Elevated Serum Trimethylamine N-Oxide Predicts Impaired Vascular Reactivity in Patients with Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Measurements
2.3. Biochemical Analysis
2.4. Assessment of Endothelial Function
2.5. Serum TMAO Quantification by HPLC-MS
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| eNOS | Endothelial nitric oxide synthase |
| FMO3 | Flavin-containing monooxygenase 3 |
| HDL-C | High-density lipoprotein cholesterol |
| HPLC-MS | High-performance liquid chromatography-mass spectrometry |
| LDL-C | Low-density lipoprotein cholesterol |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| NO | Nitric oxide |
| SBP | Systolic blood pressure |
| TCH | Total cholesterol |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| VRI | Vascular reactivity index |
References
- Fryar, C.D.; Kit, B.; Carroll, M.D.; Afful, J. Hypertension prevalence, awareness, treatment, and control among adults age 18 and older: United States, August 2021–August 2023. NCHS Data Brief 2024, 511, CS35423. [Google Scholar]
- Sudharsanan, N.; Theilmann, M.; Kirschbaum, T.K.; Manne-Goehler, J.; Azadnajafabad, S.; Bovet, P.; Chen, S.; Damasceno, A.; De Neve, J.W.; Dorobantu, M.; et al. Variation in the proportion of adults in need of blood pressure-lowering medications by hypertension care guideline in low- and middle-income countries: A cross-sectional study of 1,037,215 individuals from 50 nationally representative surveys. Circulation 2021, 143, 991–1001. [Google Scholar] [CrossRef]
- Ma, J.; Chen, X. Advances in pathogenesis and treatment of essential hypertension. Front. Cardiovasc. Med. 2022, 9, 1003852. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial dysfunction in hypertension: Current concepts and clinical implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Savoia, C.; D’Agostino, M.; Lauri, F.; Volpe, M. Angiotensin type 2 receptor in hypertensive cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 125–132. [Google Scholar] [CrossRef]
- Murray, E.C.; Nosalski, R.; MacRitchie, N.; Tomaszewski, M.; Maffia, P.; Harrison, D.G.; Guzik, T.J. Therapeutic targeting of inflammation in hypertension: From novel mechanisms to translational perspective. Cardiovasc. Res. 2021, 117, 2589–2609. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and cardiovascular disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, W.H.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef]
- Geng, J.; Yang, C.; Wang, B.; Zhang, X.; Hu, T.; Gu, Y.; Li, J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018, 97, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-oxide: The good, the bad and the unknown. Toxins 2016, 8, 8110326. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Modulation of endothelial function by TMAO, a gut microbiota-derived metabolite. Int. J. Mol. Sci. 2023, 24, 5806. [Google Scholar] [CrossRef]
- Naghavi, M.; Yen, A.A.; Lin, A.W.; Tanaka, H.; Kleis, S. New indices of endothelial function measured by digital thermal monitoring of vascular reactivity: Data from 6084 patients registry. Int. J. Vasc. Med. 2016, 2016, 1348028. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Lin, Y.L.; Chen, Y.H.; Hung, S.C.; Liou, H.H.; Tsai, J.P.; Hsu, B.G. The association between serum trimethylamine N-oxide and arterial stiffness in chronic peritoneal dialysis patients: A cross-sectional study. Toxins 2024, 16, 523. [Google Scholar] [CrossRef]
- Chen, T.-L.; Lee, M.-C.; Ho, C.-C.; Hsu, B.-G.; Tsai, J.-P. Serum Adipocyte Fatty Acid-Binding Protein Level is Negatively Associated with Vascular Reactivity Index Measured by Digital Thermal Monitoring in Kidney Transplant Patients. Metabolites 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Busse, R.; Fleming, I. Vascular endothelium and blood flow. Handb. Exp. Pharmacol. 2006, 176 Pt 2, 43–78. [Google Scholar]
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of endothelial dysfunction, injury, and death. Annu. Rev. Pathol. 2009, 4, 71–95. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Parise, H.; Vita, J.A.; Larson, M.G.; Warner, E.; Keaney, J.F., Jr.; Keyes, M.J.; Levy, D.; Vasan, R.S.; Benjamin, E.J. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension 2004, 44, 134–139. [Google Scholar] [CrossRef]
- Higashi, Y. Endothelial function in dyslipidemia: Roles of LDL-cholesterol, HDL-cholesterol and triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef]
- Steinberg, D. Arterial metabolism of lipoproteins in relation to atherogenesis. Ann. N. Y. Acad. Sci. 1990, 598, 125–135. [Google Scholar] [CrossRef]
- Tsimikas, S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr. Atheroscler. Rep. 2006, 8, 55–61. [Google Scholar] [CrossRef]
- Kaplan, M.; Aviram, M. Oxidized low density lipoprotein: Atherogenic and proinflammatory characteristics during macrophage foam cell formation. An inhibitory role for nutritional antioxidants and serum paraoxonase. Clin. Chem. Lab. Med. 1999, 37, 777–778. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Hsu, B.G.; Lu, C.W.; Wang, J.H. Serum adiponectin level is positively associated with vascular reactivity index by digital thermal monitoring in patients with coronary artery disease. Tzu Chi Med. J. 2023, 35, 348–354. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Richey, J.J.; Zigler, M.C.; Cuevas, L.M.; Gonzalez, A.; Vázquez-Baeza, Y.; Battson, M.L.; Smithson, A.T.; Gilley, A.D.; et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019, 597, 2361–2378. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Beckman, J.A.; Shibao, C.A. Trimethylamine-N-Oxide, more red meat for the vascular scientists. Hypertension 2020, 76, 40–41. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Ren, J.; Zou, H.; Liu, Y.; Chen, Y.; Qiu, Y.; Zhuang, W.; Tao, J.; Yang, J. Association of enhanced circulating trimethylamine N-oxide with vascular endothelial dysfunction in periodontitis patients. J. Periodontol. 2022, 93, 770–779. [Google Scholar] [CrossRef]
- Boini, K.M.; Hussain, T.; Li, P.L.; Koka, S. Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell. Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef]
| Characteristics | All Participants (n = 110) | Good Vascular Reactivity (n = 43) | Intermediate Vascular Reactivity (n = 57) | Poor Vascular Reactivity (n = 10) | p Value |
|---|---|---|---|---|---|
| Age (years) | 63.29 ± 8.43 | 61.98 ± 7.61 | 63.18 ± 8.87 | 69.61 ± 6.87 | 0.010 * |
| Height (cm) | 164.96 ± 6.85 | 164.12 ± 7.00 | 165.45 ± 6.98 | 165.80 ± 5.43 | 0.487 |
| Body weight (kg) | 72.22 ± 11.79 | 71.42 ± 9.52 | 73.26 ± 13.45 | 69.77 ± 10.95 | 0.693 |
| Body mass index (kg/m2) | 26.47 ± 3.59 | 26.50 ± 3.13 | 26.66 ± 4.00 | 25.27 ± 2.99 | 0.330 |
| Vascular reactivity index | 1.86 ± 0.61 | 2.43 ± 0.35 | 1.64 ± 0.24 | 0.64 ± 0.19 | <0.001 * |
| Systolic BP (mmHg) | 136.95 ± 18.28 | 138.33 ± 18.14 | 135.58 ± 18.28 | 138.80 ± 20.13 | 0.942 |
| Diastolic BP (mmHg) | 80.31 ± 11.27 | 82.16 ± 10.86 | 79.28 ± 11.91 | 78.20 ± 8.75 | 0.319 |
| Total cholesterol (mg/dL) | 161.88 ± 39.29 | 159.95 ± 35.17 | 157.25 ± 37.02 | 196.60 ± 53.88 | 0.007 * |
| Triglyceride (mg/dL) | 131.50 (103.50–188.50) | 122.00 (102.00–184.00) | 131.00 (103.00–194.50) | 153.50 (101.50–201.75) | 0.926 |
| HDL-C (mg/dL) | 46.51 ± 11.85 | 47.51 ± 9.24 | 45.84 ± 14.18 | 46.00 ± 6.63 | 0.719 |
| LDL-C (mg/dL) | 90.44 ± 30.77 | 90.56 ± 28.29 | 85.49 ± 26.91 | 118.10 ± 47.15 | 0.009 * |
| Fasting glucose (mg/dL) | 109.00 (91.00–141.50) | 114.00 (91.00–139.00) | 105.00 (91.50–148.00) | 97.00 (77.00–136.75) | 0.321 |
| Blood urea nitrogen (mg/dL) | 17.00 (14.00–20.00) | 16.00 (14.00–18.00) | 17.00 (14.00–22.00) | 17.50 (12.75–22.75) | 0.343 |
| Creatinine (mg/dL) | 1.00 (0.90–1.10) | 1.00 (0.80–1.10) | 1.00 (0.90–1.20) | 1.00 (0.90–1.05) | 0.385 |
| eGFR (mL/min) | 77.47 ± 18.19 | 79.99 ± 16.21 | 75.34 ± 19.08 | 78.75 ± 21.35 | 0.846 |
| TMAO (μg/L) | 18.65 (11.45–28.34) | 11.86 (9.05–18.61) | 21.33 (13.92–28.35) | 49.73 (34.91–58.97) | <0.001 * |
| Male, n (%) | 96 (87.3) | 37 (86.0) | 50 (87.7) | 9 (90.0) | 0.935 |
| Diabetes mellitus, n (%) | 51 (46.4) | 21 (48.8) | 24 (42.1) | 6 (60.0) | 0.530 |
| Coronary artery disease, n (%) | 83 (75.5) | 35 (81.4) | 40 (70.2) | 8 (80.0) | 0.409 |
| Smoking, n (%) | 23 (20.9) | 12 (27.9) | 8 (14.0) | 3 (30.0) | 0.183 |
| ACE inhibitor use, n (%) | 20 (18.2) | 8 (18.6) | 11 (19.3) | 1 (10.0) | 0.778 |
| ARB use, n (%) | 51 (46.4) | 23 (53.5) | 24 (42.1) | 4 (40.0) | 0.483 |
| β-blocker use, n (%) | 55 (50.0) | 19 (44.2) | 29 (50.9) | 7 (70.0) | 0.333 |
| CCB use, n (%) | 44 (40.0) | 20 (46.5) | 19 (33.3) | 5 (50.0) | 0.328 |
| Statin use, n (%) | 87 (79.1) | 30 (69.8) | 49 (86.0) | 8 (80.0) | 0.143 |
| Fibrate use, n (%) | 6 (5.5) | 3 (7.0) | 2 (3.5) | 1 (10.0) | 0.603 |
| Model | TMAO (per 1 μg/L of Increase) for Vascular Reactivity Dysfunction | TMAO (per 1 μg/L of Increase) for Poor Vascular Reactivity | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Crude model | 1.098 (1.047–1.150) | <0.001 * | 1.176 (1.084–1.275) | <0.001 * |
| Model 1 | 1.100 (1.048–1.154) | <0.001 * | 1.174 (1.079–1.278) | <0.001 * |
| Model 2 | 1.100 (1.047–1.155) | <0.001 * | 1.580 (1.002–2.492) | 0.049 * |
| Variables | Vascular Reactivity Index | ||||
|---|---|---|---|---|---|
| Simple Regression | Multivariable Regression | ||||
| r | p Value | Beta | Adjusted r2 Change | p Value | |
| Age (years) | −0.233 | 0.014 * | – | – | – |
| Height (cm) | −0.085 | 0.375 | – | – | – |
| Body weight (kg) | −0.028 | 0.768 | – | – | – |
| Body mass index (kg/m2) | 0.027 | 0.778 | – | – | – |
| Systolic blood pressure (mmHg) | 0.038 | 0.692 | – | – | – |
| Diastolic blood pressure (mmHg) | 0.154 | 0.109 | – | – | – |
| Total cholesterol (mg/dL) | −0.229 | 0.016 * | – | – | – |
| Log-Triglyceride (mg/dL) | −0.031 | 0.748 | – | – | – |
| HDL-C (mg/dL) | 0.059 | 0.544 | – | – | – |
| LDL-C (mg/dL) | −0.176 | 0.065 | – | – | – |
| Log-Glucose (mg/dL) | 0.162 | 0.091 | – | – | – |
| Log-BUN (mg/dL) | −0.152 | 0.112 | – | – | – |
| Log-Creatinine (mg/dL) | −0.094 | 0.329 | – | – | – |
| eGFR (mL/min) | 0.089 | 0.357 | – | – | – |
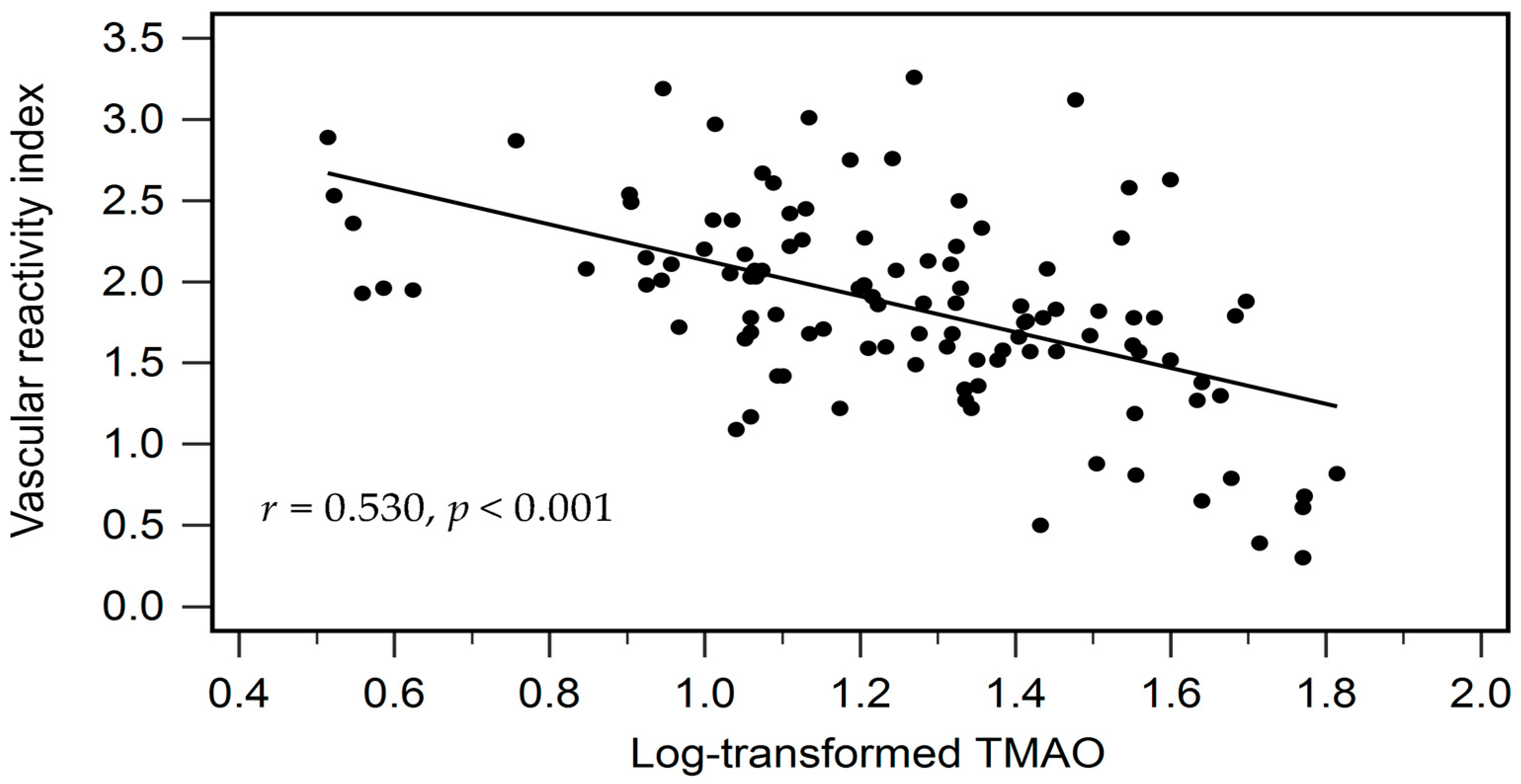
| Log-TMAO (μg/L) | −0.530 | <0.001 * | −0.530 | 0.274 | <0.001 * |
| Variables | Spearman Coefficient of Correlation | p Value |
|---|---|---|
| Age (years) | 0.191 | 0.046 * |
| Body mass index (kg/m2) | −0.066 | 0.495 |
| Vascular reactivity index | −0.530 | <0.001 * |
| Systolic blood pressure (mmHg) | −0.077 | 0.427 |
| Diastolic blood pressure (mmHg) | −0.130 | 0.176 |
| Total cholesterol (mg/dL) | 0.145 | 0.130 |
| Log-Triglyceride (mg/dL) | −0.035 | 0.714 |
| HDL-C (mg/dL) | −0.047 | 0.624 |
| LDL-C (mg/dL) | 0.060 | 0.531 |
| Log-Glucose (mg/dL) | −0.048 | 0.620 |
| eGFR (mL/min) | −0.246 | 0.010 * |
| Vascular Reactivity Dysfunction | |||||||
|---|---|---|---|---|---|---|---|
| AUC (95% CI) | p Value | Cut-Off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| TMAO (μg/L) | 0.770 (0.681–0.859) | p < 0.001 * | 13.62 | 80.60 | 65.12 | 78.26 | 68.30 |
| Poor Vascular Reactivity | |||||||
| AUC (95% CI) | p Value | Cut-Off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| TMAO (μg/L) | 0.950 (0.898–1.000) | p < 0.001 * | 26.23 | 100.0 | 79.00 | 32.26 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, I.-M.; Wang, J.-H.; Liu, C.-H.; Hsu, B.-G. Elevated Serum Trimethylamine N-Oxide Predicts Impaired Vascular Reactivity in Patients with Hypertension. Diagnostics 2025, 15, 2400. https://doi.org/10.3390/diagnostics15182400
Su I-M, Wang J-H, Liu C-H, Hsu B-G. Elevated Serum Trimethylamine N-Oxide Predicts Impaired Vascular Reactivity in Patients with Hypertension. Diagnostics. 2025; 15(18):2400. https://doi.org/10.3390/diagnostics15182400
Chicago/Turabian StyleSu, I-Min, Ji-Hung Wang, Chin-Hung Liu, and Bang-Gee Hsu. 2025. "Elevated Serum Trimethylamine N-Oxide Predicts Impaired Vascular Reactivity in Patients with Hypertension" Diagnostics 15, no. 18: 2400. https://doi.org/10.3390/diagnostics15182400
APA StyleSu, I.-M., Wang, J.-H., Liu, C.-H., & Hsu, B.-G. (2025). Elevated Serum Trimethylamine N-Oxide Predicts Impaired Vascular Reactivity in Patients with Hypertension. Diagnostics, 15(18), 2400. https://doi.org/10.3390/diagnostics15182400







