Impact of a Major Earthquake on Glycemic Control in Adults with Type 2 Diabetes: A Retrospective Cohort Study from Türkiye
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
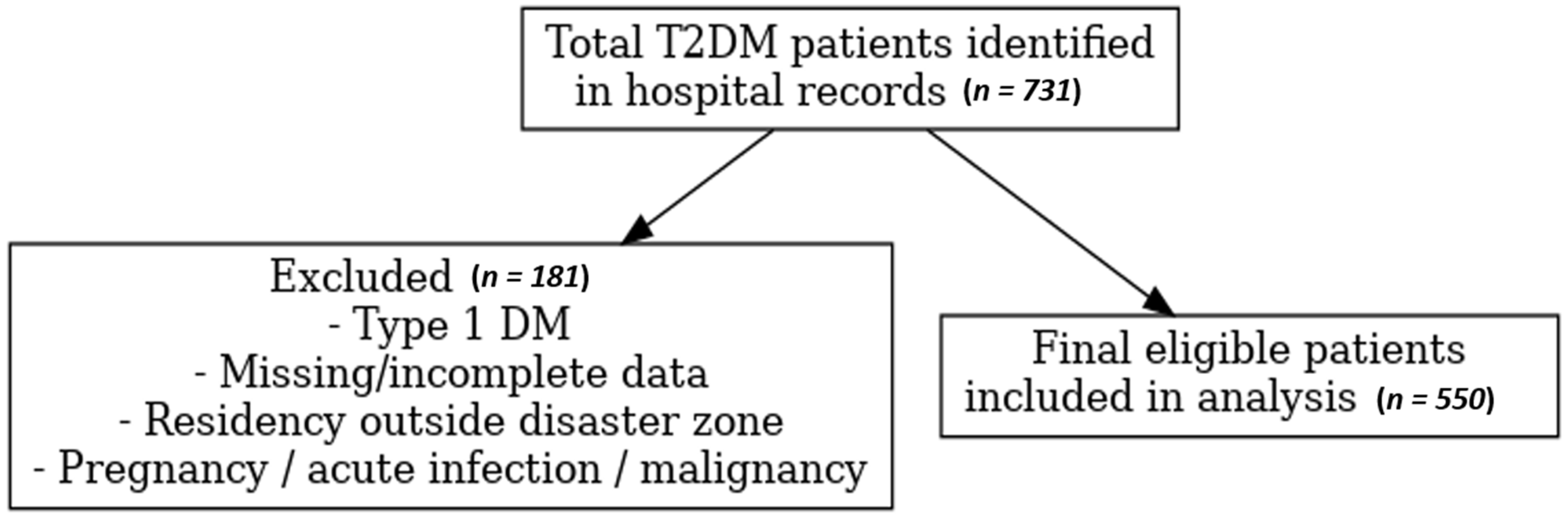
2.2. Study Population and Inclusion Criteria
- ⮚
- Documented T2DM diagnosis in the hospital information system prior to 6 February 2023;
- ⮚
- Availability of at least one recorded HbA1c measurement and accompanying biochemical tests during both the three months before and the three to five months after the earthquake;
- ⮚
- Residency in the disaster-affected region.
- ⮚
- Type 1 diabetes mellitus;
- ⮚
- Gestational diabetes;
- ⮚
- Secondary diabetes (e.g., pancreatogenic or steroid-induced diabetes);
- ⮚
- Acute metabolic complications (e.g., diabetic ketoacidosis [DKA], hyperosmolar hyperglycemic state [HHS]);
- ⮚
- Missing or incomplete laboratory data;
- ⮚
- Residency outside the disaster zone;
- ⮚
- Pregnancy, severe acute infection, or active malignancy at the time of testing.
2.3. Data Collection
- ✓
- Demographics: age, sex, nationality, and residential location (central Gaziantep or Islahiye).
- ✓
- Laboratory parameters (pre- and post-earthquake): HbA1c (%), fasting plasma glucose (mg/dL), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, albumin, creatinine, blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate transaminase (AST), white blood cell count (WBC), platelet count (PLT), hemoglobin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), vitamin D, vitamin B12, and thyroid-stimulating hormone (TSH).
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douglas, F.E.; Shroff, C.; Meiser-Stedman, R. Treatment of children and adolescents with post-traumatic stress in humanitarian crises: Systematic review and meta-analysis. Bull. World Health Organ. 2025, 103, 445–461. [Google Scholar] [CrossRef]
- Yamashita, M.; Seino, S.; Nofuji, Y.; Sugawara, Y.; Okamura, T.; Kawakubo, K.; Shinkai, S.; Fujiwara, Y. Examining apathy prevalence and associated factors among older adults after the Great East Japan Earthquake: A mixed-methods study. BMC Geriatr. 2025, 25, 498. [Google Scholar] [CrossRef]
- Tokumaru, O.; Fujita, M.; Nagai, S.; Minamikawa, Y.; Kumatani, J. Medical Problems and Concerns with Temporary Evacuation Shelters after Great Earthquake Disasters in Japan: A Systematic Review. Disaster Med. Public Health Prep. 2022, 16, 1645–1652. [Google Scholar] [CrossRef]
- Aycicek, H.B.; Ozdemir, E.C. The Essential Role of Early Rehabilitation in Disasters: A Single Center Experience in Turkiye-Syria Earthquake. Disaster Med. Public Health Prep. 2025, 19, e188. [Google Scholar] [CrossRef]
- Cansel, N.; Sandikli, H.; Melez, S.N.I.; Sandikli, M.; Balikci Cicek, I.; Kayhan Tetik, B. Evaluation of Post-traumatic Stress, Depression, and Anxiety Levels in Survivors of the 2023 Kahramanmaras Turkiye Earthquakes at the 12th Month After the Event. Disaster Med. Public Health Prep. 2025, 19, e176. [Google Scholar] [CrossRef]
- Ghazanchaei, E.; Khorasani-Zavareh, D.; Aghazadeh-Attari, J.; Mohebbi, I. Identifying and describing impact of disasters on non-communicable diseases: A systematic review. Iran. J. Public Health 2021, 50, 1143. [Google Scholar] [CrossRef] [PubMed]
- Kurt, N.B.; Yilmaz, H.O. The relationship between post-traumatic stress and eating disorders in disaster survivors: A sample of February 2023 Kahramanmaras earthquake. Psychol. Health Med. 2025, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.P.; Luk, A.O.; Chan, J.C. Detecting people at high risk of type 2 diabetes- How do we find them and who should be treated? Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 345–355. [Google Scholar] [CrossRef]
- Caturano, A.; Galiero, R.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Sardu, C.; Vetrano, E.; Monda, M.; et al. Modern Challenges in Type 2 Diabetes: Balancing New Medications with Multifactorial Care. Biomedicines 2024, 12, 2039. [Google Scholar] [CrossRef]
- Rodríguez, J.H. Generalities about natural disasters and diabetes mellitus. Rev. Cuba. De Med. Gen. Integral 2021, 37, 1–10. [Google Scholar]
- Watanabe, H.; Takahara, M.; Katakami, N.; Matsuoka, T.-a.; Shimomura, I. Glycemic control of people with diabetes over months after the 2018 North Osaka Earthquake. Diabetol. Int. 2021, 12, 80–86. [Google Scholar] [CrossRef]
- Tarçın, G.; Dilek, S.Ö.; Kılıç, S.; Ata, A. Effect of continuous glucose monitoring device assistance on glycemic control of 2023 Kahramanmaraş doublet earthquake survivors with type 1 diabetes in Adana, Turkey. Turk. Arch. Pediatr. 2023, 58, 653. [Google Scholar] [CrossRef]
- Fonseca, V.A.; Smith, H.; Kuhadiya, N.; Leger, S.M.; Yau, C.L.; Reynolds, K.; Shi, L.; McDuffie, R.H.; Thethi, T.; John-Kalarickal, J. Impact of a natural disaster on diabetes: Exacerbation of disparities and long-term consequences. Diabetes Care 2009, 32, 1632–1638. [Google Scholar] [CrossRef]
- Tanaka, M.; Imai, J.; Satoh, M.; Hashimoto, T.; Izumi, T.; Sawada, S.; Uno, K.; Hasegawa, Y.; Kaneko, K.; Yamada, T. Glycemic control in diabetic patients with impaired endogenous insulin secretory capacity is vulnerable after a natural disaster: Study of Great East Japan Earthquake. Diabetes Care 2014, 37, e212–e213. [Google Scholar] [CrossRef]
- Tanaka, M.; Imai, J.; Satoh, M.; Hashimoto, T.; Izumi, T.; Sawada, S.; Uno, K.; Hasegawa, Y.; Kaneko, K.; Yamada, T. Impacts of the G reat E ast J apan E arthquake on diabetic patients. J. Diabetes Investig. 2015, 6, 577–586. [Google Scholar] [CrossRef]
- Trabzon, G.; Sarı, S.A.; Yüce, S.; Bilaloğlu, S.; Güllü, Ş.D. The Impact of the 2023 Turkey Earthquakes on Glycemic Control and Stress Levels in Children with Type 1 Diabetes: Single-center Experience. J. Clin. Res. Pediatr. Endocrinol. 2025, 17, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E. Diabetes Self-Management and Health Care Demand Procrastination Behavior Among Earthquake Victims with Type 2 Diabetes in Earthquake Zone. Disaster Med. Public Health Prep. 2025, 19, e82. [Google Scholar] [CrossRef]
- Fujihara, K.; Saito, A.; Heianza, Y.; Gibo, H.; Suzuki, H.; Shimano, H.; Saito, K.; Kodama, S.; Yamada, N.; Sone, H. Impact of psychological stress caused by the Great East Japan Earthquake on glycemic control in patients with diabetes. Exp. Clin. Endocrinol. Diabetes 2012, 120, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Tsubokura, M.; Takita, M.; Matsumura, T.; Hara, K.; Tanimoto, T.; Kobayashi, K.; Hamaki, T.; Oiso, G.; Kami, M.; Okawada, T. Changes in metabolic profiles after the Great East Japan Earthquake: A retrospective observational study. BMC Public Health 2013, 13, 1–10. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Advance Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. New Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Miyakawa, N.; Motoshima, H.; Hanatani, S.; Ishii, N.; Igata, M.; Yoshinaga, K.; Kukidome, D.; Senokuchi, T.; Kawashima, J.; et al. Impacts of the 2016 Kumamoto Earthquake on glycemic control in patients with diabetes. J. Diabetes Investig. 2019, 10, 521–530. [Google Scholar] [CrossRef]
- Odhaib, S.A.; Masood, S.N.; Belkhadir, J.; Sandid, M.; Shaikh, Z.; Farooq, F.N.; Naz, F.; Masood, F.T.; Ayub, A.; Bilal, A. Diabetes Care during Humanitarian Crises due to Floodings and Earthquakes in IDF-MENA Region: Pakistan Experience. J. Diabetol. 2022, 13, S62–S67. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Fukuda, Y.; Tsubokura, M.; Kato, S.; Nomura, S.; Saito, Y. Managing Type 2 Diabetes Mellitus through Periodical Hospital Visits in the Aftermath of the Great East Japan Earthquake Disaster: A Retrospective Case Series. PLoS ONE 2015, 10, e0125632. [Google Scholar] [CrossRef]
- Ciocca, G.; Carosa, E.; Stornelli, M.; Limoncin, E.; Gravina, G.L.; Iannarelli, R.; Sperandio, A.; Di Sante, S.; Lenzi, A.; Lauro, D.; et al. Post-traumatic stress disorder, coping strategies and type 2 diabetes: Psychometric assessment after L'Aquila earthquake. Acta Diabetol. 2015, 52, 513–521. [Google Scholar] [CrossRef]
- Edelhoff, D.; Liebermann, A.; Schubert, O.; Guth, J.F. Prospective Clinical Split-Mouth Study of Two-Wing-Retained Resin-Bonded Anterior Fixed Dental Prostheses with Metallic and Ceramic Frameworks: 5-year Results. Int. J. Prosthodont. 2023, 36, 253–261. [Google Scholar] [CrossRef]
- Hirai, H.; Nagao, M.; Ohira, T.; Maeda, M.; Okazaki, K.; Nakano, H.; Hayashi, F.; Harigane, M.; Suzuki, Y.; Takahashi, A. Psychological burden predicts new-onset diabetes in men: A longitudinal observational study in the Fukushima Health Management Survey after the Great East Japan Earthquake. Front. Endocrinol. 2022, 13, 1008109. [Google Scholar] [CrossRef] [PubMed]
- Vozarova, B.; Weyer, C.; Lindsay, R.S.; Pratley, R.E.; Bogardus, C.; Tataranni, P.A. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002, 51, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Afek, A.; Shamiss, A.; Derazne, E.; Tzur, D.; Gordon, B.; Tirosh, A. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care 2013, 36, 276–282. [Google Scholar] [CrossRef]
- Ma, E.; Ohira, T.; Fukasawa, M.; Yasumura, S.; Miyazaki, M.; Suzuki, T.; Furuyama, A.; Kataoka, M.; Hosoya, M. Prevalence trends of metabolic syndrome in residents of postdisaster Fukushima: A longitudinal analysis of Fukushima Health Database 2012–2019. Public Health 2023, 217, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Liu, L.; Xu, W. Association of Albumin-To-Creatinine Ratio With Diabetic Retinopathy Among US Adults (NHANES 2009–2016). Endocrinol. Diabetes Metab. 2025, 8, e70029. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Z.; Yu, Y.; He, Y.; Yuan, Y.; Wu, X.; Xu, Q.; Niu, J.; Wu, X.; Tan, J. Predictive Model for In-Hospital Death in Older Patients with Type 2 Diabetes Mellitus: A Multicenter Retrospective Study in Southwest China. Diabetes Metab. Syndr. Obes. 2025, 18, 1873–1889. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± SD, n (%) |
|---|---|
| Age (years) | 56.2 ± 11.0 |
| Gender | |
| Male | 238 (43.3%) |
| Female | 312 (56.7%) |
| Nationality | |
| Turkish | 513 (93.3%) |
| Syrian | 37 (6.7%) |
| Place of Residence | |
| Central Gaziantep | 252 (45.8%) |
| Islahiye | 298 (54.2%) |
| Comorbidities | |
| Hypertension | 170 (30.9%) |
| Hyperlipidemia | 311 (56.5%) |
| Coronary artery disease | 26 (4.7%) |
| Obesity (BMI > 30 kg/m2) | 184 (33.5%) |
| Chronic kidney disease | 39 (7.1%) |
| Duration of T2DM (years) | |
| <5 years | 103 (18.7%) |
| 5–10 years | 196 (35.6%) |
| >10 years | 251 (45.6%) |
| Treatment Modality | |
| Oral antidiabetics | 321 (58.4%) |
| Insulin therapy | 96 (17.5%) |
| Combined (oral + insulin) | 133 (24.2%) |
| Parameter | Pre-Earthquake (Mean ± SD) | Post-Earthquake (Mean ± SD) | p-Value |
| White Blood Cell (WBC) (×103/μL) | 8.49 ± 5.1 | 9.42 ± 5.6 | 0.003 ** |
| Hemoglobin (g/dL) | 14.2 ± 1.8 | 13.9 ± 2.1 | 0.321 |
| Platelet (PLT) (×103/μL) | 272.3 ± 88.7 | 292.8 ± 87.1 | <0.001 ** |
| Urea (mg/dL) | 32.8 ± 5.8 | 30.9 ± 13.5 | 0.023 * |
| Creatinine (mg/dL) | 1.25 ± 7.1 | 0.81 ± 0.3 | 0.181 |
| ALT (U/L) | 23.6 ± 16.5 | 20.9 ± 14.6 | 0.002 ** |
| Albumin (g/dL) | 27.5 ± 17.5 | 28.8 ± 18.4 | 0.150 |
| Total Cholesterol (mg/dL) | 190.6 ± 45.7 | 193.5 ± 43.7 | 0.239 |
| LDL (mg/dL) | 106.2 ± 46.3 | 108.7 ± 44.9 | 0.354 |
| Triglyceride (TG) (mg/dL) | 202.5 ± 170.4 | 180.6 ± 113.1 | 0.010 * |
| HDL (mg/dL) | 53.2 ± 46.4 | 45.1 ± 10.7 | <0.001 ** |
| Glucose (mg/dL) | 167.4 ± 71.8 | 178.2 ± 79.4 | <0.001 ** |
| HbA1c (%) | 8.34 ± 1.9 | 8.87 ± 5.5 | 0.012 * |
| ESR (mm/h) | 22.8 ± 36.7 | 16.9 ± 21.1 | 0.636 |
| CRP (mg/L) | 7.58 ± 10.8 | 7.18 ± 17.5 | 0.863 |
| Vitamin D (ng/mL) | 14.3 ± 18.1 | 15.1 ± 18.0 | 0.191 |
| Vitamin B12 (pg/mL) | 421.9 ± 369.9 | 395.4 ± 228.6 | 0.499 |
| TSH (μIU/mL) | 2.99 ± 4.6 | 2.36 ± 2.0 | 0.192 |
| Parameter | Correlation Coefficient (r) | p-Value |
|---|---|---|
| White Blood Cell (WBC) | −0.112 | 0.009 ** |
| Hemoglobin | 0.053 | 0.240 |
| Platelet (PLT) | −0.064 | 0.157 |
| Urea | 0.035 | 0.451 |
| Creatinine | 0.040 | 0.384 |
| ALT | 0.002 | 0.973 |
| Albumin | −0.332 | 0.029 * |
| Total Cholesterol | 0.107 | 0.032 * |
| LDL | 0.173 | <0.001 ** |
| Triglyceride (TG) | 0.323 | <0.001 ** |
| HDL | −0.175 | <0.001 ** |
| Glucose | 0.362 | <0.001 ** |
| ESR | 0.116 | 0.706 |
| CRP | −0.057 | 0.643 |
| Vitamin D | −0.535 | 0.137 |
| Vitamin B12 | −0.046 | 0.689 |
| TSH | 0.050 | 0.659 |
| Independent Variable | Unstandardized β | Standard Error (SE) | Standardized β (Beta) | t-Value | p-Value |
|---|---|---|---|---|---|
| Age (years) | 0.002 | 0.001 | 0.065 | 1.92 | 0.055 |
| Sex (male vs. female) | −0.048 | 0.032 | −0.058 | −1.50 | 0.135 |
| BMI (kg/m2) | 0.006 | 0.003 | 0.082 | 2.01 | 0.045 * |
| Diabetes duration 5–10 y (vs. <5 y) | 0.051 | 0.028 | 0.067 | 1.83 | 0.068 |
| Diabetes duration >10 y (vs. <5 y) | 0.094 | 0.036 | 0.101 | 2.61 | 0.009 ** |
| Insulin use (any vs. none) | 0.121 | 0.041 | 0.112 | 2.95 | 0.003 ** |
| ΔFasting Glucose (mg/dL) | 0.008 | 0.002 | 0.256 | 4.12 | <0.001 ** |
| ΔTriglyceride (mg/dL) | 0.005 | 0.001 | 0.221 | 3.84 | <0.001 ** |
| ΔHDL (mg/dL) | −0.011 | 0.004 | −0.142 | −2.71 | 0.007 * |
| ΔAlbumin (g/dL) | −0.017 | 0.006 | −0.168 | −2.94 | 0.004 * |
| ΔWBC (×103/μL) | −0.010 | 0.003 | −0.124 | −2.36 | 0.019 * |
| Constant (Intercept) | 0.342 | 0.092 | — | 3.72 | <0.001 ** |
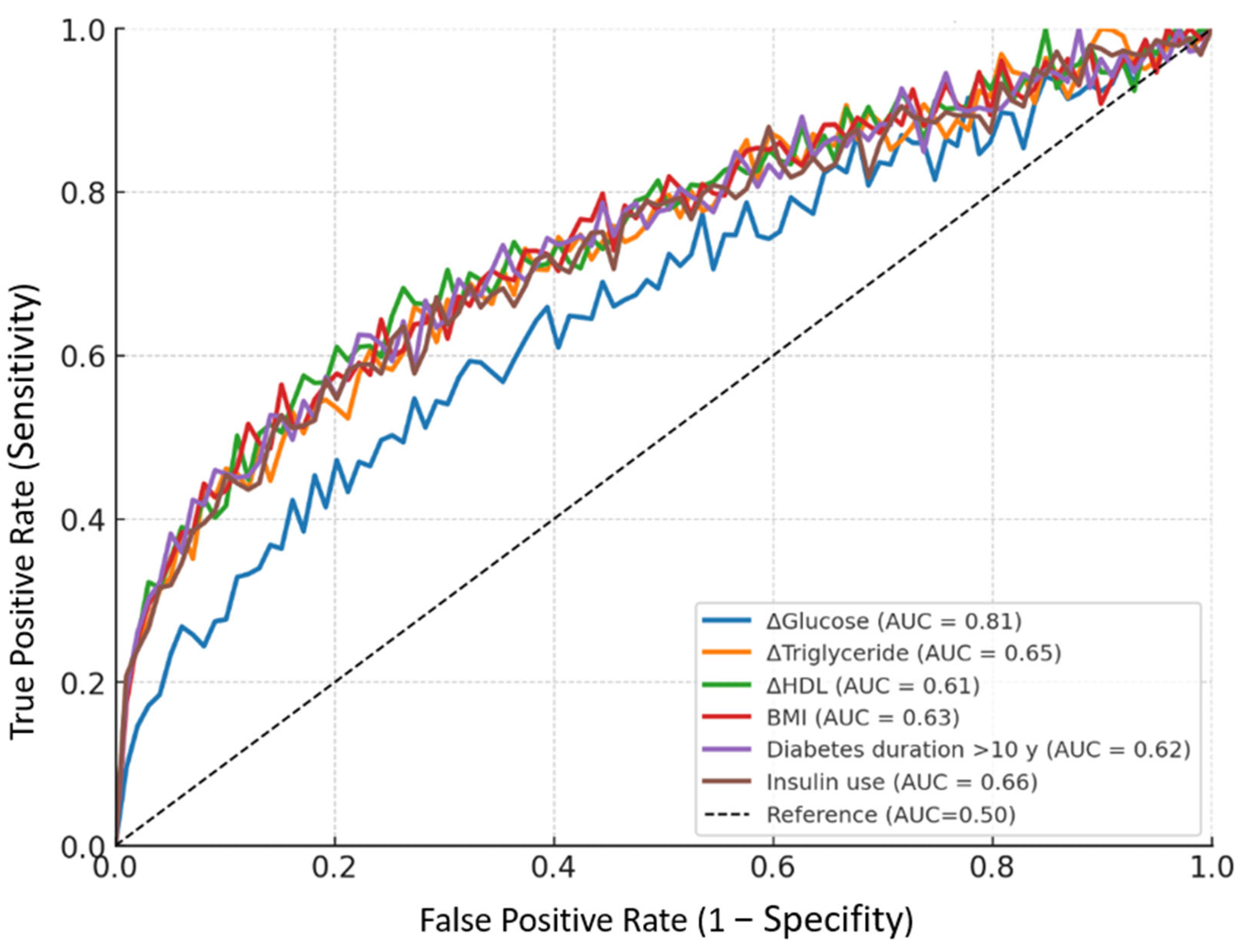
| Parameter | Cut-Off | Sensitivity | Specifity | AUC (95% CI) | p Value |
|---|---|---|---|---|---|
| ΔGlucose (mg/dL) | >12.5 | 82.1% | 73.4% | 0.81 (0.74–0.84) | <0.001 ** |
| ΔTriglyceride (mg/dL) | >25.0 | 70.3% | 61.8% | 0.65 (0.59–0.71) | <0.001 ** |
| ΔHDL (mg/dL) | <−3.0 | 62.5% | 55.2% | 0.61 (0.55–0.69) | 0.016 * |
| BMI (kg/m2) | >29.0 | 68.2% | 60.7% | 0.63 (0.57–0.69) | 0.002 ** |
| Diabetes duration >10 y (vs. <5 y) | — | 65.4% | 59.1% | 0.62 (0.56–0.68) | 0.004 ** |
| Insulin use (any vs. none) | — | 71.7% | 64.3% | 0.66 (0.60–0.72) | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir, S.; Ozturk, S. Impact of a Major Earthquake on Glycemic Control in Adults with Type 2 Diabetes: A Retrospective Cohort Study from Türkiye. Diagnostics 2025, 15, 2361. https://doi.org/10.3390/diagnostics15182361
Ozdemir S, Ozturk S. Impact of a Major Earthquake on Glycemic Control in Adults with Type 2 Diabetes: A Retrospective Cohort Study from Türkiye. Diagnostics. 2025; 15(18):2361. https://doi.org/10.3390/diagnostics15182361
Chicago/Turabian StyleOzdemir, Sedat, and Sadettin Ozturk. 2025. "Impact of a Major Earthquake on Glycemic Control in Adults with Type 2 Diabetes: A Retrospective Cohort Study from Türkiye" Diagnostics 15, no. 18: 2361. https://doi.org/10.3390/diagnostics15182361
APA StyleOzdemir, S., & Ozturk, S. (2025). Impact of a Major Earthquake on Glycemic Control in Adults with Type 2 Diabetes: A Retrospective Cohort Study from Türkiye. Diagnostics, 15(18), 2361. https://doi.org/10.3390/diagnostics15182361







