carpal Tunnel Syndrome Work Group; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons/ASSH Clinical Practice Guideline Summary Management of Carpal Tunnel Syndrome. JAAOS-J. Am. Acad. Orthop. Surg. 2022, 33, e356–e366. [Google Scholar] [CrossRef] [PubMed]
- Osiak, K.; Mazurek, A.; Pękala, P.; Koziej, M.; Walocha, J.A.; Pasternak, A. Electrodiagnostic studies in the surgical treatment of carpal tunnel syndrome—A systematic review. J. Clin. Med. 2021, 10, 2691. [Google Scholar] [CrossRef]
- Alanazy, M.H. Clinical and electrophysiological evaluation of carpal tunnel syndrome: Approach and pitfalls. Neurosci. J. 2017, 22, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, A.; Stelitano, C.; Bollani, P.; Giardini, A.; Vanzetti, E.; Ferrari, M. Carpal tunnel sonography. J. Ultrasound 2020, 23, 337–347. [Google Scholar] [CrossRef]
- McDonagh, C.; Alexander, M.; Kane, D. The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: A new paradigm. Rheumatology 2015, 54, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Gaughan, J.P.; Ilyas, A.M. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: A meta-analysis. Clin. Orthop. Relat. Res.® 2011, 469, 1089–1094. [Google Scholar] [CrossRef]
- Di Cosmo, M.; Fiorentino, M.C.; Villani, F.P.; Frontoni, E.; Smerilli, G.; Filippucci, E.; Moccia, S. A deep learning approach to median nerve evaluation in ultrasound images of carpal tunnel inlet. Med. Biol. Eng. Comput. 2022, 60, 3255–3264. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-J.; Chang, K.-V.; Lou, Y.-M.; Wu, W.-T.; Özçakar, L. Can ultrasound imaging be used for the diagnosis of carpal tunnel syndrome in diabetic patients? A systemic review and network meta-analysis. J. Neurol. 2020, 267, 1887–1895. [Google Scholar] [CrossRef]
- Ikumi, A.; Yoshii, Y.; Kudo, T.; Kohyama, S.; Ogawa, T.; Hara, Y.; Ishii, T. Potential relationships between the median nerve cross-sectional area and physical characteristics in unilateral symptomatic carpal tunnel syndrome patients. J. Clin. Med. 2023, 12, 2515. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.-E.; Cho, J.M.; Yang, J.W.; Kim, M.; Kwon, H.D. Characteristics of diabetic and non-diabetic carpal tunnel syndrome in terms of clinical, electrophysiological, and Sonographic features: A cross-sectional study. BMC Musculoskelet. Disord. 2023, 24, 739. [Google Scholar] [CrossRef]
- Martikkala, L.; Pemmari, A.; Himanen, S.; Mäkelä, K. Median Nerve Shear Wave Elastography Is Associated with the Neurophysiological Severity of Carpal Tunnel Syndrome. J. Ultrasound Med. 2024, 43, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Holováčová, D.; Kužma, M.; Killinger, Z.; Payer, J. Cross-sectional area of the median nerve is increased in primary autoimmune hypothyroidism and decreases upon treatment with thyroxine. Eur. J. Endocrinol. 2016, 175, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.; Wang, T.; Qin, K.; Teng, J.; Qi, H. Comparison of ultrasound and magnetic resonance imaging of the median nerve’s recurrent motor branch and the value of its diameter in diagnosing carpal tunnel syndrome. Quant. Imaging Med. Surg. 2024, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Won, H.-C.; Oh, J.-Y.; Kim, D.-H.; Hwang, S.-C.; Yoo, J.-I. Value of cross-sectional area of median nerve by MRI in carpal tunnel syndrome. Asian J. Surg. 2020, 43, 654–659. [Google Scholar] [CrossRef]
- Tezcan, E.A.; Levendoglu, F.; Durmaz, M.S.; Kara, H.; Batur, E.B.; Gezer, I.A.; Korez, M.K. Carpal tunnel syndrome in patients with psoriatic arthritis: Ultrasonography and magnetic resonance imaging findings. J. Rheum. Dis. 2023, 30, 36–44. [Google Scholar] [CrossRef]
- Pulikkottil, B.J.; Schub, M.; Kadow, T.R.; Wang, W.; Fowler, J.R. Correlating median nerve cross-sectional area with nerve conduction studies. J. Hand Surg. 2016, 41, 958–962. [Google Scholar] [CrossRef]
- Sezgin, M.; Incel, N.A.; Serhan, S.; Camdeviren, H.; As, I.; Erdoğan, C. Assessment of symptom severity and functional status in patients with carpal tunnel syndrome: Reliability and validity of the Turkish version of the Boston Questionnaire. Disabil. Rehabil. 2006, 28, 1281–1286. [Google Scholar] [CrossRef]
- Ng, A.; Griffith, J.; Lee, R.; Tse, W.; Wong, C.; Ho, P. Ultrasound carpal tunnel syndrome: Additional criteria for diagnosis. Clin. Radiol. 2018, 73, 214.e11–214.e18. [Google Scholar] [CrossRef]
- Cartwright, M.S.; Hobson-Webb, L.D.; Boon, A.J.; Alter, K.E.; Hunt, C.H.; Flores, V.H.; Werner, R.A.; Shook, S.J.; Thomas, T.D.; Primack, S.J.; et al. Evidence-based guideline: Neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve 2012, 46, 287–293. [Google Scholar] [CrossRef]
- Stevens, J.C. AAEM minimonograph# 26: The electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve 1997, 20, 1477–1486. [Google Scholar] [PubMed]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Aktürk, S.; Büyükavcı, R.; Ersoy, Y. Median nerve ultrasound in carpal tunnel syndrome with normal electrodiagnostic tests. Acta Neurol. Belg. 2020, 120, 43–47. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, H.K.; Kim, K.H.; Yun, H.S. Ultrasonography of median nerve and electrophysiologic severity in carpal tunnel syndrome. Ann. Rehabil. Med. 2012, 36, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kerasnoudis, A.; Pitarokoili, K.; Behrendt, V.; Gold, R.; Yoon, M. Multifocal motor neuropathy: Correlation of nerve ultrasound, electrophysiological, and clinical findings. J. Peripher. Nerv. Syst. 2014, 19, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Koh, Y.D.; Kim, J.O.; Choi, S.W. Changes in clinical symptoms, functions, and the median nerve cross-sectional area at the carpal tunnel inlet after open carpal tunnel release. Clin. Orthop. Surg. 2016, 8, 298–302. [Google Scholar] [CrossRef]
- Tajika, T.; Kobayashi, T.; Yamamoto, A.; Kaneko, T.; Takagishi, K. Diagnostic utility of sonography and correlation between sonographic and clinical findings in patients with carpal tunnel syndrome. J. Ultrasound Med. 2013, 32, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.J.T.; Chandrasekaran, R.; Prakash, A.; Mogali, S.R. A systematic review: Normative reference values of the median nerve cross-sectional area using ultrasonography in healthy individuals. Sci. Rep. 2022, 12, 9217. [Google Scholar] [CrossRef]
- Roll, S.C.; Evans, K.D.; Li, X.; Freimer, M.; Sommerich, C.M. Screening for carpal tunnel syndrome using sonography. J. Ultrasound Med. 2011, 30, 1657–1667. [Google Scholar] [CrossRef]
- Marciniak, C.; Caldera, F.; Welty, L.; Lai, J.; Lento, P.; Feldman, E.; Sered, H.; Sayeed, Y.; Plastaras, C. High-resolution median nerve sonographic measurements: Correlations with median nerve conduction studies in healthy adults. J. Ultrasound Med. 2013, 32, 2091–2098. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghasemi-Rad, M.; Mladkova-Suchy, N.; Ansari, S. Correlation between the severity of carpal tunnel syndrome and color Doppler sonography findings. Am. J. Roentgenol. 2012, 198, W181–W184. [Google Scholar] [CrossRef]
- Shiri, R. Hypothyroidism and carpal tunnel syndrome: A meta-analysis. Muscle Nerve 2014, 50, 879–883. [Google Scholar] [CrossRef]
- Yıldoğan, A.T.; Öngün, G.; Eren, F.; Kara, H.; Akkulak, A.E.; Kiraç, C.O.; Baldane, S.; Albayrak, I.; Aygül, R. Nerve Conduction Studies and Measurement of Median Nerve Cross-Sectional Area in Patients Newly Diagnosed with Hypothyroidism. Med. Rec. 2023, 5, 367–371. [Google Scholar] [CrossRef]
- Riccò, M.; Signorelli, C. Personal and occupational risk factors for carpal tunnel syndrome in meat processing industry workers in Northern Italy. Med. Pract. 2017, 68, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Güneş, M.; Büyükgöl, H. Correlation of neutrophil/lymphocyte and platelet/lymphocyte ratios with the severity of idiopathic carpal tunnel syndrome. Muscle Nerve 2020, 61, 369–374. [Google Scholar] [CrossRef]
- Altun, Y. Are C-reactive protein, neutrophil to lymphocyte and platelet to lymphocyte ratios related to the severity of idiopathic carpal tunnel syndrome? Ann. Med. Res. 2022, 29, 915–919. [Google Scholar] [CrossRef]
- Uçar, M.; Vatansever, F.; Tanık, N.; Çebiçci, M.; Sütbeyaz, S.; Sarp, Ü.; Gürbüz, K. Karpal tünel sendromu olan hastalarda ortalama trombosit hacmi ve diğer hemogram sonuçlarının karşılaştırılması. J. Clin. Exp. Investig. 2015, 6, 154–158. [Google Scholar]
| Variable | Overall (N: 62 Patients) |
|---|---|
| Age, years | 51.56 ± 9.34 |
| Gender, Female/Male, n (%) | 45/17 (72.6/27.4) |
| BMI, kg/m2 | 31.02 ± 5.26 |
| Dominant hand, Right/Left, n (%) | 58/4 (93.5/6.5) |
| Educational Status, n (%) | |
| 8 (12.9) |
| 34 (54.8) |
| 15 (24.2) |
| 5 (8.1) |
| Occupational Status, n (%) | |
| 38 (61.3) |
| 1 (1.6) |
| 4 (6.5) |
| 5 (8.1) |
| 9 (14.4) |
| 5 (8.1) |
| Comorbidity, n (%) | |
| 33 (53.2) |
| 15 (24.2) |
| 5 (8.1) |
| 4 (6.5) |
| 3 (4.8) |
| 2 (3.2) |
| Symptom duration, months | 19.9 ± 15.9 |
| Laterality, Bilateral/Unilateral, n (%) | 53/9 (85.5/14.5) |
| VAS pain (0–10) | 6.85 ± 2.46 |
| BCTQ-SSS (1–5) | 2.58 ± 0.72 |
| BCTQ-FSS (1–5) | 2.57 ± 0.90 |
| Median nerve CSA, mm2—right | 13.42 ± 4.26 (range: 6.73–26.2) |
| Median nerve CSA, mm2—left | 13.42 ± 3.84 (range: 7.88–24.4) |
| Median nerve CSA, mm2—most symptomatic side | 14.24 ± 4.34 (range: 7.43–26.2) |
| Severity Category | Hands, n (%) |
|---|---|
| Mild | 58 (52.3) |
| Moderate | 45 (40.5) |
| Severe | 8 (7.2) |
| Total | 111 (100) |
| Side | Variable | ρ (Spearman) | 95% CI | n | p | q (FDR) |
|---|---|---|---|---|---|---|
| EDX-Right | Motor wrist latency | 0.557 | 0.334–0.733 | 58 | <0.001 | <0.001 |
| EDX-Right | Sensory latency | 0.486 | 0.222–0.688 | 57 | <0.001 | 0.003 |
| EDX-Right | Motor F-latency | 0.288 | 0.003–0.544 | 56 | 0.033 | 0.039 |
| EDX-Right | Sensory amplitude | −0.411 | −0.639 to −0.144 | 60 | 0.002 | 0.004 |
| EDX-Right | Sensory velocity | −0.419 | −0.628 to −0.157 | 57 | 0.001 | 0.002 |
| EDX-Right | Motor wrist amplitude | −0.330 | −0.548 to −0.057 | 60 | 0.014 | 0.020 |
| EDX-Left | Motor wrist latency | 0.318 | 0.022–0.578 | 49 | 0.030 | 0.042 |
| EDX-Left | Motor F-latency | 0.321 | 0.043–0.570 | 49 | 0.028 | 0.042 |
| EDX-Left | Motor wrist amplitude | −0.327 | −0.572 to −0.049 | 50 | 0.025 | 0.042 |
| EDX-Left | Sensory amplitude | −0.363 | −0.596 to −0.068 | 50 | 0.012 | 0.042 |
| EDX-Left | Sensory latency | 0.319 | 0.051–0.581 | 47 | 0.029 | 0.042 |
| Cross-Sectional Area | Motor Wrist Latency | Motor Wrist Amplitude | Motor Wrist–Elbow Velocity | Motor F-Latency | Sensory Latency | Sensory Amplitude | Sensory Velocity | |
|---|---|---|---|---|---|---|---|---|
| Cross-Sectional Area | 1 | 0.557 | −0.33 | 0.021 | 0.288 | 0.486 | −0.411 | −0.419 |
| Motor Wrist Latency | <0.001 | 1 | −0.376 | −0.101 | 0.528 | 0.706 | −0.496 | −0.72 |
| Motor Wrist Amplitude | 0.014 | 0.005 | 1 | 0.166 | −0.258 | −0.34 | 0.371 | 0.328 |
| Motor Wrist–Elbow Velocity | 0.881 | 0.463 | 0.227 | 1 | −0.194 | −0.188 | −0.102 | 0.101 |
| Motor F-Latency | 0.033 | <0.001 | 0.058 | 0.155 | 1 | 0.59 | −0.505 | −0.426 |
| Sensory Latency | <0.001 | <0.001 | 0.011 | 0.17 | <0.001 | 1 | −0.433 | −0.892 |
| Sensory Amplitude | 0.002 | <0.001 | 0.005 | 0.457 | <0.001 | 0.001 | 1 | 0.378 |
| Sensory Velocity | 0.001 | <0.001 | 0.015 | 0.461 | 0.001 | <0.001 | 0.004 | 1 |
 | ||||||||
| Cross-Sectional Area | Motor Wrist Latency | Motor Wrist Amplitude | Motor Wrist–Elbow Velocity | Motor F-Latency | Sensory Latency | Sensory Amplitude | Sensory Velocity | |
|---|---|---|---|---|---|---|---|---|
| Cross-Sectional Area | 1 | 0.318 | −0.327 | −0.276 | 0.321 | 0.319 | −0.363 | −0.227 |
| Motor Wrist Latency | 0.03 | 1 | −0.401 | −0.093 | 0.67 | 0.805 | −0.683 | −0.766 |
| Motor Wrist Amplitude | 0.025 | 0.005 | 1 | 0.352 | −0.366 | −0.21 | 0.329 | 0.265 |
| Motor Wrist–Elbow Velocity | 0.06 | 0.536 | 0.015 | 1 | −0.334 | −0.101 | 0.77 | 0.26 |
| Motor F-Latency | 0.028 | <0.001 | 0.011 | 0.22 | 1 | 0.58 | −0.533 | −0.393 |
| Sensory Latency | 0.029 | <0.001 | 0.157 | 0.497 | <0.001 | 1 | −0.675 | −0.879 |
| Sensory Amplitude | 0.012 | <0.001 | 0.024 | 0.605 | <0.001 | <0.001 | 1 | 0.635 |
| Sensory Velocity | 0.124 | <0.001 | 0.072 | 0.865 | 0.006 | <0.001 | <0.001 | 1 |
 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, H.; Kaplan, H.; Aba, F.N.; Karaca, S.; Cüce, İ. Ultrasonographic Median Nerve Cross-Sectional Area and Clinical, Electrodiagnostic, and Laboratory Biomarkers in Electrodiagnostically Confirmed Carpal Tunnel Syndrome: A Single-Center Correlational Study. Diagnostics 2025, 15, 2407. https://doi.org/10.3390/diagnostics15182407
Kara H, Kaplan H, Aba FN, Karaca S, Cüce İ. Ultrasonographic Median Nerve Cross-Sectional Area and Clinical, Electrodiagnostic, and Laboratory Biomarkers in Electrodiagnostically Confirmed Carpal Tunnel Syndrome: A Single-Center Correlational Study. Diagnostics. 2025; 15(18):2407. https://doi.org/10.3390/diagnostics15182407
Chicago/Turabian StyleKara, Hasan, Hüseyin Kaplan, Fatma Nur Aba, Servin Karaca, and İsa Cüce. 2025. "Ultrasonographic Median Nerve Cross-Sectional Area and Clinical, Electrodiagnostic, and Laboratory Biomarkers in Electrodiagnostically Confirmed Carpal Tunnel Syndrome: A Single-Center Correlational Study" Diagnostics 15, no. 18: 2407. https://doi.org/10.3390/diagnostics15182407
APA StyleKara, H., Kaplan, H., Aba, F. N., Karaca, S., & Cüce, İ. (2025). Ultrasonographic Median Nerve Cross-Sectional Area and Clinical, Electrodiagnostic, and Laboratory Biomarkers in Electrodiagnostically Confirmed Carpal Tunnel Syndrome: A Single-Center Correlational Study. Diagnostics, 15(18), 2407. https://doi.org/10.3390/diagnostics15182407






