Early Detection of Subclinical Myocardial Dysfunction in Familial Dilated Cardiomyopathy Using Myocardial Work Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Asymptomatic status with either positive (G+) or negative (G−) genotype (no history of heart failure symptoms, and no activity limitation as per clinical records).
- No echocardiographic evidence of left ventricular dilatation, defined as left ventricular (LV) end-diastolic dimension (EDD) of >56 mm and/or LV end-diastolic volume indexed (EDVi) > 79 mL/m2 in males, and LV EDD > 51 mm and/or LV EDVi > 70 mL/m2 in females, or systolic impairment with a LV ejection fraction (EF) measured on echocardiography using Simpson’s biplane of <53%.
- Available genetic screening results.
2.2. Transthoracic Echocardiography
2.3. Statistical Analysis
2.4. Groups Comparison and Correlation Analysis
2.5. Regression Analysis
2.6. ROC Curve Analysis
2.7. Statistical Significance
3. Results
3.1. Myocardial Work in Genotype-Positive, Phenotype-Negative Individuals
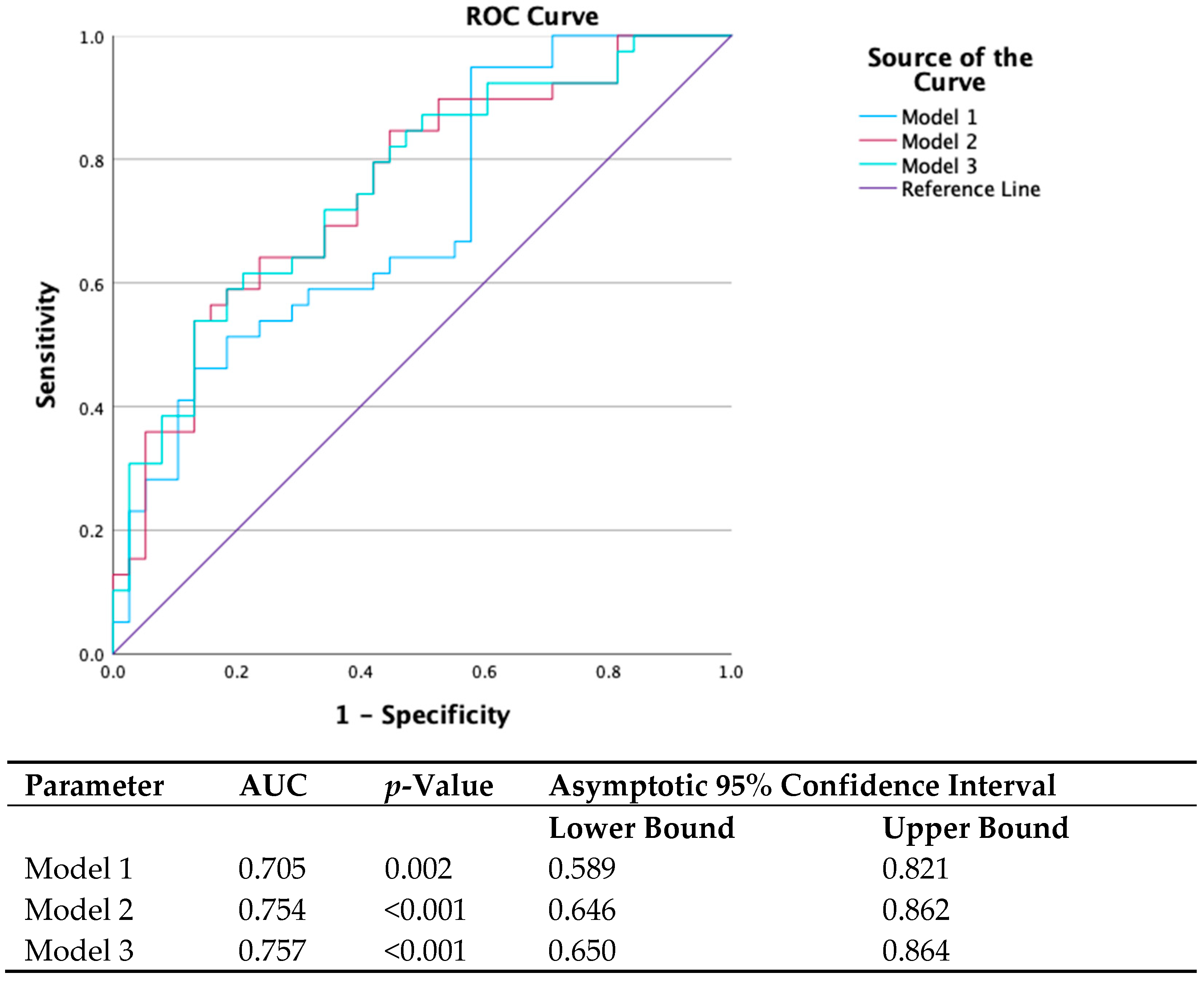
3.2. Added Value of Myocardial Work Beyond Traditional Echocardiographic Parameters
- Model 1: s′ septal, s′ average, e′ lateral, E max, and E/A (parameters found to be correlated with gene status in univariate analysis).
- Model 2: Model 1 plus GWE.
- Model 3: Model 1 plus GWW.
3.3. Prognostic Value of Myocardial Work for the Development of Cardiomyopathy
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Peak late mitral inflow velocity. |
| ACEi | Angiotensin-converting enzyme inhibitor. |
| AF | Atrial fibrillation. |
| ARB | Angiotensin receptor blocker. |
| AUC | Area under the curve. |
| AVC | Aortic valve closure. |
| BP | Blood pressure. |
| BSA | Body surface area. |
| CAD | Coronary artery disease. |
| CMR | Cardiac magnetic resonance imaging (cardiac MRI). |
| DCM | Dilated cardiomyopathy. |
| DT | Deceleration time. |
| E | Peak early mitral inflow velocity. |
| EDD | End-diastolic dimension. |
| EDVi | End-diastolic volume indexed. |
| EF | Ejection fraction. |
| e′ | Early diastolic tissue Doppler velocity. |
| G+ | Genotype positive. |
| G− | Genotype negative. |
| GCW | Global constructive work. |
| GLS | Global longitudinal strain. |
| GWE | Global work efficiency. |
| GWI | Global work index. |
| GWW | Global wasted work. |
| IVS | Interventricular septum. |
| LA | Left atrium. |
| LP/P | Likely pathogenic/pathogenic. |
| LV | Left ventricle/ventricular. |
| LVDD | Left ventricular end-diastolic diameter. |
| LVDDi | Left ventricular end-diastolic diameter indexed to BSA. |
| LVDVi | Left ventricular end-diastolic volume indexed to BSA. |
| LVEF | Left ventricular ejection fraction. |
| LVPWt | Left ventricular posterior wall thickness. |
| MW | Myocardial work. |
| NSVT | Non-sustained ventricular tachycardia. |
| OR | Odds ratio. |
| ROC | Receiver-operator characteristic. |
| s′ | Systolic tissue Doppler velocity. |
Appendix A
References
- Charron, P.; Arad, M.; Arbustini, E.; Basso, C.; Bilinska, Z.; Elliott, P.; Helio, T.; Keren, A.; McKenna, W.J.; Monserrat, L.; et al. Genetic counselling and testing in cardiomyopathies: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2010, 31, 2715–2726. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Badano, L.P.; Lang, R.M.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; Baroni, M.; Cardim, N.; de Diego, J.J.G.; Derumeaux, G.; et al. Normal Reference Ranges for Echocardiography: Rationale, study design, and methodology (NORRE Study). Eur. Heart J. Cardiovasc. Imaging 2013, 14, 303–308. [Google Scholar] [CrossRef]
- Cosyns, B.; Garbi, M.; Separovic, J.; Pasquet, A.; Lancellotti, P.; Education Committee of the European Association of Cardiovascular Imaging Association (EACVI). Update of the echocardiography core syllabus of the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2013, 14, 837–839. [Google Scholar]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the american heart association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Lindenfeld, J.; Mestroni, L.; Seidman, C.E.; Taylor, M.R.; Towbin, J.A. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J. Card. Fail. 2009, 15, 83–97. [Google Scholar] [CrossRef]
- Mahon, N.G.; Murphy, R.T.; MacRae, C.A.; Caforio, A.L.P.; Elliott, P.M.; McKenna, W.J. Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease. Ann. Intern. Med. 2005, 143, 108–115. [Google Scholar] [CrossRef]
- Paldino, A.; De Angelis, G.; Ferro, M.D.; Faganello, G.; Porcari, A.; Barbati, G.; Korcova, R.; Gentile, P.; Artico, J.; Cannatà, A.; et al. High prevalence of subtle systolic and diastolic dysfunction in genotype-positive phenotype-negative relatives of dilated cardiomyopathy patients. Int. J. Cardiol. 2021, 324, 108–114. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Thune, J.J.; Colan, S.D.; Cirino, A.L.; Farrohi, F.; Rivero, J.; McDonough, B.; Sparks, E.; Orav, E.J.; Seidman, J.G.; et al. Subtle abnormalities in contractile function are an early manifestation of sarcomere mutations in dilated cardiomyopathy. Circ. Cardiovasc. Genet. 2012, 5, 503–510. [Google Scholar] [CrossRef]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.F.; Shah, D.J.; Jue, J.; White, B.E.; Indorkar, R.; et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients with Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2018, 11, 1419–1429. [Google Scholar] [CrossRef]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef]
- van der Bijl, P.; Bootsma, M.; Hiemstra, Y.L.; Marsan, N.A.; Bax, J.J.; Delgado, V. Left ventricular 2D speckle tracking echocardiography for detection of systolic dysfunction in genetic, dilated cardiomyopathies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 694–699. [Google Scholar] [CrossRef]
- Verdonschot, J.A.; Merken, J.J.; Rocca, H.-P.B.-L.; Hazebroek, M.R.; Eurlings, C.G.; Thijssen, E.; Wang, P.; Weerts, J.; van Empel, V.; Schummers, G.; et al. Value of Speckle Tracking-Based Deformation Analysis in Screening Relatives of Patients with Asymptomatic Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2020, 13, 549–558. [Google Scholar] [CrossRef]
- Moya, A.; Buytaert, D.; Penicka, M.; Bartunek, J.; Vanderheyden, M. State-of-the-Art: Noninvasive Assessment of Left Ventricular Function Through Myocardial Work. J. Am. Soc. Echocardiogr. 2023, 36, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Le Rolle, V.; Leclercq, C.; Galli, E.; Samset, E.; Casset, C.; Mabo, P.; Hernandez, A.; Donal, E. Estimation of myocardial work from pressure-strain loops analysis: An experimental evaluation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Leclercq, C.; Fournet, M.; Hubert, A.; Bernard, A.; Smiseth, O.A.; Mabo, P.; Samset, E.; Hernandez, A.; Donal, E. Value of myocardial work estimation in the prediction of response to cardiac resynchronization therapy. J. Am. Soc. Echocardiogr. 2018, 31, 220–230. [Google Scholar] [CrossRef]
- Meucci, M.C.; Butcher, S.C.; Galloo, X.; van der Velde, E.T.; Marsan, N.A.; Bax, J.J.; Delgado, V. Noninvasive Left Ventricular Myocardial Work in Patients with Chronic Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. J. Am. Soc. Echocardiogr. 2022, 35, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Vannuccini, F.; Mandoli, G.E.; Lisi, M.; Iuliano, M.A.; Santoro, A.; Niglio, F.P.; Diviggiano, E.E.; Lorenz, V.; Montesi, G.; et al. Myocardial work and left heart deformation parameters across primary mitral regurgitation severity. Int. J. Cardiol. 2024, 399, 131772. [Google Scholar] [CrossRef]
- Peters, M.; Jan, M.F.; Ashraf, M.; Sanders, H.; Roemer, S.; Schweitzer, M.; Adefisoye, J.; Galazka, P.; Jain, R.; Jahangir, A.; et al. Myocardial work in apical hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, N.; Sun, Q.; Wei, H.; Fu, T.; Shang, Z.; Sun, Y.; Cong, T.; Xia, Y.; Xie, F.; et al. Association Between Segmental Noninvasive Myocardial Work and Microvascular Perfusion in ST-Segment Elevation Myocardial Infarction: Implications for Left Ventricular Functional Recovery and Clinical Outcomes. J. Am. Soc. Echocardiogr. 2023, 36, 1055–1063. [Google Scholar] [CrossRef]
- Fortuni, F.; Butcher, S.C.; van der Kley, F.; Lustosa, R.P.; Karalis, I.; de Weger, A.; Priori, S.G.; van der Bijl, P.; Bax, J.J.; Delgado, V.; et al. Left Ventricular Myocardial Work in Patients with Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 257–266. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef]
- Cui, C.; Liu, L.; Li, Y.; Liu, Y.; Huang, D.; Hu, Y.; Zhang, L. Left Ventricular Pressure-Strain Loop-Based Quantitative Examination of the Global and Regional Myocardial Work of Patients with Dilated Cardiomyopathy. Ultrasound Med. Biol. 2020, 46, 2834–2845. [Google Scholar] [CrossRef] [PubMed]
- Chahal, N.S.; Lim, T.K.; Jain, P.; Chambers, J.C.; Kooner, J.S.; Senior, R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: A population-based study. Eur. J. Echocardiogr. 2010, 11, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; De Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Jankajova, M.; Singh, R.B.; Hristova, K.; Elkilany, G.; Fatima, G.; Singh, J.; Fedacko, J. Identification of Pre-Heart Failure in Early Stages: The Role of Six Stages of Heart Failure. Diagnostics 2024, 14, 2618. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar]
- Truong, V.T.; Vo, H.Q.; Ngo, T.N.M.; Mazur, J.; Nguyen, T.T.H.; Pham, T.T.M.; Le, T.K.; Phan, H.; Palmer, C.; Nagueh, S.F.; et al. Normal Ranges of Global Left Ventricular Myocardial Work Indices in Adults: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2022, 35, 369–377. [Google Scholar] [CrossRef] [PubMed]

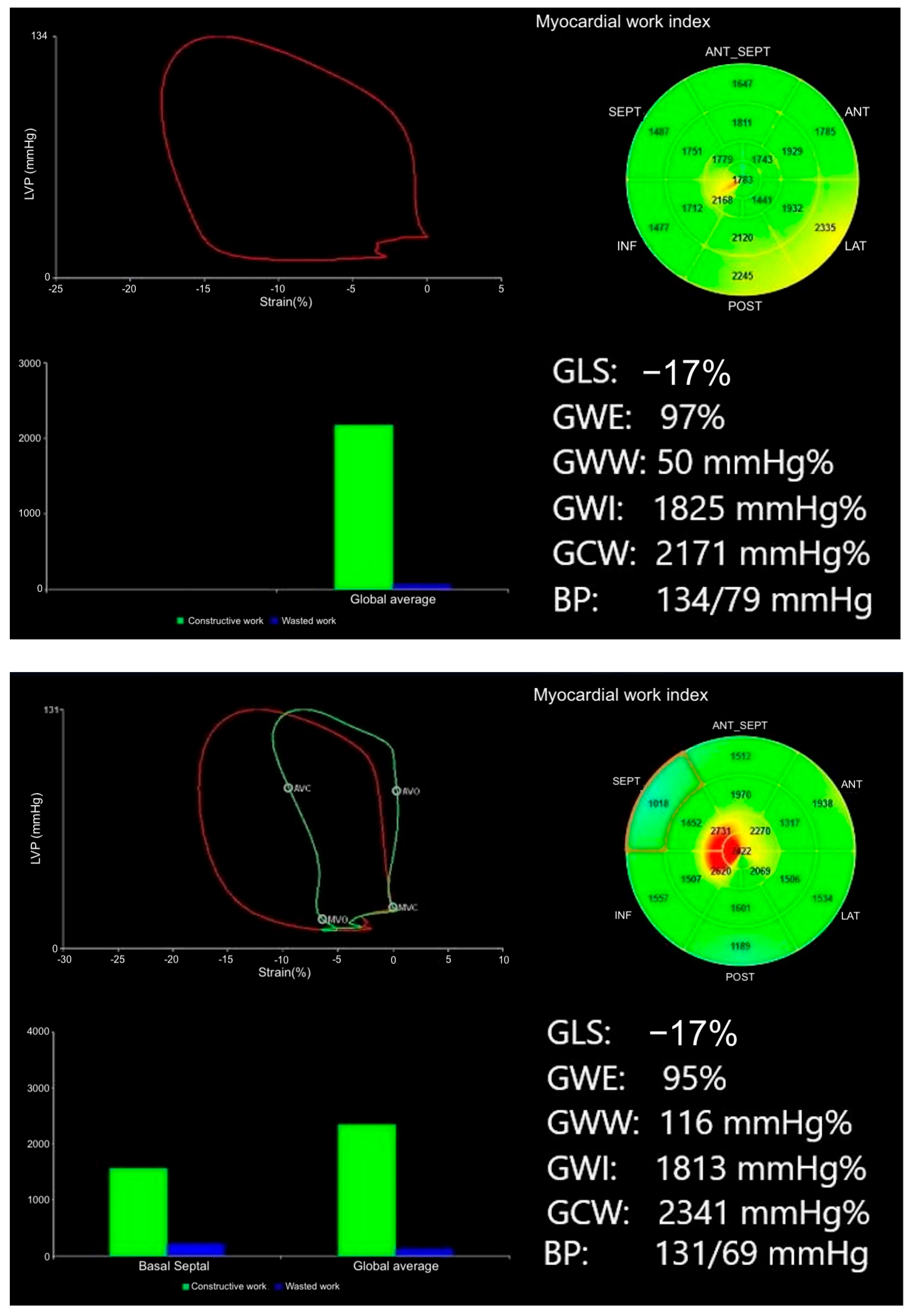
| Baseline Characteristics | G− (N = 38) | G+ (N = 39) | p-Value |
|---|---|---|---|
| Age (±SD) | 33 (±12.8) | 38 (±13.7) | 0.082 |
| Female (%) | 18 (47%) | 22 (55%) | 0.503 |
| BSA | 1.91 (±0.2) | 1.91 (±0.2) | 0.963 |
| Systolic BP (±SD) | 121 (±15.8) | 120 (±16) | 0.656 |
| Diastolic BP (±SD) | 74 (±10.6) | 72 (±11.3) | 0.490 |
| Smoking | 2 (5.3%) | 5 (12.5%) | 0.249 |
| Known CAD | 0 | 0 | - |
| Hyperlipidemia | 1 (2.6%) | 4 (10%) | 0.175 |
| Hypertension (%) | 3 (7.9%) | 4 (10) | 0.747 |
| Diabetes (%) | 0 | 2 (5%) | 0.165 |
| ACEi/ARB (%) | 4 (10.5%) | 8 (20.5%) | 0.230 |
| BB | 2 (5.3%) | 4 (10%) | 0.414 |
| Loop diuretics | 0 | 0 | - |
| NSVT (%) | 0 | 3 (7.5%) | 0.087 |
| AF | 0 | 1 (2.5%) | 0.320 |
| QRS (ms) | 97 ms | 95 ms | 0.459 |
| Myocardial work parameters | |||
| GWE (%) | 96 (±1.5) | 94 (±2.4) | <0.001 |
| GWI (mmHg%) | 1920 (±292) | 1839 (±424) | 0.125 |
| GCW (mmHg%) | 2250 (±325) | 2217 (±474) | 0.282 |
| GWW (mmHg%) | 80 (±35) | 113 (±53) | 0.001 |
| Traditional echo parameters | |||
| LVDD (mm) | 52 (±6) | 53 (±5) | 0.363 |
| LVDDi (mm/m2) | 27 (±2) | 28 (±3) | 0.262 |
| LVDVi | 63 (±13) | 61 (±12) | 0.213 |
| LVEF (%) | 59 (±4) | 58 (±4) | 0.093 |
| GLS (%) | 18.7 (±1.7) | 18.0 (±2.7) | 0.089 |
| Mitral s′ sep (cm/s) | 8.3 (±1.4) | 7.8 (±1.1) | 0.019 |
| Mitral s′ lat (cm/s) | 10.7 (±2.6) | 9.9(±2.5) | 0.066 |
| Mitral s′ avg | 9.6 (±1.7) | 8.8 (±1.5) | 0.023 |
| E max | 73 (±15.4) | 67 (±14.9) | 0.061 |
| A max | 51(±16) | 56(±16) | 0.098 |
| E/A ratio | 1.6 (±0.5) | 1.3 (±0.3) | 0.005 |
| E/e′ | 7.4 (±6.7) | 6.2 (±1.9) | 0.258 |
| Mitral e′ septal | 10.5 (±2.5) | 9.6 (±2.3) | 0.062 |
| Mitral e′ lateral (cm/s) | 15.2 (±3.9) | 13.1 (±3.9) | 0.011 |
| AVC (ms) | 405 (±44) | 408 (±41) | 0.735 |
| LA area | 18.6 (±3.7) | 19 (±3.9) | 0.626 |
| LV posterior wall | 7 (±1.2) | 7 (±1.1) | 0.722 |
| IVS | 8 (±1.4) | 8 (±1.4) | 0.647 |
| Gene | G+ (N = 39) |
|---|---|
| BAG3 | 3 |
| DSP | 17 |
| FLNC | 3 |
| LMNA | 3 |
| RBM20 | 3 |
| TNNT2 | 1 |
| TPM1 | 1 |
| TTN | 8 |
| Genotype Classification G+/G− | ||||
|---|---|---|---|---|
| Variable | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Unadjusted | Adjusted | |||
| GWE | 0.671 (0.511–0.881) | 0.004 | 0.746 (0.560–0.994) | 0.045 |
| GWW | 1.017 (1.005–1.028) | 0.004 | 1.012 (1.002–1.024) | 0.047 |
| GWE | p-Value | GWI | p-Value | GCW | p-Value | GWW | p-Value | |
|---|---|---|---|---|---|---|---|---|
| LVDD | −0.023 | 0.844 | −0.209 | 0.069 | −0.227 | 0.048 | −0.050 | 0.663 |
| LVDDi | −0.980 | 0.399 | −0.115 | 0.320 | −0.119 | 0.303 | 0.034 | 0.766 |
| EF | 0.653 | <0.001 | 0.342 | 0.002 | 0.307 | 0.007 | −0.453 | <0.001 |
| GLS | 0.354 | 0.002 | 0.339 | 0.003 | 0.349 | 0.002 | −0.275 | 0.015 |
| S′ septal | 0.428 | <0.001 | 0.225 | 0.049 | 0.171 | 0.137 | −0.373 | <0.001 |
| E max | 0.072 | 0.532 | 0.267 | 0.019 | 0.239 | 0.036 | −0.010 | 0.932 |
| E/A ratio | 0.259 | 0.023 | 0.093 | 0.419 | 0.078 | 0.498 | −0.245 | 0.032 |
| e′ lateral | 0.212 | 0.064 | 0.040 | 0.731 | 0.020 | 0.865 | −0.198 | 0.084 |
| AVC | −0.074 | 0.524 | −0.116 | 0.315 | −0.045 | 0.700 | 0.105 | 0.365 |
| DT | −0.084 | 0.467 | −0.115 | 0.321 | −0.088 | 0.446 | 0.021 | 0.857 |
| LA area | 0.099 | 0.394 | −0.162 | 0.160 | −0.155 | 0.180 | −0.118 | 0.308 |
| LVPWt | 0.024 | 0.834 | −0.187 | 0.104 | −0.156 | 0.177 | −0.069 | 0.553 |
| Genotype Group and Development of Cardiomyopathy Phenotype | |||
|---|---|---|---|
| Follow-up data (N = 77) | G− (N = 38) | G+ (N = 39) | p-Value |
| Cardiomyopathy Phenotype Positive (+) (N = 16) * | 1 (3%) | 15 (38%) | <0.001 |
| Cardiomyopathy Phenotype Negative (−) (N = 61) | 37 (97%) | 24 (62%) | <0.001 |
| Myocardial work parameters and development of cardiomyopathy phenotype * | |||
| Myocardial work parameters | Phenotype Negative (−) (N = 61) | Phenotype Positive (+) (N = 16) | p-Value |
| GWE (%) | 96 (±1.7) | 94 (±3.0) | <0.001 |
| GWI (mmHg%) | 1915 (±337) | 1706 (±417) | 0.268 |
| GCW (mmHg%) | 2247 (±366) | 2130 (±510) | 0.091 |
| GWW (mmHg%) | 91 (±41) | 121 (±65) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettos, A.; Monteiro, R.P.; Triantafyllou, M.; Gul, U.; Bhattacharyya, S.; Lopes, L.R.; Antonopoulos, A.; Protonotarios, A.; Lloyd, G.; Gossios, T.; et al. Early Detection of Subclinical Myocardial Dysfunction in Familial Dilated Cardiomyopathy Using Myocardial Work Analysis. Diagnostics 2025, 15, 2363. https://doi.org/10.3390/diagnostics15182363
Vrettos A, Monteiro RP, Triantafyllou M, Gul U, Bhattacharyya S, Lopes LR, Antonopoulos A, Protonotarios A, Lloyd G, Gossios T, et al. Early Detection of Subclinical Myocardial Dysfunction in Familial Dilated Cardiomyopathy Using Myocardial Work Analysis. Diagnostics. 2025; 15(18):2363. https://doi.org/10.3390/diagnostics15182363
Chicago/Turabian StyleVrettos, Apostolos, Ricardo Prista Monteiro, Miltiadis Triantafyllou, Uzma Gul, Sanjeev Bhattacharyya, Luís R. Lopes, Alexios Antonopoulos, Alexandros Protonotarios, Guy Lloyd, Thomas Gossios, and et al. 2025. "Early Detection of Subclinical Myocardial Dysfunction in Familial Dilated Cardiomyopathy Using Myocardial Work Analysis" Diagnostics 15, no. 18: 2363. https://doi.org/10.3390/diagnostics15182363
APA StyleVrettos, A., Monteiro, R. P., Triantafyllou, M., Gul, U., Bhattacharyya, S., Lopes, L. R., Antonopoulos, A., Protonotarios, A., Lloyd, G., Gossios, T., & Savvatis, K. (2025). Early Detection of Subclinical Myocardial Dysfunction in Familial Dilated Cardiomyopathy Using Myocardial Work Analysis. Diagnostics, 15(18), 2363. https://doi.org/10.3390/diagnostics15182363






