2. Case Report
A 54-year-old right-handed male, with no previous history of neurological, epileptic, or neoplastic disease, presented to our neurosurgical clinic for evaluation of a progressive neurologic syndrome marked by the subacute emergence of expressive language deficits, seizure activity, and signs of intracranial hypertension. His past medical history included grade II essential hypertension, managed inconsistently over more than a decade, and associated grade II hypertensive retinopathy. He also had a background of cervical spondylodiscopathy (radiological stage IV) and multilevel lumbar degenerative disk disease, neither of which were symptomatic at the time of presentation. There was no family history of CNS tumors, epilepsy, neurocutaneous syndromes, or hereditary cancer predisposition.
Symptom onset occurred approximately two months before evaluation, with the gradual appearance of diffuse, dull, pressure-like bifrontal headaches. Initially infrequent and responsive to NSAIDs, the headaches evolved into daily episodes, pronounced in the early morning, often accompanied by nausea, non-bilious vomiting, and brief posture-related visual obscurations. The patient did not report photophobia, phonophobia, or fever. Over time, the headaches became refractory to over-the-counter analgesics, and his wife noted a subtle change in demeanor, with increasing fatigue, reduced social interaction, and diminished verbal output. The positional nature of symptoms, in conjunction with episodic visual symptoms, raised early concern for compensated intracranial hypertension, particularly in the context of his known hypertensive microangiopathy. Approximately three weeks prior to presentation, the patient began exhibiting clear signs of expressive language dysfunction. His speech became notably hesitant, with increased word-finding difficulty, simplified grammatical structure, and frequent pauses. Despite these expressive deficits, auditory comprehension and self-monitoring were preserved. The pattern was clinically consistent with a Broca-type non-fluent aphasia, suggesting involvement of the dominant posterior inferior frontal gyrus, possibly extending into the pars opercularis and pars triangularis, with suspected disruption of connected subcortical white matter tracts—including the frontal aslant tract (FAT) and anterior segment of the arcuate fasciculus. These pathways are known to subserve fluency, initiation of speech, and syntactic integrity, and may account for the progressive deterioration observed. Ten days prior to admission, the patient experienced a first unprovoked generalized tonic–clonic seizure, reportedly beginning with right perioral paresthesia and transient speech arrest, followed by loss of consciousness and bilateral tonic–clonic movements lasting under two minutes. He remained confused for several hours and was unable to speak meaningfully during the immediate postictal period. A second seizure occurred four days later, with similar semiology, including a focal onset in the right upper limb. No antiseizure therapy was initiated before clinic evaluation, and no prior EEG or neuroimaging had been performed. The clinical semiology strongly suggested a focal seizure of left frontal origin, most likely involving the dominant opercular-insular region, with secondary generalization. At the time of clinical assessment, the patient was afebrile, normoglycemic, and hemodynamically stable, though hypertensive (BP 162/98 mmHg). He was fully alert and oriented, with no signs of confusion or delirium. His mood and affect were appropriate, and there was no evidence of disinhibition, apathy, or psychomotor slowing. General physical examination was unremarkable. Dermatologic evaluation revealed no cutaneous lesions suggestive of neurofibromatosis or other phakomatoses.
Neurological examination confirmed a moderate to severe Broca-type aphasia, characterized by non-fluent, effortful speech, marked reduction in propositional language, impaired repetition, and word-finding pauses. Naming was delayed, and sentence construction was reduced to brief, telegraphic utterances. Comprehension remained intact for simple and moderately complex verbal instructions. Reading aloud was severely impaired, though silent reading was reportedly preserved. Writing was not assessed in detail at this stage. The language profile supported cortical involvement of the dominant inferior frontal gyrus, with possible extension into nearby frontal operculum and anterior insula. Cranial nerve examination was unremarkable. Visual fields were full to confrontation. Extraocular movements were intact, and no gaze deviation or nystagmus was observed. Pupils were equal and reactive to light. Fundoscopic examination revealed bilateral grade II papilledema, with blurred optic disk margins, venous congestion, and increased vascular tortuosity, but no hemorrhages or exudates—findings supportive of evolving intracranial hypertension. Motor examination revealed mild right-sided hemiparesis, with grade 4+/5 strength in both the right upper and lower extremities, spastic tone, brisk deep tendon reflexes, and a positive Babinski sign. A pronator drift was noted in the right upper limb. No cerebellar signs were elicited. Coordination was preserved bilaterally. Sensory examination was normal for all modalities, including fine touch, pinprick, vibration, and proprioception. Gait was not assessed due to recent seizures and the need to minimize fall risk.
Despite the expressive language impairment, the patient engaged effectively with non-verbal tasks. He demonstrated preserved attention, abstract reasoning, visuospatial organization, and pattern recognition, suggesting sparing of dorsolateral prefrontal, parietal, and temporoparietal integrative networks. His behavior remained appropriate, and insight was preserved. There was no evidence of mesial frontal, orbitofrontal, or limbic disinhibition. Formal neuropsychological testing—including fluency tasks, semantic categorization, and symbolic sequencing—was planned but deferred until stabilization, given the need for urgent neuroimaging and seizure control. The patient’s systemic profile, notably his chronic hypertension and established microvascular disease, warranted consideration of small-vessel ischemic comorbidity, which could contribute to perilesional white matter changes or seizure threshold reduction. However, the pattern of clinical evolution—including progressive non-fluent aphasia, localizing seizures, right corticospinal signs, and objective papilledema—was most consistent with a space-occupying lesion in the dominant frontobasal region, exerting mass effect, producing cortical and subcortical dysfunction, and likely causing subfalcine shift. A rapidly expanding high-grade glial neoplasm, such as a GBM (IDH-wildtype), was considered the leading diagnostic possibility, based on age, semiology, and time course. Differential diagnoses included anaplastic astrocytoma, oligodendroglioma with secondary transformation, and —though less likely—primary CNS lymphoma or tumefactive demyelinating disease. Basic laboratory workup—including CBC, serum electrolytes, and renal and hepatic panels—was within normal limits, and there were no signs of systemic inflammation, metabolic instability, or infection.
The patient was admitted for further diagnostic workup, seizure prophylaxis, and early surgical planning. Contrast-enhanced magnetic resonance imaging (MRI) (
Figure 1) was immediately scheduled to characterize the lesion’s size, location, vascular behavior, potential infiltration of eloquent structures, and operability.
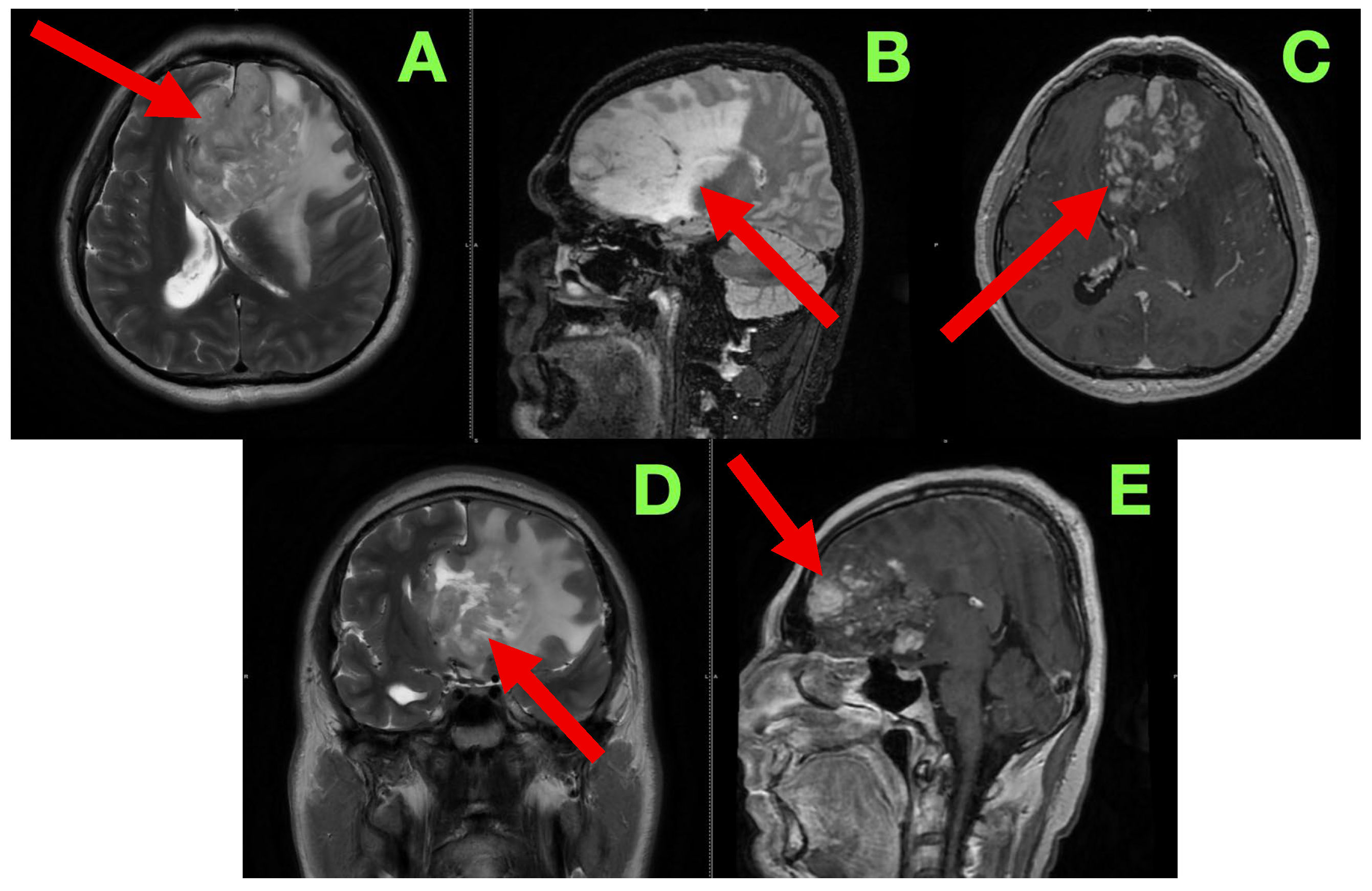
In order to better characterize the lesion and its relationship to eloquent brain structures, we performed a contrast-enhanced cranial MRI following commonly accepted practices and standard high-resolution protocol, which included T1-weighted, T2-weighted, FLAIR, and post-contrast T1 sequences, acquired in the axial, sagittal, and coronal planes. Our goal was to evaluate not only the lesion in terms of size and morphology, but patterns of infiltrative growth, behavior of contrast, edema in the perilesional area, and any early signs of herniation or distortion of local tissue—all useful information in formulating a surgical approach. The study showed a large intra-axial lesion, centrally located in the left inferior frontal lobe, extending medially across the interhemispheric fissure, and abutting potentially or involving structures including the genu of the corpus callosum, anterior cingulate gyrus, and the subcallosal area. The heterogeneity of the lesion suggested infiltrative or infiltrating capacity, with indistinct margins and associated mass effect on surrounding gyri and white matter tracts. In the axial sequences on T2-weighted images (
Figure 1A), the lesion exhibited heterogeneous hyperintensity, made up of heterogeneous, irregular, infiltrative borders. The lesion extended from the inferior frontal gyrus into the middle frontal gyrus (pars triangularis and opercularis), with evidence of perilesional signal change indicating vasogenic edema, extending inferiorly from the deep portions of the frontal lobe into the critical centro-semiovale, frontal horn, and anterior portions of the corona radiata. The left frontal horn of the lateral ventricle was compressed, and a midline shift of approximately 7 mm was observed. There was also some subtle effacement of the left Sylvian fissure and perisylvian cortex evident. The overall findings suggested not only cortical but also MCU of some important white matter tracts, particularly the FAT and pairing superior longitudinal fasciculus (SLF), and potentially less so of the anterior limb of the internal capsule. The sagittal FLAIR images offered further insight regarding vertical and anteroposterior extent of the lesion. There appeared to be deformation of the genu of the corpus callosum as it looked bowed posteriorly and superiorly. Edema extended anteriorly toward the cingulate gyrus and there was narrowing of the interhemispheric fissure. The lesion was clearly in contact with the subcallosal area and there appeared borderline indistinctness from the gyrus rectus and the olfactory sulcus as well as possible involvement of ventromedial prefrontal regions. The basal cisterns were patent but due to evident asymmetries in midline structures, likely early subfalcine shift was occurring. Axial post-contrast T1-weighted images (
Figure 1C) showed multifocal nodular enhancement but without any significant or well-formed ring enhancement or associated central necrosis. Areas of contrast uptake were seen at the superior and medial margins of the lesion and there was an area extending towards the callosal–cingulate junction. These findings could represent frank blood–brain barrier disruption and absent necrotic transformational features. There was importantly no enhancement of the ependyma or ventricular dissemination or leptomeningeal spread. Coronal T2-weighted images (
Figure 1D) confirmed inferior extent of the lesion and effacement towards the orbitofrontal cortex and adjacent anterior insula. There was good correlation of sulcal effacement to these areas. The finding appeared to laterally displace the medial frontobasal cisterns, and surrounding edema was located toward the limen insulae. These findings raised the question of possible subpial invasion with respect to language relevant structures in the perisylvian area. Beyond the limitations of the axials, the sagittal post-contrast T1 (
Figure 1E) images also demonstrated blurring between the cortical–subcortical transition in the convexity of the frontoorbital region and focal enhancement along the gyral surfaces, perhaps indicative of either superficial cortical spread or diffuse perivascular pathology. Spatially, the observed enhancement was patchy and irregular—reasonably suggestive of an infiltrative neoplastic process, but not specific. There were no signs of enhancement of the basal meningeal layers or of infratentorial structures. The third and fourth ventricles were symmetric and non-dilated.
Read in conjunction with the clinical presentation, the radiologic findings shaped our impression of an infiltrative high-grade glioma—most likely a GBM (IDH-wildtype). Out of an abundance of caution with diagnosis, we also considered the option of an anaplastic astrocytoma, as well as the potential for secondary transformation of a lower-grade glioma, and, with the least likelihood, primary CNS lymphomas or tumefactive demyelinating lesions, given the absence of necrosis and ring enhancement. The degree of mass effect and the nature of the enhancement noted seemed excessive relative to contrast uptake, which we reasoned as representing a high-grade glioma likely to have diffuse infiltrative behavior with minimal central breakdown.
The involvement of the dominant inferior frontal gyrus, in particular, with adjacent extension in and around regions absolutely necessary for speech initiation and fluency, aligns with the patient’s presentation of non-fluent aphasia. Also, even the surrounding edema encroaches toward the anterior limb of the internal capsule. This correlates with the positive right-sided corticospinal findings on examination. Additionally, we were concerned about the radiologic appearance suggesting ‘early’ subfalcine herniation. That said, at this phase, there was no evidence of distortion of the brainstem structures or uncal shift.
To prepare for surgery, we hoped to coordinate these aspects into a prognosis that yielded a functional-expanding approach. We suspected it would be difficult due to the vascularity of the lesion, the infiltrative margin, and the proximity to language cortex and the related tracts. Surgical resection was performed with the goal of achieving maximal safe debulking and was entirely reliant on knowledge of anatomy and intraoperative observation. In light of the infiltrative character of the lesion, the frontobasal depth of the lesion, and the patient’s dominant hemisphere, a traditional microsurgical approach was selected. We intentionally performed the procedure without neuronavigation, intraoperative mapping, and 5-ALA fluorescence techniques. This was with the intent of maintaining our attention on tactile and visual motors of the operation and respecting the anatomy revealed in real time, an approach honed through years of experience treating lesions in this region of the brain that often present with subtle topographic nuances. The patient was placed in a supine position with the head turned about 25–30 degrees right relative to the table and held in a three-pin cranial fixation device. The head was modestly extended to make the left frontal convexity orthogonal to the surgical field. This also satisfied our goal of using the retraction of gravity to expose the basal frontal structures with minimal displace. Our efforts were to create a working corridor to the inferior frontal gyrus, frontal pole, and subcallosal area without anteriorly deviating the hemisphere to put non-desired tension in the bridging veins and possibly separating the basal cistern structures. A left frontotemporal curvilinear scalp incision was made and a single-piece frontobasal craniotomy was raised with three burr holes (keyhole, frontal squama, and pterion) located to facilitate maximum bone removal inferiorly. The extent of the craniotomy enabled exposure from the frontal pole to the Sylvian fissure, sustained exposure to the inferior frontal sulcus, and allowed subsequent access to the adjacent opercular cortex. During elevation of the frontobasal craniotomy, the frontal sinus was carefully avoided, and no entry into the sinus cavity occurred. This minimized the risk of post-operative CSF leak or infection, which can complicate anterior cranial fossa approaches. With exposure to the cranial cavity, the dura was observed to be tense and no pulsatility could be observed, validating our interpretations from the modality imaging of suspected intracranial pressures issues. The dura mater was opened in a C-shaped anteriorly based flap, which was reflected anterolaterally and fixed to the bone lip. The brain just below it was globally edematous with pale, flattened gyri and poor sulcal definition. There was no visible exophytic component or surface discoloration, as expected for deeply seated, infiltrative lesions. To decrease cortical tension and avoid excessive retraction, the left opto-carotid cistern was opened to allow controlled CSF drainage, with direct and careful vision and microdissection of the fine arachnoid, respecting the optic nerve sheath and anterior perforators. The operating microscope was positioned and microsurgical dissection commenced. A subpial entry point was selected on the posterior segment of the left inferior frontal gyrus, carefully 1.5–2 cm anterior to the presumed precentral sulcus, lateral to the midline, and posterior to the frontal pole—a trajectory that balanced reasonably safe access and preserving motor and supplementary speech regions.
Corticotomy was performed along the inferior frontal sulcus using a fine bipolar tip and microsuction. Upon accessing the tumor tissue, it was gray-white, friable and somewhat vascular, and without any discernible capsule or margin. The consistency was soft, and aspiration brought bits of granular tissue and some microvessel ooze, suggesting a possible high grade astrocytic lesion. There was no plane of cleavage between tumor and brain, and so excision was performed carefully, developing a centripetal envelope in a piecemeal fashion, using alternating bipolar coagulation, fine suction, and blunt microdissectors.
As resection continued, the temptation to evaluate how much tumor was being resected was constantly modified by dependence upon visual, tactile, and anatomical reflexes rather than technology. Tumor tissue was described as soft, gray-white tissue, as opposed to elastic, organized tissue of spared parenchyma, and this was the first indication of operative limits. When the distinction of tumor and parenchyma became unclear and the plane of cleavage between infiltrated tissue and inflamed but functioning cortex was lost, resection would inevitably slow, and ultimately cease, as the risk of resecting eloquent tissue was now greater than any potential oncological benefit. The resection progressed in the surgical field, and the texture of the tissue and organization of spared medullary fibers provided other indications. For example, increasing resistance and firmness indicated that one had most likely entered tracts that were eloquent, especially along the posterior margin where the appearance of the anatomy located the anterior limb of the internal capsule. At this point in the operation, the method went from aspiration to preservation, where even a slight transgression could translate to a permanent and profound deficit.
Vascular structures also provided important reference points for surgical judgment and decision-making. In addition, the existence of small perforators from the anterior cerebral artery along the cingulate sulcus and small vessels off the recurrent artery of Heubner—along with veins returning to the anterior perforated substance—created anatomical demarcation lines indicating limits that can be approached but not crossed. Each of the vessels was preserved by careful sharp dissection and low energy bipolar coagulation, and the presence of the vessels represented that we had stopped resections in those planes. The anatomical borders delineated by the risks in the midline and subcallosal area were equally self-evident. At this point, the dissection was close to the genu of the corpus callosum and area of the subcallosum and was moving into the risk of crossing fornical columns or crossing ependymal surfaces. It was important to respect these deep structures because their injury would compromise memory and executive function and overall quality of life. The risk of uncontrolled bleeding was also an important limiting factor. In the areas that would have required a lot of coagulation where very delicate perforators or venous collectors were in the way to carry out the next step to aspirate, we simply left it alone. The unilateral decision was made much easier in the absence of intraoperative mapping: without awake speech and language testing, any depth of dissection that was concerning on some level for resistance, fiber orientation, and distortion of anatomy as it related to involvement of the frontal aslant tract and superior longitudinal fasciculus constituted a functional red line and was treated as such. Under the contingencies provided in this section, the decision-making evolved through intraoperative evaluations made by composite tactile sensations, visual observations, and vascular–anatomical cautions in a process that allowed the resection to proceed until all margins were determined—not on the basis of technical ability, but rather on risk related to function or anatomy.
The freedom to respect the superficial and lateral aspects of tumor mass to a level that produced decompression and a normalization of contour was possible, while the deep frontobasal and medial extensions of tumor mass were acknowledged and intentionally left in place. While the cavity itself looked grossly clean in the fields we could visualize, we made the explicit decision to respect the anatomical limitations and end up with a subtotal rather than a gross total resection. Thus, the disease that was left was not a technical limitation, but a conscious decision to respect a surgical limitation, to protect the eloquent cortex as well as vascularity, and facilitate the patient to proceed to adjuvant therapy without additional risk.
Medial advancement provided access to the pericallosal area, with the genu of the corpus callosum clearly angled posteriorly, which resulted from medial bulk from the tumor, likely. We intentionally stayed within callosal fibers and cingulate gyrus, stopping just short of direct transcallosal violation. The dissection in this corridor was particularly interesting in that it required very sharp definition of planes since many vascular landmarks were obscured by edema and gliotic reaction. Along the cingulate sulcus, we encountered three small pial perforating vessels from the anterior cerebral artery that we preserved through very sharp dissection and low energy bipolar. Inferiorly, the lesion tapped into the gyrus rectus and medial orbitofrontal cortex, with swelling almost differentiating the olfactory sulcus. We preserved the olfactory tract, noting compression, but not displacement. Special care was taken to ensure its preservation, and post-operatively the patient did not report subjective anosmia, with olfactory function appearing clinically preserved. As we continued deeper with dissection, we paid particular attention to our dissection planes with respect to the subcallosal area, where the anatomical realizations of margins would almost challenge any use of a microscope. We did not directly visualize the fornical columns but were estimating through depth and trajectory; while cognizant of our functional restraint throughout our depth, we also respected midline ependymal structures at all costs. As we dissected laterally, we were approaching the anterior insular border and did not formally enter the Sylvian fissure. At the posterior extent, the tumor was at what appeared to be an anterior limb of the internal capsule, based on tissue tensile firmness, but also the orientation of any surviving medullary fibers and the deep perforating vessels coming off a Heubner artery and anterior perforated substance. This part we treated as a silent surgical margin, where any resistance at depth and perception of risk for bleeding was interpreted as functional stop point—which again we chose to respect, stopping all surgical activity at both the deep and medial tumor extents.
We were able to safely grossly resect the more superficial and lateral portion of the tumor; however, due to vascularity, the anatomic distortion of surrounding structures, and their proximity to eloquent subcortical tracts, we intentionally left small volumes of the deep-seated frontobasal lesions intact. Although the resection cavity appeared macroscopically clean in its superficial and lateral aspects, small volumes of deep frontobasal tumor were intentionally preserved. Based on intraoperative judgment and post-operative imaging, the overall extent of resection was estimated at approximately 90–92%, corresponding to a subtotal resection. It is well recognized that earlier surgical intervention, performed before severe edema or neurological deterioration, generally facilitates safer cytoreduction and smoother post-operative recovery, whereas delayed surgery in the presence of worsening deficits or raised intracranial pressure may increase the risk of morbidity. In this case, surgery was timed promptly after diagnosis, enabling maximal safe debulking while preserving function.
A hemostatic environment was created through staged bipolar coagulation, and as required also using oxidized cellulose (Surgicel) and hemostatic matrix. After irrigating the cavity with warm Ringer’s lactate and observing no breach of an ependymal barrier or compromise of venous access or return, we conducted a final microscopic inspection and noted a clean, satisfactory hemostatic bed without any residual exophytic component. The dura was closed in a primary fashion using interrupted nonabsorbable sutures, followed by a replacement of the bone flap and layered closure. A subgaleal closed suction drain was placed. There were no complications at surgery.
The patient was transferred, intubated and hemodynamically stable, from the operating theater to the neurosurgical intensive care unit for vigilant monitoring in the immediate post-operative period. He was sedated for one hour after arrival and clinically extubated without incident, exhibiting spontaneous respiratory effort, persistent full consciousness, and a Glasgow Coma Scale score of 15. The patient was alert and oriented to person, place, and time, and able to follow a series of complex commands. The initial neurological examination yielded no new motor deficits. There was no drift, with the right hemibody strength (5/5) preserved, and no focal paresis. The cranial nerve examination was unremarkable. The patient’s language output remained hypophonic and slow, and reflective of the baseline level of expressive aphasia, with no further deterioration. There was no evidence of dysphasia, ideomotor apraxia, neglect, or visual field deficit. Pupils were equal and reactive, and there was no evidence of papilledema on fundoscopic examination. The surgical site dressing was clean, dry, and intact.
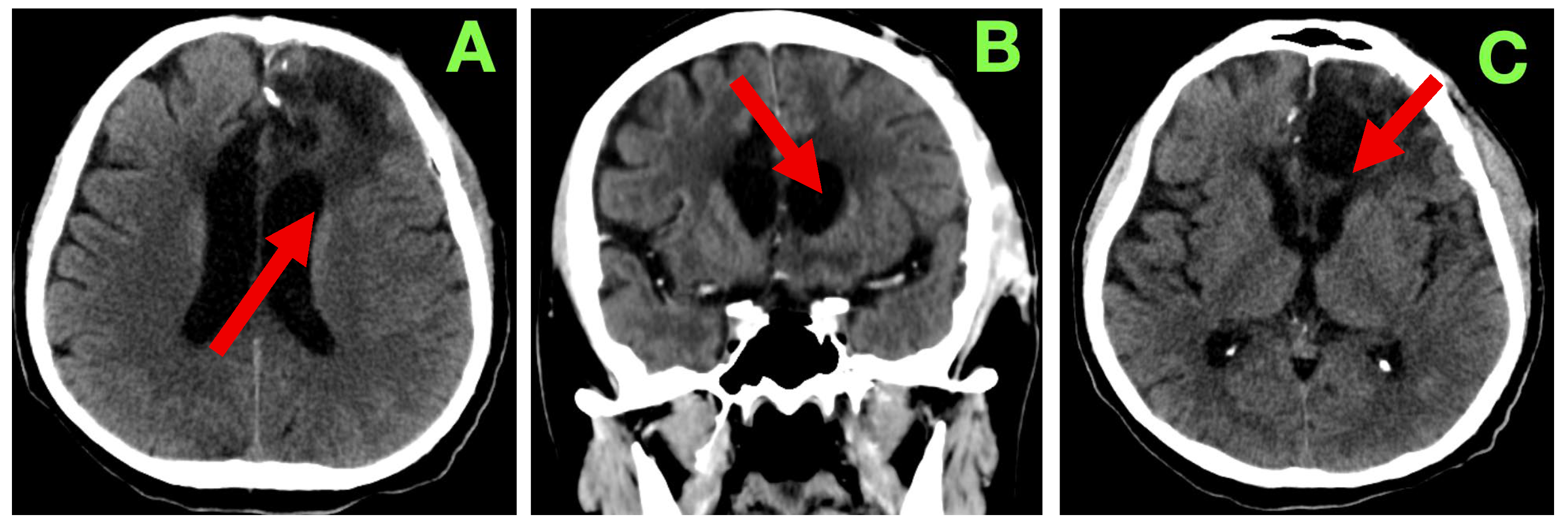
A non-contrast cranial CT scan was obtained within 4 h of the procedure to evaluate the resection cavity and rule out acute complications (
Figure 2).
The patient followed a predictable and favorable clinical course through the expected first 24 h of recovery following the procedure. Hemodynamically, all parameters were physiological: blood pressure was normotensive (MAP 85–95 mmHg), heart rate was regular (72–76 bpm), he was spontaneously breathing (unlabored at 16–18/min), oxygen saturation remained above 97% on room air, and he was afebrile. Additionally, the patient had a GCS of 15, exhibited an intact attention span, and was fully oriented in serial neurological examinations. He had no motor asymmetry, cranial nerve palsies, and no new focal deficits. There was no change in the phasic characteristics of previously observed non-fluent expressive aphasia (decreased verbal output with intact comprehension, repetition, and naming), and there was no disinhibition, irritability, or apathy. Pupils were equal and direct-light reactive bilaterally. Fundoscopy on post-operative day 1 revealed no papilledema, and there were no new visual complaints reported by the patient. A small volume of serosanguinous fluid (<20 mL) was drained from the subgaleal drain over the 24 h period. Given that there was no evidence of CSF leak, subgaleal tension or wound dehiscence, the drain was safely removed on post-operative day 2. The dressing was dry and intact, with normal apposition of the wound, and no evidence of dehiscence, infection, hematoma, or subcutaneous emphysema. Oral intake was resumed within the first 24 h, with normal swallowing and restored appetite. The patient was continent and did not experience urinary retention or bowel dysfunction. The patient was autonomously able to transition from standing to sitting in bed with assistance as needed without any orthostatic symptoms when verticalized. Laboratory values collected on day 1 post-operatively demonstrated a CRP value of 13.2 mg/L and a D-dimer value of 765 ng/mL, which were expected and within normal parameters for the early post-operative time frame. The patient’s hemoglobin value remained stable at 12.4 g/dL. The patient’s platelets, INR, aPTT, and electrolytes were normal. The patient’s liver and renal function results were within normal limits. The patient did not require any antipyretic or antimicrobial drugs. Thromboprophylaxis was started with enoxaparin 0.4 mL/day on post-operative day 1 and continued without complication. The patient remained on therapeutic anticonvulsant prophylaxis with 1000 mg/day of levetiracetam without any sedation or adverse outcomes relating to behavior. Dexamethasone was also continued at 8 mg/day and was tapered over the next ten days. The patient did not experience agitation, hyperglycemia or gastrointestinal intolerance that might have resulted in discontinuing treatment. The patient ambulated in the corridor with minimal assistance on post-operative day 3. The patient was engaged in supervised neurorehabilitation exercises to try and initiate language and executive function tasks. The patient showed good affective tone with no indications of frontal lobe dysfunction or apathy. His speech remained limited and spontaneous but purposeful. The patient was compliant and showed appreciation for his clinical course. He continued to ask questions and was fully aware of his prognosis. The patient was monitored overnight and did not experience any seizures, confusion or agitated periods. No anti-emetics were given. There were no signs of hyponatremia, and the fluid intake/output was normal. On day 5 after surgery the patient had returned to all self-care with minimal supervision. Upon examination, the surgical incision was dry, well-apposed, and was free of erythema, swelling or discharge. The staples were left intact, and no new dressing was to be applied. There was no CSF leak, subgaleal collection or delayed hemorrhage. While the clinical team anticipated a contrast-based MRI to rule out the possibility of new lesions, the team was delayed in scheduling the MRI. The clinical team ultimately decided to wait until a 3-month post-operative scan given stability neurologically, the initial clean scan, and lack of complications. On post-operative day 7 the patient was discharged home in stable condition, fully oriented cognitively, could mobilize independently, and had no new focal deficits. Upon discharge, the patient exhibited the same aphasia as prior with some level of comprehension intact and was able to produce a good deal of phrases at the same level. The patient was at full motor strength and coordination on the right (unilateral). There was no spasticity, clonus, or evidence of a pyramid release. The medications upon discharge included levetiracetam 1000 mg/day, dexamethasone (taper to 0), pantoprazole 20 mg/day, prophylactic enoxaparin, and outpatient follow-ups scheduled with neurosurgery, neurology, and neuro-oncology.
At our 3-month follow-up the patient showed clinical stability and evidence of recovery. The family indicated that there had been gradual improvement in verbal fluency; particularly, they reported spontaneous talk during routine interactions with family support. The patient was able to produce a few grammatically correct sentences but was still producing at a slower rate relatively. There were also no new neurological deficits, and the patient reported no seizures, syncope, or natural headaches. The patient was fully self-sufficient with their care, had returned to ambling without an assistive device, and the patient was contributing in a limited capacity to household items and chores.
On examination, the strength of motor function was intact bilaterally with no noticeable asymmetries or fasciculations or evident fatigability in the exam. The deep tendon reflexes were symmetric and there were flexor responses on the Planta response testing. Gait and coordination were normal on testing. On language examination, the patient showed fluent expressive output with mild anomia, but observed accuracy with respect to syntax, repetition, and reading comprehension. On cognitive assessment there was intact short-term memory, attention span, and executive function testing. As part of standard follow-up, a non-contrast cranial CT (
Figure 3) was completed at 3 months. This scan showed a stable post-operative cavity without any new enhancement in the cavity or hemorrhagic complication or indication of any new hydrocephalus. There was also considerable reduction in perilesional edema. The ventricular appearance had returned to their apparent normal appearance, with no evident midline shift/sub-dural collection/sub-acute delayed infarct. Relative to the original report, they interpreted the findings as consistent with radiological stability of the surgical site and that there continued no progression, particularly with the absence of any new findings.
With a satisfactory clinical and radiological status on assessment, and with final molecular data pending, the case was put forward for multidisciplinary review to organize adjuvant oncological therapy. The purpose of this report is to illustrate a patient’s entire clinical, surgical, and immediate follow-up history with a large left frontal intra-axial tumor including the rationale for management, anatomical considerations, and the post-operative course as is typical in neurosurgical practice. A major emphasis will be on the degree of detail in microsurgical technique that is required to achieve maximal safe resection while retaining neurological function and quality of life. By describing the patient’s journey from presentation through to three-month follow-up demonstrating radiological stability, it is hoped that the importance of multidisciplinary care and coordination of medical professionals in achieving optimal outcomes and preparing for adjuvant therapy will be exemplified.
As of this report, three-month follow-up has been established and the patient remains alive, neurologically stable, and radiologically free of progression. The functional outcomes have been good: motor strength and cognition were indexed as preserved; language abilities continue to improve; and independence of activities of daily living have been maintained. The family have reported meaningful reintegration into every day and social life reflecting an acceptable quality of life.
After multidisciplinary discussion, the patient proceeded according to standard adjuvant therapy treatment and received adjuvant chemoradiotherapy according to the Stupp protocol, which included external beam radiotherapy in combination with concomitant and adjuvant temozolomide chemotherapy. The excellent neurological recovery following surgery allowed timely initiation of therapy with no further delay, which created continuity of care in a way that met internationally accepted standards of care. Transitioning from surgery to oncological treatment is made seamless when surgical methods of tumor resection depend upon knowledge of relevant anatomy as achieved through the use of microsurgical techniques and safe cytoreduction and can be completed while preserving functional state and eligibility for standard multimodal treatment of GBM.