Clinical, Bone Mineral Density and Spinal Remodelling Responses to Zoledronate Treatment in Chronic Recurrent Multifocal Osteomyelitis
Abstract
1. Introduction
2. Aims
3. Objective
4. Methods
4.1. Study Setting
4.2. Treatment Protocol
4.3. Subjects
4.4. Data Gathering
4.5. Statistics
4.6. Ethics
5. Results
5.1. Baseline Characteristics
5.2. Indication for Treatment
5.3. Pre-Bisphosphonate Treatment
5.4. Bisphosphonate Treatment Details
5.5. Treatment Response
5.6. Clinical Response
5.7. Change in BMD
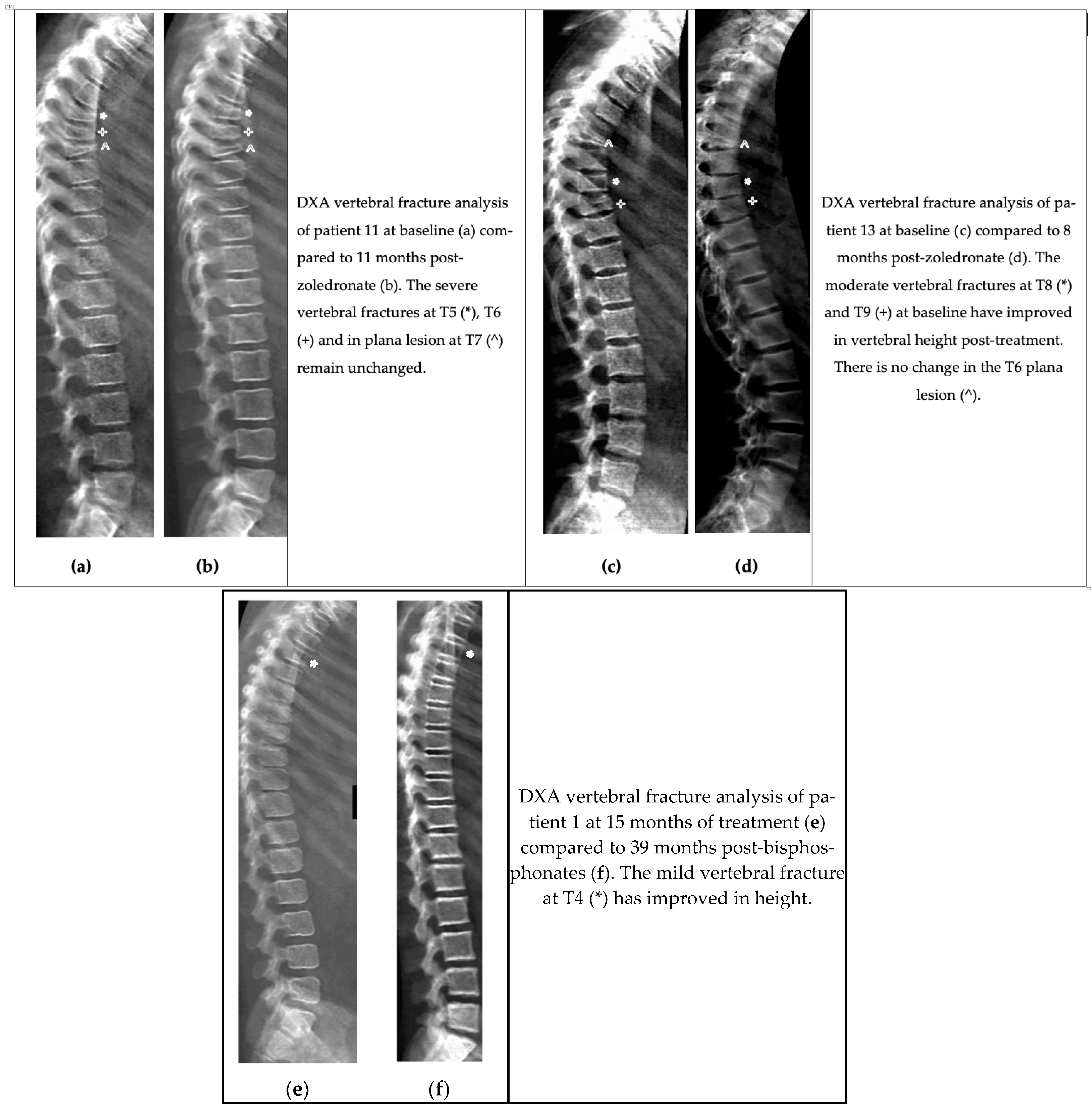
5.8. Radiographic Remodelling Response
5.9. Safety
6. Discussion
Limitations and Future Work
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giedion, A.; Holthusen, W.; Masel, L.F.; Vischer, D. Subacute and chronic “symmetrical” osteomyelitis. Ann. Radiol. 1972, 15, 329–342. [Google Scholar] [PubMed]
- Orphanet. Chronic Nonbacterial Osteomyelitis/Chronic Recurrent Multifocal Osteomyelitis. Available online: https://www.orpha.net/en/disease/detail/324964 (accessed on 22 June 2025).
- Chia, D.T.; Toms, A.P.; Sanghrajka, A.; Ramanan, A.V.; Killeen, O.G.; Ilea, C.; Mahmood, K.; Compeyrot-Lacassagne, S.; Bailey, K.; Martin, N.; et al. Incidence of chronic recurrent multifocal osteomyelitis in children and adolescents in the UK and Republic of Ireland. Rheumatology 2024, 64, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, Y.J.; Greenwood, S.J.; Cribb, G.; Davies, K.; Cassar-Pullicino, V.N. Complete resolution and remodelling of chronic recurrent multifocal osteomyelitis on MRI and radiographs. Skelet. Radiol. 2018, 47, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Sato, T.S.; Ferguson, P. Not all benign: Disease course, complications, and sequalae of chronic recurrent multifocal osteomyelitis in children. Curr. Opin. Rheumatol. 2022, 34, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, E.Y.; Oliver, M.S.; Cooper, A.M.; Basiaga, M.L.; Vora, S.S.; Lee, T.C.; Fox, E.; Amarilyo, G.; Stern, S.M.; et al. Chronic Nonbacterial Osteomyelitis/Chronic Recurrent Multifocal Osteomyelitis Study Group and the Childhood Arthritis and Rheumatology Research Alliance Scleroderma, Vasculitis, Autoinflammatory and Rare Diseases Subcommittee. Consensus Treatment Plans for Chronic Nonbacterial Osteomyelitis Refractory to Nonsteroidal Antiinflammatory Drugs and/or With Active Spinal Lesions. Arthritis Care Res. 2018, 70, 1228–1237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kerrison, C.; Davidson, J.E.; Cleary, A.G.; Beresford, M.W. Pamidronate in the treatment of childhood SAPHO syndrome. Rheumatology 2004, 43, 1246–1251. [Google Scholar] [CrossRef]
- Simm, P.; Allen, R.; Zacharin, M. Bisphosphonate Treatment in Chronic Recurrent Multifocal Osteomyelitis. J. Pediatr. 2008, 152, 571–575. [Google Scholar] [CrossRef]
- Miettunen, P.M.; Wei, X.; Kaura, D.; Reslan, W.A.; Aguirre, A.N.; Kellner, J.D. Dramatic pain relief and resolution of bone inflammation following pamidronate in 9 pediatric patients with persistent chronic recurrent multifocal osteomyelitis (CRMO). Pediatr. Rheumatol. Online J. 2009, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, H.; Wiltshire, E.; Briody, J.; Hall, J.; Chaitow, J.; Sillence, D.; Cowell, C.; Munns, C. Childhood Chronic Recurrent Multifocal Osteomyelitis: Pamidronate Therapy Decreases Pain and Improves Vertebral Shape. J. Rheumatol. 2008, 35, 707–712. [Google Scholar] [PubMed]
- Hospach, T.; Langendoerfer, M.; Kalle Tvon Maier, J.; Dannecker, G.E. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur. J. Pediatr. 2010, 169, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Roderick, M.; Shah, R.; Finn, A.; Ramanan, A.V. Efficacy of pamidronate therapy in children with chronic non-bacterial osteitis: Disease activity assessment by whole body magnetic resonance imaging. Rheumatology 2014, 53, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Range, U.; Hahn, G.; Berner, R.; Hedrich, C.M. Treatment Response and Longterm Outcomes in Children with Chronic Nonbacterial Osteomyelitis. J. Rheumatol. 2017, 44, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P.; Wenkert, D.; Clements, K.L.; McAlister, W.H.; Mumm, S. Bisphosphonate-induced osteopetrosis. N. Engl. J. Med. 2003, 349, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Simm, P.J.; Biggin, A.; Zacharin, M.R.; Rodda, C.P.; Tham, E.; Siafarikas, A.; Jefferies, C.; Hofman, P.L.; Jensen, D.E.; Woodhead, H.; et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J. Paediatr. Child Health 2018, 54, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.E.; Sbrocchi, A.M.; Scuccimarri, R. Improvement in spinal involvement with zoledronic acid in pediatric patients with chronic recurrent multifocal osteomyelitis: A case series. Bone Abstr. 2017, 6, 134. [Google Scholar] [CrossRef]
- Jansen, R.B.; Nilsson, J.; Buch-Larsen, K.; Kofod, T.; Schwarz, P. Treatment Effect of Zoledronic Acid in Chronic Non-bacterial Osteomyelitis of the Jaw: A Case Series. Calcif. Tissue Int. 2024, 114, 129–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1562. [Google Scholar] [CrossRef] [PubMed]
| Patient Details | Lesions | Pre-BP Treatment | BP Indication | Treatment Details | Clinical Response | Spinal Response | Year 1 Delta BMD | Post-Year 1 Annual Delta BMD Average | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9y Male | Mild spinal 1 Upper limb 3 Lower limb 3 Pelvic 5 | NSAID Steroids | Pain | 4 years of treatment with pamidronate then zoledronate Treatment was stopped due to escalating BMD Suspected monogenic mimic of CRMO | Improved pain Treatment de-escalation | Yes | No baseline | LS BMAD +0.45 per year TBLH BMD +0.25 per year |
| 2 | 14y Female | Mild spinal 1 Lower limb 1 Pelvic 2 | NSAID Steroids | Pain | 1 year of Treatment with Zoledronate Stopped due to Inefficacy Repeat MRI showed no active CRMO lesions Suspected pain amplification syndrome | None | N/A (sacral) | LS BMAD +0.1 TBLH BMD + 0.5 | N/A |
| 3 | 15y Female | Mild spinal 5 Upper limb 2 Lower limb 5 Pelvic 2 | NSAID Steroids | Spinal Lesions Pain | 1 year of Treatment with Zoledronate Stopped BP for inefficacy Suspected monogenic mimic Suspected pain amplification syndrome | Improved pain Treatment de-escalation | No | No baseline | N/A |
| 4 | 18y Female | Lower limb 6 Pelvic 1 | NSAID Opioid | Pain | 18 months of Treatment with Zoledronate Transferred to adult services—no further data | Improved pain Treatment de-escalation | N/A | No baseline | No follow-up scan |
| 5 | 12y Female | Lower limb 3 | NSAID Steroid | Pain | 18 months of treatment with Zoledronate Treatment complete | Improved pain Treatment de-escalation | N/A | LS BMAD +1 TBLH BMD +0.5 | No follow-up scan |
| 6 | 13y Female | Severe spinal 2 Mild spinal 1 Upper limb 2 Lower limb 4 Pelvic 2 | NSAID | Spinal lesions | 30 months of treatment with Zoledronate Treatment complete | Treatment de-escalation | No | LS BMAD + 1.7 TBLH BMD +0.9 | LS BMAD + 0.1 per year TBLH BMD +0.7 per year |
| 7 | 11y Male | Lower limb 4 Pelvic 1 | Missing data | Pain | 30 months of treatment Pamidronate—every 3 months then every 4 months Treatment complete | Improved pain | N/A | No baseline | No follow-up scan |
| 8 | 10y Female | Lower limb 5 Clavicle 1 | NSAID Steroid | Pain | 18 months of treatment with Zoledronate Treatment complete | Improved pain Treatment de-escalation | N/A | LS BMAD +2.1 TBLH BMD +1.6 | No follow-up scan |
| 9 | 12y Male | Mild spinal 3 Lower limb 5 Pelvic 1 | NSAID Steroid DMARD | Spinal lesions Pain | 30 months of treatment Pamidronate for 12 months then zoledronate for 18 months Treatment stopped due to BMD increase | Improved pain Treatment de-escalation | None | No baseline scan | Not known [When checked at Year 3: LS BMAD +3.9 TBLH BMD +2.5] |
| 10 | 14y Male | Severe spinal 2 Moderate Spinal 2 Mild spinal 8 Lower limb 5 | NSAID | Spinal lesions Pain | 18 months of treatment with Zoledronate Treatment complete | Improved pain | None | LS BMAD +1.6 TBLH BMD +0.3 | LS BMAD −0.3 per year TBLH BMD −0.1 per year |
| 11 | 9y Female | Plana spinal 1 Severe spinal 2 Upper limb 1 Lower limb 4 | NSAID | Spinal lesions | Ongoing treatment Zoledronate Doses halved due to escalating BMD | Not known (data missing) | No | LS BMAD +2.6 TBLH BMD +0.4 | Not known (in first year of treatment) |
| 12 | 7y Female | Clavicle 1 | NSAID Steroid DMARD | Pain | 27 months of treatment Zoledronate dose interval increased for escalating BMD Treatment stopped due to escalating BMD | Improved Pain Treatment de-escalation | N/A | LS BMAD + 0.8 TBLH BMD +0.3 | LS BMAD + 0.3 per year TBLH BMD +0.3 per year |
| 13 | 16y Male | Plana spinal 1 Moderate spinal 2 Upper limb 1 Lower limb 1 Pelvic 1 | NSAID Opioid | Spinal lesions Pain | 12 months of treatment with Zoledronate Treatment complete Suspected pain amplification syndrome | Improved pain Treatment de-escalation | Yes (except plana lesion) | LS BMAD + 1.4 TBLH BMD +0.6 | N/A |
| 14 | 11y Male | Severe spinal 3 | NSAID Opioid | Spinal lesions Pain | 18 months of treatment (ongoing) Zoledronate Doses halved for escalating BMD | Improved pain Treatment de-escalation | Yes | LS BMAD + 1.3 TBLH BMD +1.1 | LS BMAD + 0.65 per year TBLH BMD +0.45 per year |
| 15 | 16y Male | Upper limb 1 Pelvic 1 | NSAID Steroid | Pain | 18 months of treatment Pamidronate for 6 months then zoledronate for 12 months Treatment complete | Improved pain Treatment de-escalation | N/A | No baseline scan | No follow-up scan |
| 16 | 14y Female | Severe spinal 2 Mild spinal 1 Clavicle 1 | NSAID Steroid | Spinal lesions Pain | 6 months of treatment (ongoing) Zoledronate Transferred to another region | Improved pain Treatment de-escalation | N/A | No follow-up scan | No follow-up scan |
| 17 | 12y Male | Plana spinal 3 | NSAID | Spinal lesions Pain | 30 months of treatment with Zoledronate Interval increased for escalating BMD Treatment complete | Improved pain Treatment de-escalation | No | LS BMAD + 1.5 TBLH BMD +1.1 | No follow-up scans |
| 18 | 10y Female | Lower limb 2 Jaw 1 | NSAID Opioid | Pain | Zoledronate—due to receive first dose | N/A (treatment not yet received) | N/A | N/A | Not yet due |
| 19 | 12y Female | Lower limb 4 Pelvic 1 Clavicle 1 | NSAID | Pain | 36 months of treatment Pamidronate for 12 months then zoledronate for 24 months Treatment complete | Improved pain Treatment de-escalation | N/A | LS BMAD + 0.8 TBLH BMD +0.4 | No follow-up scan |
| 20 | 14y Female | Lower limb 3 Pelvic 2 | NSAID | Pain | 12 months of treatment with Pamidronate Treatment discontinued—no improvement in pain Suspected pain amplification syndrome | None | N/A | No baseline scan | No follow-up scan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, F.; Davis, P.J.C.; Crabtree, N.; Uday, S. Clinical, Bone Mineral Density and Spinal Remodelling Responses to Zoledronate Treatment in Chronic Recurrent Multifocal Osteomyelitis. Diagnostics 2025, 15, 2320. https://doi.org/10.3390/diagnostics15182320
Patel F, Davis PJC, Crabtree N, Uday S. Clinical, Bone Mineral Density and Spinal Remodelling Responses to Zoledronate Treatment in Chronic Recurrent Multifocal Osteomyelitis. Diagnostics. 2025; 15(18):2320. https://doi.org/10.3390/diagnostics15182320
Chicago/Turabian StylePatel, Fahim, Penelope J.C. Davis, Nicola Crabtree, and Suma Uday. 2025. "Clinical, Bone Mineral Density and Spinal Remodelling Responses to Zoledronate Treatment in Chronic Recurrent Multifocal Osteomyelitis" Diagnostics 15, no. 18: 2320. https://doi.org/10.3390/diagnostics15182320
APA StylePatel, F., Davis, P. J. C., Crabtree, N., & Uday, S. (2025). Clinical, Bone Mineral Density and Spinal Remodelling Responses to Zoledronate Treatment in Chronic Recurrent Multifocal Osteomyelitis. Diagnostics, 15(18), 2320. https://doi.org/10.3390/diagnostics15182320






