1. Introduction
Basal cell carcinoma (BCC) is a type of non-melanocytic skin cancer (NMSC). BCC is a malignancy of the skin originating in the basal layer of the epidermis. It develops from pluripotent stem cells in the hair follicles or interfollicular dermis without producing precancerous lesions. BCC tends to occur in areas exposed to UV radiation over a long period of time, with a predilection for the head and neck, followed by the thoracic region and the upper and lower limbs. In the literature, rare cases of BCC occurring on mucosa and soles have also been reported [
1].
The European incidence of non-melanocytic skin cancers is 14.2 per 100,000 people, with a mortality rate of 0.5, thus ranking Europe third in the world in terms of incidence and mortality rate according to the WHO Global Cancer Observatory [
2]. In Romania, the incidence is 8.6 per 100,000 inhabitants, according to the WHO Global Cancer Observatory [
2]. A closer analysis of these data shows that men have a much higher incidence and mortality rate than women. While the incidence in women is only 5.8, that in men is more than double, at 12.3, and the mortality rate for women is only 0.66 compared with 1.7 for men, which is almost 3 times greater [
2].
Over 26 different subtypes of BCC are mentioned in the literature [
3], each with distinct clinical and histological features [
4,
5]. Of these, the most common and frequently diagnosed histopathological subtypes are the nodular, micronodular, superficial, pigmented, infiltrative, and fibroepithelial (Pinkus fibroepithelioma), infundibulocystic, sclerosing (morpheaform-like), basosquamous or metatypical, and sarcomatoid subtypes [
6]. Ulceration may be present within these lesions. A lesion may also contain several of the subtypes listed above [
1,
5,
6].
In addition to the standard classification of BCC, an important characteristic of this type of skin lesion is the absence of melanin. However, some subtypes may have minimal traces of melanin [
5].
The WHO classifies (
Figure 1) BCC according to the risk of tumour recurrence [
3]. Thus, the superficial, nodular, pigmented, infundibulocystic, and fibroepithelial subtypes are included in the low risk of tumour recurrence category, while the subtypes sclerosing/morpheaform, infiltrative, basosquamous, sarcomatoid, and micronodular are included in the high risk of recurrence category. According to the 2023 classification, the presence or absence of perineural invasion may represent a risk factor. TNM staging is not used in the diagnosis of BCC due to the extremely low risk of metastasis [
1,
6].
The pathophysiology of BCC is complex and is determined by the interaction between environmental and individual factors. Among the most important aetiologies of BCC are prolonged exposure to UV rays, sunburns at a young age, and mutations in the embryonic phase of the Hedgehog (HH) pathway, which result in malignant tissue proliferation in BCC.
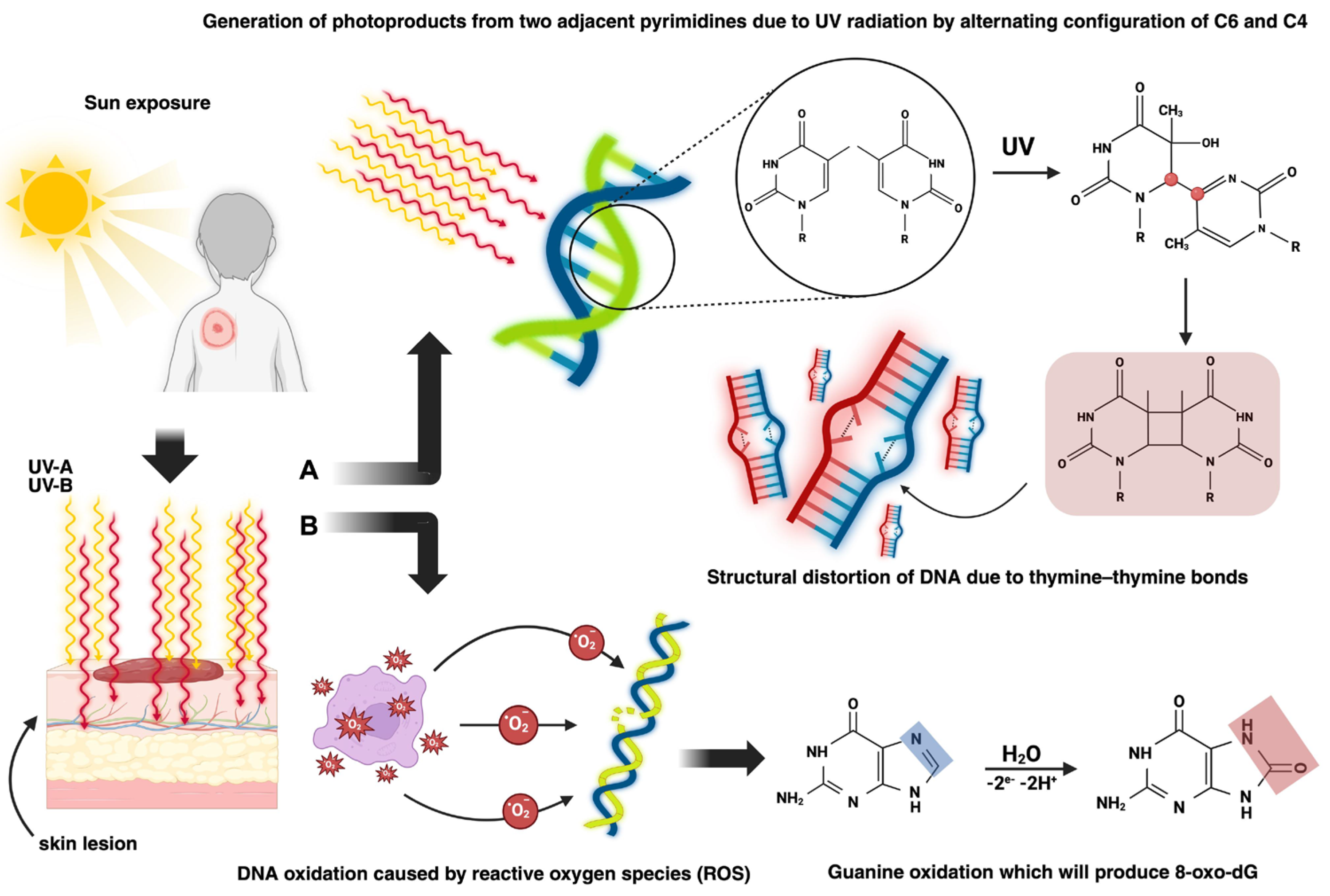
In terms of pathophysiologic mechanisms, there are two pathways through which BCC can develop (
Figure 2): exogenous causes such as UV radiation, which leads to both direct skin lesions due to the formation of cyclobutene pyrimidine dimers (CPDs) and photoproducts and indirect skin lesions produced by the interaction of reactive oxygen species (ROS) with DNA, and endogenous causes, primarily genetic mutations [
7,
8,
9,
10].
According to the concentrations of the Hedgehog protein (P-HH), in the Hedgehog (HH) pathway (
Figure 3), the synthesis of different genes is induced. Even if the HH pathway plays an important role in embryonic development, it can also influence tumoural homeostasis [
12,
13,
14,
15].
In the Canonical Hedgehog pathway (CHHP), the action of three types of P-HH occurs: Sonic (SHH), Indian (IHH), and Desert (DHH). The expression of these ligands is tissue-dependent. When one of the ligands binds to PTCH, SMO will no longer be inhibited and can phosphorylate and induce the transcription of Gli factors (Gli-1-activator, Gli-2 and Gli-3-suppressor), which is related to cell development and differentiation [
8,
9,
15].
The non-canonical Hedgehog pathway (nCHHP) can be SMO-independent, in which case both PTCH1-PHH-mediated and SMO-dependent cell proliferation affect calcium channels, chemotacticity, and cell migration, thus leading to tumoural survival and proliferation [
8,
9,
15].
BCC treatment requires a multidisciplinary and individualised approach. The treatment management strategy involves considering a variety of factors, such as location, size, and histological subtype, which may indicate a possible outcome.
Even though surgical excision is the gold standard, topical treatments with Imiquimod or 5-Fluorouracil may be considered depending on the situation when the BCC subtype has a low risk of recurrence and is less than 2 mm deep. In the case of inoperable lesions, radiotherapy and treatment with local destructive procedures such as cryotherapy or laser therapy may be taken into consideration.
Systemic treatment consists of targeted therapies against the Hedgehog pathway, represented by Hedgehog pathway inhibitors (iHHs). This treatment is used primarily in advanced BCC and when radiotherapy and surgery are not feasible options. Two molecules are approved for this category: Vismodegib, which inhibits the SMO pathway, and Sonidegib, which is similar to Vismodegib [
17].
The particularity of this study is emphasised by the high number of cases included, after the COVID-19 pandemic. Moreover, all the parameters that were analysed represent a unique combination of epidemiological and demographic characteristics in correlation with macroscopic appearance and histological characteristics. Even though in comparison to other malignancies, BCC is not as life-threatening as other cancers, this study presents correlations that might reveal a predictive pathway of evolution for each patient.
The objective of this study was to highlight the histological, epidemiological, and clinicopathological aspects of BCCs diagnosed in the Clinical Pathology Department of the Mures Clinical County Hospital between January 2021 and December 2024.
2. Materials and Methods
This was a retrospective, descriptive, and observational study conducted within the Clinical Pathology Department of the Mureș Clinical County Hospital, Targu Mureș, Romania, by analysing data from reports and histopathological slides from patients who presented a positive diagnosis of BCC.
A total of 540 lesions originating from different surgical and non-surgical wards were included in this study, which showed different macroscopic shapes of both excised fragments and tumours.
The inclusion criteria for this study consisted of patients who presented a histopathological diagnosis of BCC during the designated study period (January 2021–December 2024). The exclusion criteria consisted of all skin lesion diagnoses that were not BCC and all patients who presented a positive diagnosis of basal cell carcinoma outside the study period. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Mures Clinical County Hospital (18310/23 January 2025).
Microsoft Excel iOS v.16 was used for data management. The data collected were as follows: epidemiological data (year of diagnosis, patient’s age and gender, patient’s environment of origin—rural or urban), macroscopic features (excision site, lesion’s shape and size), microscopical characteristics (histological subtype, the presence or absence of recurrence, the presence or absence of different types of differentiation or the presence or absence of ulceration, the presence or absence of peritumoural inflammatory infiltrate, and the presence or absence of perivascular invasion or excision of the tumour formation within safe surgical limits). BCCs were grouped into simple (single histologic subtype) and mixed (more than one histologic subtype) groups.
The lesion size was converted to tumour (or excision) volume using the following calculation formula: length × width × height. When the value of height and/or width/length was missing, a value of 1 mm was arbitrarily assigned. Volumes that did not have sufficient data or at least 2 out of the 3 necessary dimensions for the calculation were excluded.
Considering the large number of head and neck formations in this study, the tumours were divided into two cohorts: the general cohort, consisting of all BCCs included in this study, and the head and neck cohort, including only BCCs in this area. The areas of tumour excision were grouped according to anatomical regions as follows: the head and neck region, anterior and posterior thoracic region, abdomino-lumbar region, upper and lower limb regions, inguinal region, perineal region, and pelvic region. Histopathological reports that did not show an excision area were put into the Unspecified category. Head and neck excision sites were further grouped as follows: Cervical (neck regions), facial (excluding the frontal region), nasal, ocular, auricular, and scalp (including the frontal region).
Statistical data analysis was performed using IBM SPSS Statistics software, version 30. The data distribution was checked by evaluating Kurtosis and Skewness parameters and considering histograms and Q-Q plots, and it corroborated Shapiro–Wilk’s test for distribution. Thus, these all confirmed the non-parametric distribution of the data. The quantitative data assessed were expressed as the median, together with the minimum–maximum range (median (min-max)), and the qualitative data were expressed as absolute values, together with relative values (n(%)).
To analyse statistically significant differences, the Spearman test for correlations of non-parametric continuous variables and the Mann–Whitney U and Kruskal–Wallis tests for comparisons of non-parametric continuous variables were performed. For categorical data, Chi
2, Monte Carlo, or Fisher’s test was used where appropriate. When more than one statistical test was performed for the same data set, the Bonferroni correction (
p′) was applied to avoid type I false positive errors, according to the following formula:
The odds ratio (OR) was calculated where appropriate with the 95% confidence interval used in medical studies (CI = 95%). The statistical significance threshold was set at α = 0.05. Only values that had relevant statistical significance were selected. For the calculation of statistical differences, an equal distribution was arbitrarily considered to obtain theoretical p-values that were deemed necessary.
To perform a statistical analysis of the categories with low frequencies (macroscopic shape, histological features, etc.), data were grouped into the category “others”.
Excel, IBM SPSS v.30, GraphPad Prism 10, and Python v.3.10 were used to perform statistical tests. Figures in the Introduction Section were generated using BioRender to visually evidence and enhance the comprehension of the underlying pathophysiological processes.
4. Discussion
BCC is a non-melanocytic skin cancer (NMSC), and it represents one of the most frequently diagnosed malignant skin lesions. Due to its paradoxical characteristics regarding the behaviour of a malignant tumour, it rarely results in metastases, and it has a mortality rate close to zero; therefore, its excision represents a type of curative therapy [
18,
19,
20].
As a result, the study of the epidemiological and histopathological aspects and the treatment of this malignancy does not garner the same scientific interest as in the case of other malignant lesions with much higher lethality, such as glioblastoma and small-cell lung carcinoma [
18,
19,
20,
21].
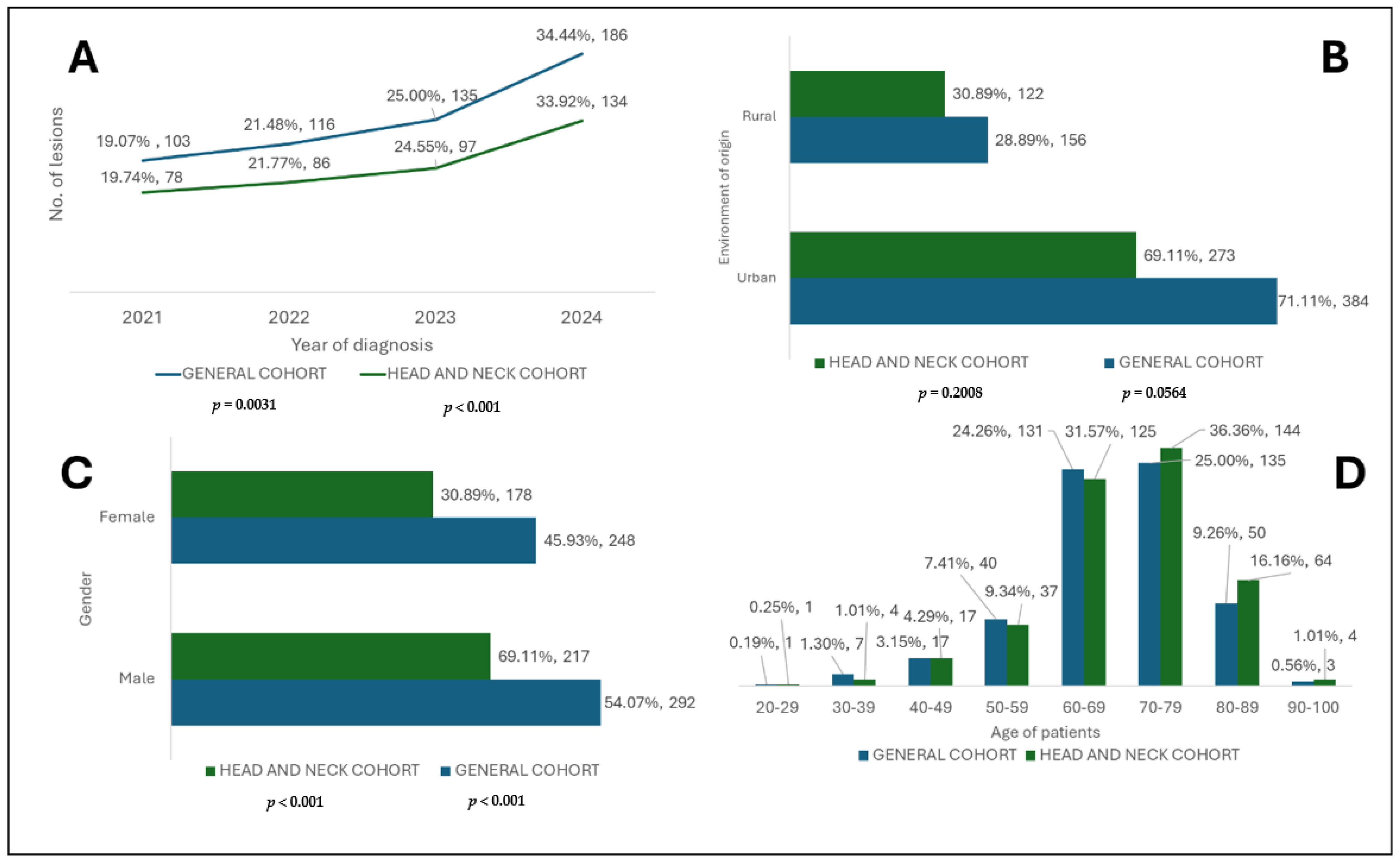
The highest incidence of BCC was registered in 2024 in both cohorts, representing more than a third of all cases (
Figure 4). This fact reflects the early screening of these lesions and a rising incidence. It can also be affirmed that the tendency of cases to be extended along with the end of the emergency state related to the COVID-19 pandemic suggests the return to normality of the healthcare system [
22,
23,
24,
25,
26]. Furthermore, climate change might also play a crucial role. Year after year, Romania registers a constant increase in temperature and critical UV sun exposure conditions and even death according to the National Institute of Meteorology (INM). If we also consider that Romanian citizens are free to move to sunnier places, this would ultimately contribute to the risks and DNA damage described [
27].
The environment of origin of the patients was significantly represented as urban areas (
p < 0.001) in both analysed cohorts. This aspect can be explained by the fact that patients from rural areas have less access to specialised medical services, and often, points of care are located at considerable distances from them compared to those from urban areas who generally benefit from easier access [
26,
28,
29].
The gender distribution of BCC between male and female patients was not significant, but a slight male predominance was observed (
Figure 4), which is consistent with the specialised literature [
30]. Furthermore, according to another study, even if at risk, males showed a positive perception about tanning, and men are mostly diagnosed with NMSC and melanocytic skin cancers [
31].
The analysis of age groups revealed a higher incidence in elderly patients, especially those in their 8th decade of life (70–79 years) in the general cohort and 7th decade (60–69 years) in the subgroup. These data underline the hypothesis of BCC carcinogenesis, in which the accumulation of cellular DNA damage caused by UV rays leads to damage, especially in elderly patients who have a substantially reduced DNA repair capacity [
32,
33]. As shown in
Figure 4, regarding the categories of younger patients, there is a relatively lower number of lesions, and the incidence starts to increase in patients over 40 years of age.
The analysis of BCC types revealed a significant predominance of the mixed form (
Table 1) in both groups (
p < 0.001), which reconfirms the morphological and architectural complexity of BCC [
34,
35,
36]. The simple or mixed form was not influenced by the patient’s gender, thus suggesting that gender cannot be considered a risk factor when it comes to a specific type of BCC, although a higher but insignificant incidence (
p > 0.05) of mixed BCC was observed among men [
34,
37,
38,
39].
The nodular histological subtype was the most common among the simple and mixed forms of BCC in both cohorts (
Table 1). The main difference was seen while analysing the second and third most common subtypes. The superficial and infiltrative types were observed in simple BCC, while the adenoid cystic and superficial ones were mainly observed in mixed BCC, and this remained the same in both cohorts. A comparative analysis of the simple and mixed types of lesion characteristics (
Table 1) showed an increased rate of tumour-free resection margins in both the general and head and neck cohorts, which is beneficial for patients.
While analysing these simple and mixed forms regarding lesion characteristics, in both the general and head and neck cohorts, the samples presented a high percentage of free resection margins, with slightly better results in the head and neck (
Table 1). With the complete excision of the lesion and free resection margins, patients no longer need to undergo further surgery, as in nearly all cases, this represents a curative intent [
7,
26,
40].
From a microscopic point of view (
Table 1), the peritumoural inflammatory infiltrate was largely present in both forms (simple and mixed BCC), as well as in the cohorts. A difference between BCC types was suggested by the presence of differentiations, squamous, sebaceous, and pillar, which were almost entirely present in the mixed forms. Ulcerations were present in a considerably higher percentage in the mixed rather than simple BCC form, which was true for both study groups [
26]. The recurrence of BCC was rare for both types—simple and mixed—independently of the analysed cohort, which is a rarity that is also confirmed by the literature [
41,
42].
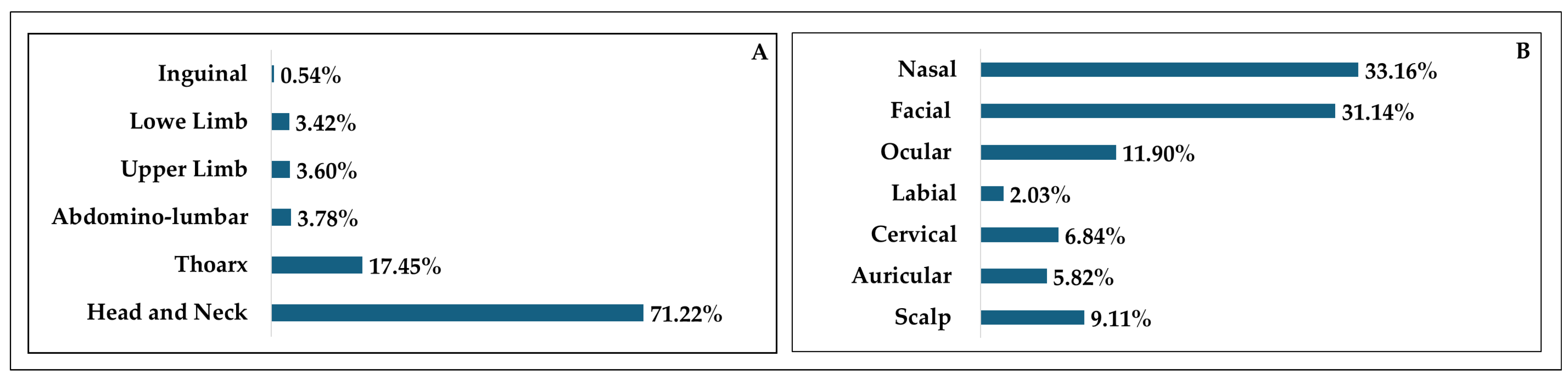
In terms of excisional sites in the general cohort (
Figure 5), it is largely seen that the head and neck area was mainly affected, followed by the thoracic region. The least affected area was the inguinal one as it is inevitably one of the most covered areas of the human body, thus shielding it from sun damage. More in depth, the head and neck cohort (
Figure 5) presented a heterogeneous distribution of excisional sites. The nasal area was mainly affected, followed by the facial regions. Interestingly, the labial area saw the lowest incidence, which might be a result of the predominance of squamous cell carcinomas rather than BCC. Furthermore, lip balms and other beauty products like lipsticks might offer slight protection against sunburns [
41].
The occurrence of histological subtypes and tumour characteristics in the simple and mixed forms of BCC were analysed in both groups (
Table 2), revealing statistically significant associations.
It was shown that the nodular subtype is significantly more common in the mixed form, which is consistent with other studies [
43]. The main difference was seen from a cohort point of view. The chance of a nodular subtype being found in mixed BCC in the head and neck was greater than 3 times more likely (OR—3.14
p < 0.001), while in the general cohort, it was double this, at 6 times more likely (OR—6.53
p < 0.001).
The micronodular subtype was present almost exclusively in the mixed form (
p < 0.001) when analysing the general cohort. It was only found in the mixed form in the head and neck, and therefore, it presented an odds ratio tending towards infinity [
44,
45].
The infiltrative subtype was observed in both the general (OR—3.58/
p = 0.005) and head and neck subgroups (OR—4.36/
p = 0.01), in which the rates of occurrence were 3 and 4, respectively. Similar results were obtained in two different studies, thus suggesting that the mixed type is associated with more aggressive forms [
34,
38].
A significant association was noted (
p < 0.001) between the mixed form of BCC and pillar differentiation, with a chance of occurrence over 5 times as high within this form in both cohorts. All these findings thus point to the architectural complexity and increased aggressiveness of the mixed type of BCC, thereby corresponding with previous research [
45].
The distribution of histological subtypes according to gender (
Table 3) did not show significant differences (
p > 0.05) in any of the analysed groups, even though some subtypes, such as the infundibulocystic and pigmented ones, were predominantly present in men, and others, such as the Pinkus subtype, were found exclusively in women. Additionally, this occurred only in the thoracic region (
Table 3), but it was above the significance threshold.
Table 3 also presents the incidence of the superficial histological subtype, in which the most affected area was the thoracic region [
39,
46,
47].
The analysis of anatomical and clinical parameters in relation to the excision sites (
Table 4) of the general cohort revealed a predominant involvement of the head and neck area but no significant differences in terms of patient age or gender (
p > 0.05) [
48]. However, there were significant differences within the general cohort in terms of environment of origin (
p = 0.0037). Even if those from both environments were predominantly affected in the head and neck area, urban patients had higher rate of chest-involved lesions compared to those from rural areas [
19,
48]. Furthermore, the superficial histological subtype had a significantly higher rate in the thoracic region compared to the other regions (
p < 0.001; the category “other regions” had the highest rate, but the number of cases in every distinct area is smaller, so we considered the thoracic region to be predominant), as shown in
Table 3 and
Table 4.
The histological characteristics and subtypes vary significantly depending on where the lesion is localised within the head and neck cohort (
p < 0.001), as shown in
Table 5. Although the nodular subtype was found in all excision sites, it predominantly affected the nasal and facial areas, while the superficial subtype showed a predilection for the same areas [
49]. The infiltrative subtype was mainly observed in the nasal and facial areas, which can be explained by the fact that these regions of the head and neck are the most exposed to UV radiation, regardless of the time of day [
50].
Histological characteristics such as non-infiltrated tumour margins and ulcerations also varied significantly (
Table 5), depending on the excision area. Ulcerations were predominantly seen in the ocular region, followed by the auricular region. This may reflect the difficulty encountered in certain anatomic areas during surgery such as the ear, in which the lowest percentage of free margins was observed [
51,
52].
The shape of the excision within the head and neck (
Table 5), as in the case of the general cohort, showed a significant association between elliptical excisional shape and free margins (
p < 0.001), thus indicating better surgical control [
53,
54]. The relationship between the macroscopic shape of the tumours and the excision areas (
Table 5) showed significant associations, as suggested by the fact that the nodular subtype was mainly associated with nodular and elevated shapes [
55], while the adenoid cystic and superficial subtypes were often associated with irregular shapes.
Ulcerated lesions were frequently observed in the nodular subtype [
6], followed by the adenoid cystic and infiltrative subtypes, which again suggests the aggressiveness and different behaviours of certain subtypes [
56].
The volumetric analysis of BCC (
Table 6) showed that in the general cohort, the tissue samples predominantly had volumes between 200 and 499 mm
3, and the tumours found in these samples predominantly had volumes smaller than 50 mm
3. These findings were also observed in the head and neck cohort even though there were slightly fewer lesions between 500 and 999 mm
3 and over 1000 mm
3, thus indicating that locations outside this subgroup have larger volumes. At the same time, the insidious nature of their appearance, marked by slow progression and reduced visibility in certain areas like the thorax or the limbs, leads to a delay in patients seeking medical attention and therefore the need for a larger excision to obtain tumour-free margins [
49,
57,
58].
Following the analysis of tumour and excision volumes according to gender, environment of origin, and year of diagnosis in both cohorts (
Table 6), it was observed that the median volumes showed significant differences in relation to certain parameters.
The gender of the patients did not influence the median tumoural volume, neither in the general cohort (
p = 0.4158) nor in the subgroup (
p = 0.6327), but men had significantly higher median volumes of excised pieces both in the general cohort (
p = 0.0189) and in the subgroup (
p = 0.0077) [
59], which may suggest a tendency for them to develop lesions with larger affected areas (
Table 6).
The median tumoural volumes of the head and neck subgroup (
Table 6) were significantly higher (
p = 0.0126) among rural patients. The general cohort also presented higher median tumoural volumes for rural patients, but the difference was only close to significance (
p = 0.0764). A further analysis of the difference in terms of the median volume of excised tissue samples in both cohorts revealed an insignificant
p-value for both the general (
p = 0.8875) and head and neck (
p = 0.1907) cohorts. Rural patients tend to have higher tumoural volumes and excisions, and it has also been widely reported that rural patients are diagnosed later, which can ultimately lead to higher tumoural volumes and dimensions compared to urban patients who have faster and easier access to healthcare providers, which might also be the case in Romania [
26,
31].
An analysis of the year of diagnosis suggested a significant downward trend in median tumour volumes in both the general cohort (
p < 0.001, decrease of 69.7%) and the subgroup (
p < 0.001, decrease of 62.7%), as can be seen in
Table 6. Even though the same was found in the case of excised tissue volumes, the differences were insignificant. This decreasing trend over the years most likely indicates a better screening campaign or easier access to treatment for skin lesions; in a digitalised world, access to information is easier and awareness can be easily raised [
60].
Table 6 supports and reinforces the observations identified. A significant negative correlation was observed in terms of tumoural volumes and the year of diagnosis, in both the general cohort and subgroup, which supports the hypothesis of earlier screening and the improved quality of medical services in this direction [
61]. The same significant negative correlation is also repeated in the case of excised tissue volumes in both groups. In terms of age, both the general and head and neck cohorts presented a positive correlation with tumour and excised tissue sample volumes. This indicates that older patients tend to have larger excisional volumes and therefore require more extensive resections and care, as supported by the literature [
62].
Tumour and excision volumes were analysed in relation to the forms of BCC (
Table 7) in both cohorts. Mixed BCC presented higher median volumes in terms of both tumour and excised tissue samples, in both the general and head and neck groups. This shows that the type of BCC (simple or mixed) does not influence tumour or excision volume. The literature suggests that the mixed type tends to be significantly larger than other forms of BCC [
34,
63], and these differences may be the result of different patient samples or even larger groups of patients. In our study, the differences found were insignificant; only the tumour volumes in the general cohort had a
p-value close to significance (
p = 0.0889).
In the general cohort, both tumoural and excised tissue sample volumes were analysed in relation to the excisional sites. Significant differences were found in both cases (
p = 0.0043 and
p < 0.0001) (
Table 7), and the largest medians were found in the thoracic region compared to other regions. This reaffirms that less obvious lesions tend to be identified later and therefore lead to larger volumes at the time of diagnosis [
64,
65]. Continuing the same volumetric analysis in the head and neck cohort, significant differences were identified in terms of excisional sites and the volumes of both tumours (
p = 0.0681) and excised tissue samples (
p < 0.0001) (
Table 7).
Labial-located lesions had the highest median volume in both tumoural and excision samples. These aspects are found in the medical literature, which confirms the rarity of labial cases, but larger volumes are present at this level [
66]. Nasal BCC presented the smallest median volume of excised tissue samples, while auricular region lesions presented the smallest median tumoural volume [
67]. The age of patients showed significant differences (
p = 0.0172) in terms of the excision area in the head and neck cohort (
Table 8). The highest median age was found in labial excisions, while the lowest was observed in the facial region. This leads to the fact that older people tend to not only have more frequent labial lesions but also present larger tumoural volumes that often result in larger excisions as well.
The tumour volume and the number of pieces in the groups were analysed in relation to histological subtypes and tumour characteristics (
Table 9). In the general cohort, no statistically significant values were found with the subtypes or characteristics, in terms of either tumour or excisional samples (
p > 0.05). Regarding the head and neck subgroup, the
p-values of the excisional volumes with both parameters were above 0.05. The same was observed for tumour volumes, but their analysis with histological subtypes was close to statistical significance (
p = 0.0573). The highest median in the general cohort was observed in BCC that presented trichilemmal differentiation, while the smallest median was observed in pigmented BCC [
68,
69].
Study limitations: Our study has several limitations. It is a retrospective study, and the variables of interest or confusion factors cannot be controlled. Furthermore, this study was conducted in a single centre on BCC cases at the Mureș County Clinical Hospital, which may lead to geographical bias. Furthermore, certain data, such as lesion volumes, were calculated arbitrarily when only two dimensions were available, which may introduce a measurement error. For lesion types that had low frequencies, they were grouped into the “others” category, which may lead to a loss of granularity. Additionally, for certain parameters, such as certain histological subtypes or anatomical areas, there were sparse data in both cohorts. Thus, there may be a significant reduction in statistical power. Another limitation is that there is no data on exposure to risk factors or the follow-up of these patients.