Kyoto Classification-Based Predictive Factors Associated with the Development of Gastric Cancer After Helicobacter pylori Eradication: A Prospective Multicenter Observational Study
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hori, M.; Matsuda, T.; Shibata, A.; Katanoda, K.; Sobue, T.; Nishimoto, H.; Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn. J. Clin. Oncol. 2015, 45, 884–891. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Herrero, R.; Park, J.Y.; Forman, D. The fight against gastric cancer—The IARC Working Group report. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1107–1114. [Google Scholar] [CrossRef]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef]
- Saka, A.; Yagi, K.; Nimura, S. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer 2016, 19, 524–530. [Google Scholar] [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Yoshida, T.; Ohara, N.; Yokota, K.; Oguma, K.; Okada, H.; Yamamoto, K. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J. Gastroenterol. 2011, 46, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P.; et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Haruma, K.; Inoue, K.; Shiotani, A. Helicobacter pylori infection and endoscopic gastritis-Kyoto classification of gastritis. Nihon Shokakibyo Gakkai Zasshi 2015, 112, 982–993. [Google Scholar] [PubMed]
- Masuyama, H.; Yoshitake, N.; Sasai, T.; Nakamura, T.; Masuyama, A.; Zuiki, T.; Kurashina, K.; Mieda, M.; Sunada, K.; Yamamoto, H.; et al. Relationship between the degree of endoscopic atrophy of the gastric mucosa and carcinogenic risk. Digestion 2015, 91, 30–36. [Google Scholar] [CrossRef]
- Spence, A.D.; Cardwell, C.R.; McMenamin, U.C.; Hicks, B.M.; Johnston, B.T.; Murray, L.J.; Coleman, H.G. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: A systematic review. BMC Gastroenterol. 2017, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Moribata, K.; Kato, J.; Iguchi, M.; Nakachi, K.; Maeda, Y.; Shingaki, N.; Niwa, T.; Deguchi, H.; Inoue, I.; Maekita, T.; et al. Endoscopic features associated with development of metachronous gastric cancer in patients who underwent endoscopic resection followed by Helicobacter pylori eradication. Dig. Endosc. 2016, 28, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Majima, A.; Dohi, O.; Takayama, S.; Hirose, R.; Inoue, K.; Yoshida, N.; Kamada, K.; Uchiyama, K.; Ishikawa, T.; Takagi, T.; et al. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest. Endosc. 2019, 90, 763–769. [Google Scholar] [CrossRef]
- Kawamura, M.; Uedo, N.; Koike, T.; Kanesaka, T.; Hatta, W.; Ogata, Y.; Oikawa, T.; Iwai, W.; Yokosawa, S.; Honda, J.; et al. Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Dig. Endosc. 2022, 34, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; Nakajima, T.; Asada, K.; Shimazu, T.; Yamamichi, N.; Maekita, T.; Yokoi, C.; Fujishiro, M.; Gotoda, T.; Ichinose, M.; et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: Results of a large-scale, multicenter cohort study in Japan. Gastric Cancer 2016, 19, 911–918. [Google Scholar] [CrossRef]
- Kamada, T.; Hata, J.; Sugiu, K.; Kusunoki, H.; Ito, M.; Tanaka, S.; Inoue, K.; Kawamura, Y.; Chayama, K.; Haruma, K. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: Results from a 9-year prospective follow-up study in Japan. Aliment. Pharmacol. Ther. 2005, 21, 1121–1126. [Google Scholar] [CrossRef]
- Majima, A.; Handa, O.; Naito, Y.; Dohi, O.; Okayama, T.; Yoshida, N.; Kamada, K.; Katada, K.; Uchiyama, K.; Ishikawa, T.; et al. Early-stage gastric cancer can be found in improved atrophic mucosa over time from successful Helicobacter pylori eradication. Digestion 2017, 95, 194–200. [Google Scholar] [CrossRef]
- Nakata, R.; Nagami, Y.; Hashimoto, A.; Sakai, T.; Ominami, M.; Fukunaga, S.; Otani, K.; Hosomi, S.; Tanaka, F.; Ohira, M.; et al. Successful eradication of Helicobacter pylori could prevent metachronous gastric cancer: A propensity matching analysis. Digestion 2021, 102, 236–245. [Google Scholar] [CrossRef]
- Yao, K. The endoscopic diagnosis of early gastric cancer. Ann. Gastroenterol. 2013, 26, 11–22. [Google Scholar]
- Kimura, K.; Takemoto, T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Tytgat, G.N. The Sydney System: Endoscopic division. Endoscopic appearances in gastritis/duodenitis. J. Gastroenterol. Hepatol. 1991, 6, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, O.; Nishizawa, T.; Koike, K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J. Gastroenterol. 2020, 26, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef]
- Shibukawa, N.; Ouchi, S.; Wakamatsu, S.; Wakahara, Y.; Kaneko, A. Gastric xanthoma is a predictive marker for early gastric cancer detected after Helicobacter pylori eradication. Intern. Med. 2019, 58, 779–784. [Google Scholar] [CrossRef]
- Yoshii, S.; Mabe, K.; Watano, K.; Ohno, M.; Matsumoto, M.; Ono, S.; Kudo, T.; Nojima, M.; Kato, M.; Sakamoto, N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig. Endosc. 2020, 32, 74–83. [Google Scholar] [CrossRef]
- Kotachi, T.; Ito, M.; Boda, T.; Kiso, M.; Masuda, K.; Hata, K.; Kawamura, T.; Sanomura, Y.; Yoshihara, M.; Tanaka, S.; et al. Clinical significance of reddish depressed lesions observed in the gastric mucosa after Helicobacter pylori eradication. Digestion 2018, 98, 48–55. [Google Scholar] [CrossRef]
- Nagata, N.; Shimbo, T.; Akiyama, J.; Nakashima, R.; Kim, H.H.; Yoshida, T.; Hoshimoto, K.; Uemura, N. Predictability of gastric intestinal metaplasia by mottled patchy erythema seen on endoscopy. Gastroenterol. Res. 2011, 4, 203–209. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M.; et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef]
- Yoshida, N.; Doyama, H.; Yano, T.; Horimatsu, T.; Uedo, N.; Yamamoto, Y.; Kakushima, N.; Kanzaki, H.; Hori, S.; Yao, K.; et al. Early gastric cancer detection in high-risk patients: A multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut 2021, 70, 67–75. [Google Scholar] [CrossRef]
- Kadota, T.; Abe, S.; Uedo, N.; Doyama, H.; Furue, Y.; Muto, M.; Nonaka, S.; Takamaru, H.; Murano, T.; Nakajo, K.; et al. Comparison of Effective Imaging Modalities for Detecting Gastric Neoplasms: A Randomized 3-Arm Phase II Trial. Am. J. Gastroenterol. 2024, 119, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Dohi, O.; Yagi, N.; Naito, Y.; Fukui, A.; Gen, Y.; Iwai, N.; Ueda, T.; Yoshida, N.; Kamada, K.; Uchiyama, K.; et al. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: A randomized controlled study. Gastrointest. Endosc. 2019, 89, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Kawada, K.; Dohi, O.; Kitamura, S.; Koike, T.; Hori, S.; Kanzaki, H.; Murao, T.; Yagi, N.; Sasaki, F.; et al. Linked Color Imaging Focused on Neoplasm Detection in the Upper Gastrointestinal Tract: A Randomized Trial. Ann. Intern. Med. 2021, 174, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Ida, K.; Terao, S.; Adachi, K.; Kato, T.; Watanabe, H.; Shimbo, T.; Research Group for Establishment of Endoscopic Diagnosis of Chronic Gastritis. Endoscopic diagnosis of gastric mucosal atrophy: Multicenter prospective study. Dig. Endosc. 2014, 26, 709–719. [Google Scholar] [CrossRef]
- Dohi, O.; Majima, A.; Naito, Y.; Yoshida, T.; Ishida, T.; Azuma, Y.; Kitae, H.; Matsumura, S.; Mizuno, N.; Yoshida, N.; et al. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig. Endosc. 2020, 32, 191–203. [Google Scholar] [CrossRef]
- Ono, S.; Kato, M.; Tsuda, M.; Miyamoto, S.; Abiko, S.; Shimizu, Y.; Sakamoto, N. Lavender color in linked color imaging enables noninvasive detection of gastric intestinal metaplasia. Digestion 2018, 98, 222–230. [Google Scholar] [CrossRef]
- Takeda, T.; Asaoka, D.; Nojiri, S.; Nishiyama, M.; Ikeda, A.; Yatagai, N.; Ishizuka, K.; Hiromoto, T.; Okubo, S.; Suzuki, M.; et al. Linked color imaging and the Kyoto classification of gastritis: Evaluation of visibility and inter-rater reliability. Digestion 2020, 101, 598–607. [Google Scholar] [CrossRef]

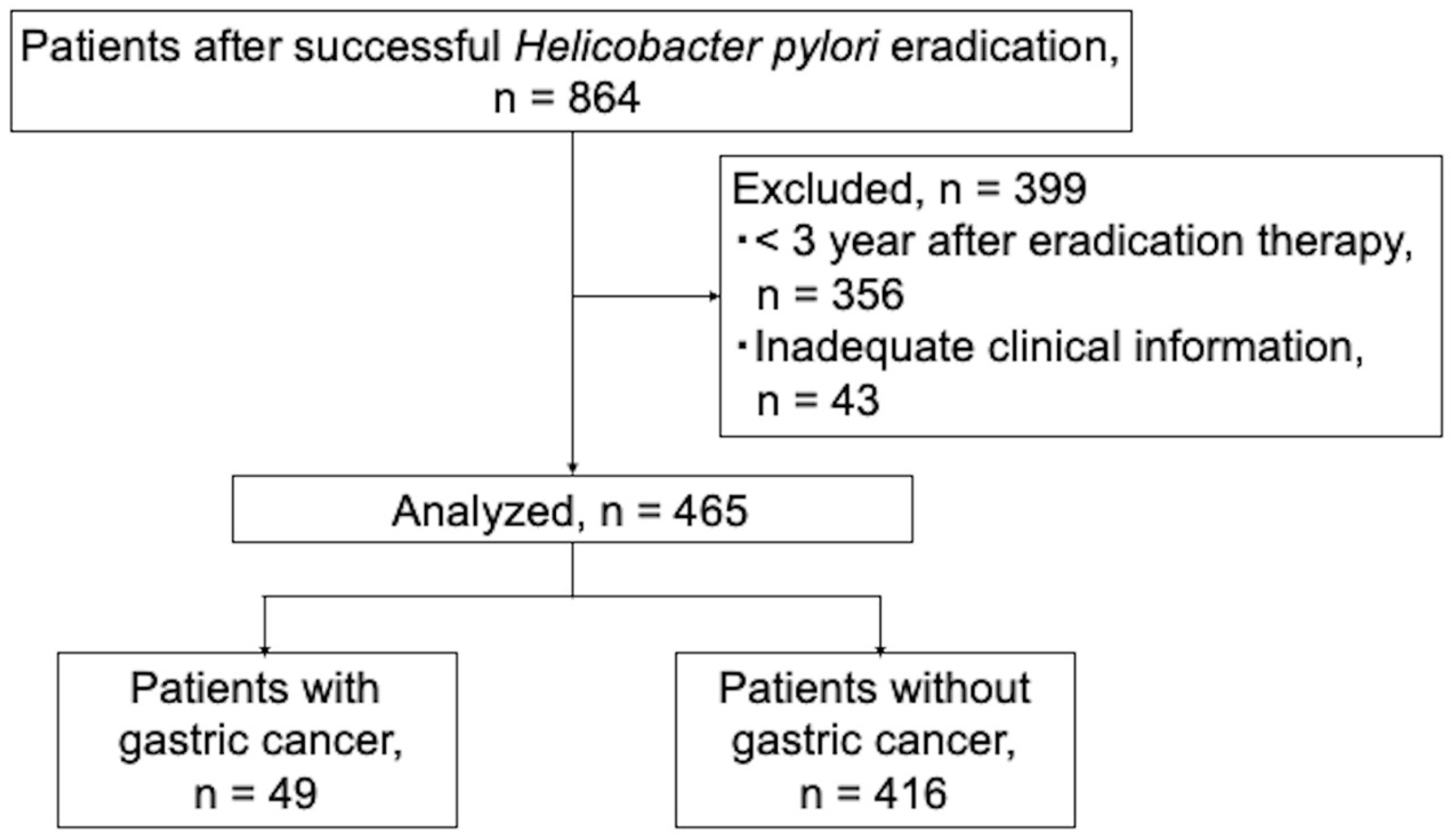

| Variables | Overall, n = 465 | Cancer, n = 49 | Non-Cancer, n = 416 | p Value, Cancer vs. Non-Cancer | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 252 (54.2) | 41 (83.7) | 211 (50.7) | <0.001 | |
| Female | 213 (45.8) | 8 (16.3) | 205 (49.3) | ||
| Age of eradication, median, years (IQR) | 65 (58–71) | 70 (66–74) | 65 (57–70) | <0.001 | |
| Body mass index, median, kg/m2 (IQR) | 22.5 (20.3–24.6) | 23.3 (20.5–25.0) | 22.4 (20.3–24.6) | 0.440 | |
| Past history, n (%) | <0.001 | ||||
| Chronic gastritis | 304 (65.4) | 15 (30.6) | 289 (69.5) | ||
| Peptic ulcer | 44 (9.5) | 2 (4.1) | 42 (10.1) | ||
| Early gastric cancer | 115 (24.7) | 32 (65.3) | 83 (19.9) | ||
| MALT lymphoma | 2 (0.4) | 0 | 2 (0.5) | ||
| Comorbidity, n (%) | |||||
| Hypertension | 141 (30.3) | 24 (49.0) | 106 (25.5) | 0.002 | |
| Dyslipidemia | 102 (21.9) | 16 (32.7) | 98 (23.6) | 0.259 | |
| Diabetes | 44 (9.5) | 8 (16.3) | 34 (8.2) | 0.119 | |
| Smoking history, n (%) | <0.001 | ||||
| None | 299 (64.3) | 18 (36.7) | 280 (67.3) | ||
| Current/former | 166 (35.7) | 31 (63.3) | 136 (32.7) | ||
| Drinking history, n (%) | 0.002 | ||||
| None | 222 (47.7) | 13 (26.5) | 208 (50.0) | ||
| Current/former | 243 (52.3) | 36 (73.5) | 208 (50.0) | ||
| Proton pump inhibitor, n (%) | 48 (10.3) | 7 (14.3) | 41 (9.9) | 0.510 | |
| Time to first endoscopy, median, year (IQR) | 0.94 (0.74–1.17) | 0.88 (0.74–1.10) | 0.97 (0.75–1.23) | 0.320 | |
| Follow-up period, median, year (IQR) | 6.06 (4.53–7.72) | 7.82 (5.73–8.59) | 6.02 (4.38–7.51) | <0.001 | |
| Endoscopic Findings | Cancer, n = 49 | Non-Cancer, n = 416 | p Value |
|---|---|---|---|
| Atrophy, A2, n (%) | 45 (91.8) | 275 (66.1) | 0.035 |
| Intestinal metaplasia, IM2, n (%) | 29 (59.2) | 146 (35.1) | 0.003 |
| Enlarged fold, n (%) | 3 (6.1) | 19 (4.6) | 0.925 |
| Nodularity, n (%) | 0 (0) | 15 (3.6) | 0.346 |
| Diffuse redness, D2, n (%) | 3 (6.1) | 29 (7.0) | 1 |
| Foveolar hyperplastic polyp, n (%) | 5 (4.9) | 22 (5.3) | 0.307 |
| Patchy redness, n (%) | 24 (49.0) | 161 (38.7) | 0.270 |
| Xanthoma, n (%) | 19 (38.8) | 93 (22.3) | 0.024 |
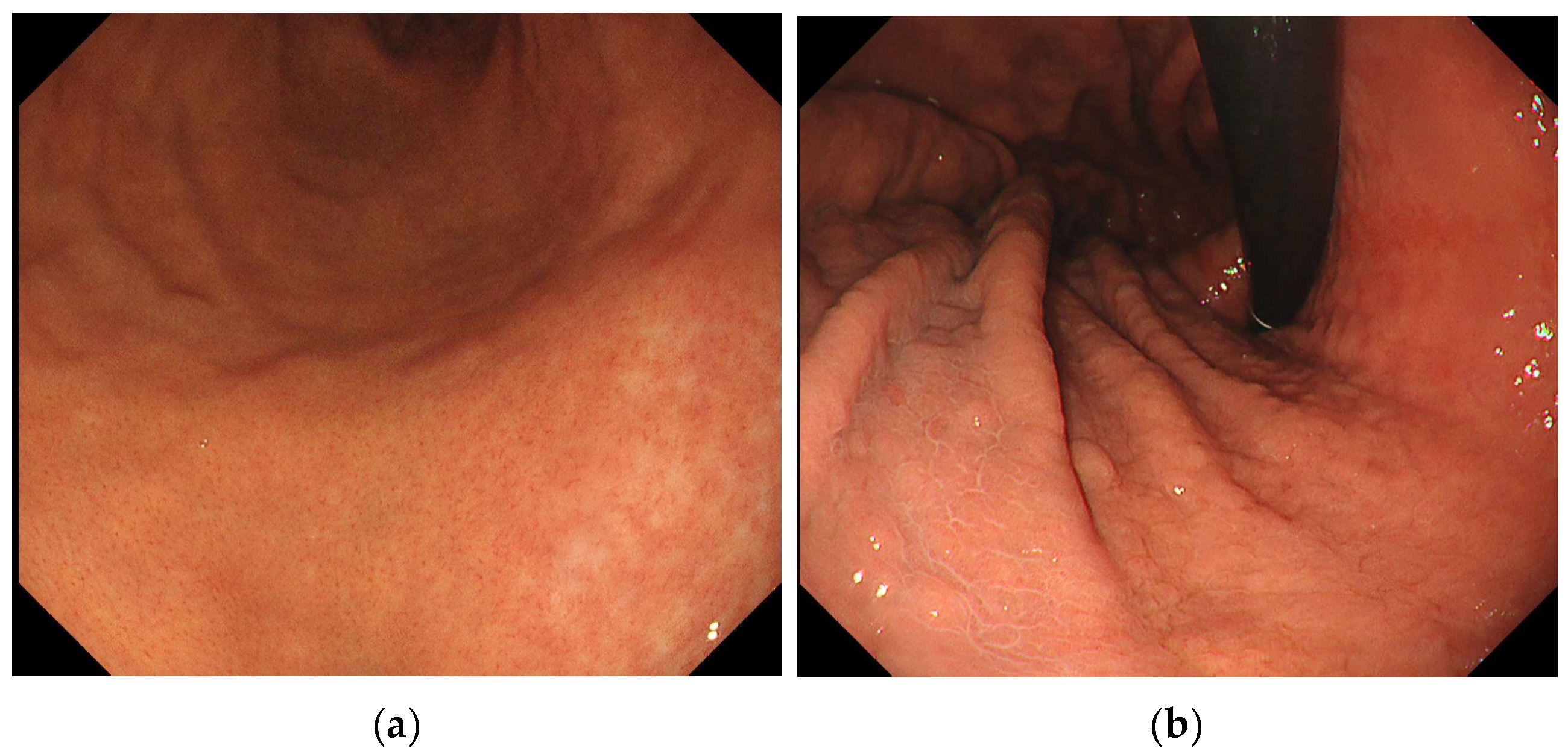
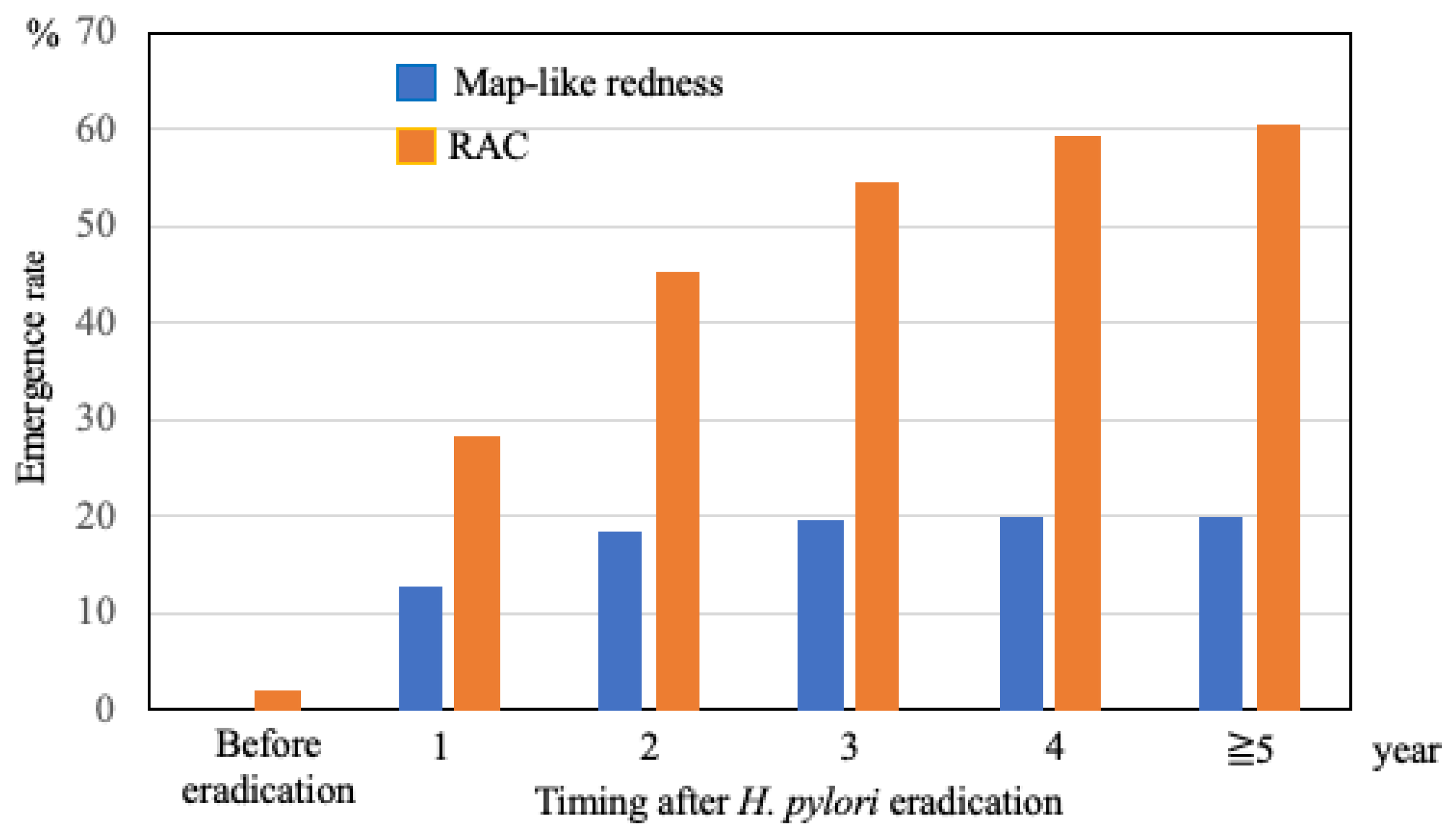
| Map-like redness, n (%) | 18 (36.7) | 47 (11.3) | <0.001 |
| Invisible RAC, n (%) | 46 (93.9) | 263 (63.2) | <0.001 |
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| Atrophy, A2 | 2.842 | 0.978–8.259 | 0.055 |
| Intestinal metaplasia, IM2 | 1.621 | 0.889–2.955 | 0.115 |
| Xanthoma | 1.548 | 0.867–2.765 | 0.140 |
| Map-like redness | 2.561 | 1.362–4.572 | 0.003 |
| Invisible RAC | 3.131 | 1.078–9.091 | 0.036 |
| Characteristics/Findings | 49 Patients with 64 Gastric Cancers | |
|---|---|---|
| Sex, n (%) | ||
| Male | 41 (83.7) | |
| Female | 8 (16.3) | |
| Age, median, years (IQR) | 70 (66–74) | |
| Lesion size, median, mm, (IQR) | 8.0 (6.0–13.8) | |
| Macroscopic type, n (%) | ||
| Elevated | 12 (18.8) | |
| Flat | 8 (12.5) | |
| Depressed | 44 (68.7) | |
| Location, n (%) | ||
| Upper third | 11 (17.2) | |
| Middle third | 34 (53.1) | |
| Lower third | 19 (29.7) | |
| Histological type, n (%) | ||
| Well-differentiated adenocarcinoma | 59 (92.1) | |
| Moderately differentiated adenocarcinoma | 4 (6.3) | |
| Poorly adenocarcinoma | 1 (1.6) | |
| Depth of invasion, n (%) | ||
| Intramucosa | 60 (93.7) | |
| Submucosa, superficial | 3 (4.7) | |
| Submucosa, deep | 1 (1.6) | |
| Treatment, n (%) | ||
| ESD | 63 (98.4) | |
| Surgery | 1 (1.6) | |
| Median time to detection, year, (range) | 3.8 (1.8–6.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayama, S.; Dohi, O.; Horie, R.; Yasuda, T.; Ochiai, T.; Iwai, N.; Imamoto, E.; Takagi, T.; Handa, O.; Konishi, H.; et al. Kyoto Classification-Based Predictive Factors Associated with the Development of Gastric Cancer After Helicobacter pylori Eradication: A Prospective Multicenter Observational Study. Diagnostics 2025, 15, 2376. https://doi.org/10.3390/diagnostics15182376
Takayama S, Dohi O, Horie R, Yasuda T, Ochiai T, Iwai N, Imamoto E, Takagi T, Handa O, Konishi H, et al. Kyoto Classification-Based Predictive Factors Associated with the Development of Gastric Cancer After Helicobacter pylori Eradication: A Prospective Multicenter Observational Study. Diagnostics. 2025; 15(18):2376. https://doi.org/10.3390/diagnostics15182376
Chicago/Turabian StyleTakayama, Shun, Osamu Dohi, Ryusuke Horie, Takeshi Yasuda, Tomoko Ochiai, Naoto Iwai, Eiko Imamoto, Tomohisa Takagi, Osamu Handa, Hideyuki Konishi, and et al. 2025. "Kyoto Classification-Based Predictive Factors Associated with the Development of Gastric Cancer After Helicobacter pylori Eradication: A Prospective Multicenter Observational Study" Diagnostics 15, no. 18: 2376. https://doi.org/10.3390/diagnostics15182376
APA StyleTakayama, S., Dohi, O., Horie, R., Yasuda, T., Ochiai, T., Iwai, N., Imamoto, E., Takagi, T., Handa, O., Konishi, H., Ando, T., Naito, Y., Takemura, T., & Itoh, Y. (2025). Kyoto Classification-Based Predictive Factors Associated with the Development of Gastric Cancer After Helicobacter pylori Eradication: A Prospective Multicenter Observational Study. Diagnostics, 15(18), 2376. https://doi.org/10.3390/diagnostics15182376








