Antineutrophil Cytoplasmic Autoantibodies Specific to Bactericidal/Permeability-Increasing Protein: A Cross-Road Between Prolonged Gram-Negative Bacterial Infections and Ulcerative Colitis/Primary Sclerosing Cholangitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. IIF for Detection of ANCA
2.3. ANCA-Profile
2.4. Concentration of BPI-ANCA
2.5. Statistics
3. Results
3.1. General Characteristics of Patients
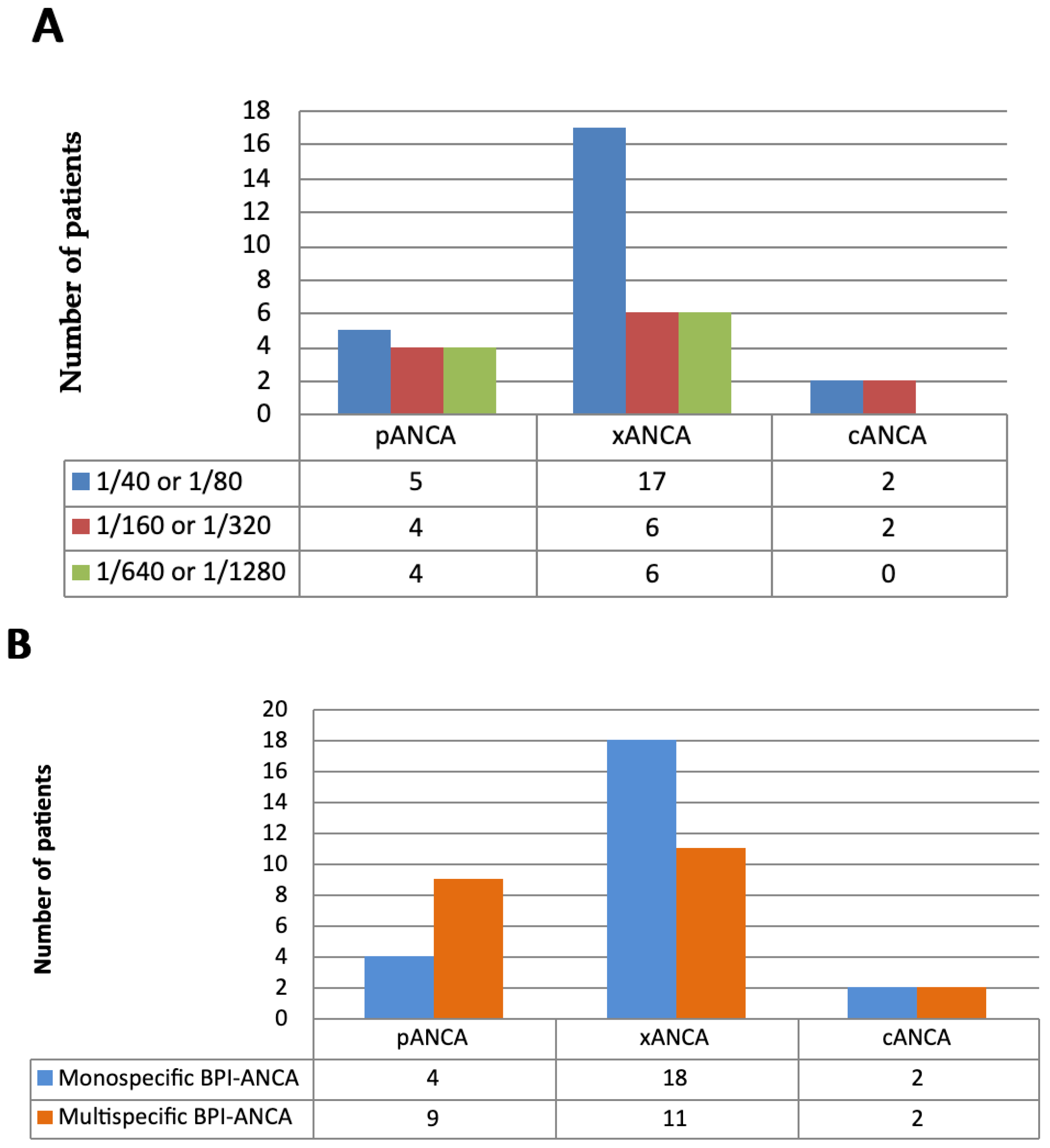
3.1.1. Type of IIF and Titer of ANCA in BPI-ANCA-Positive Patients
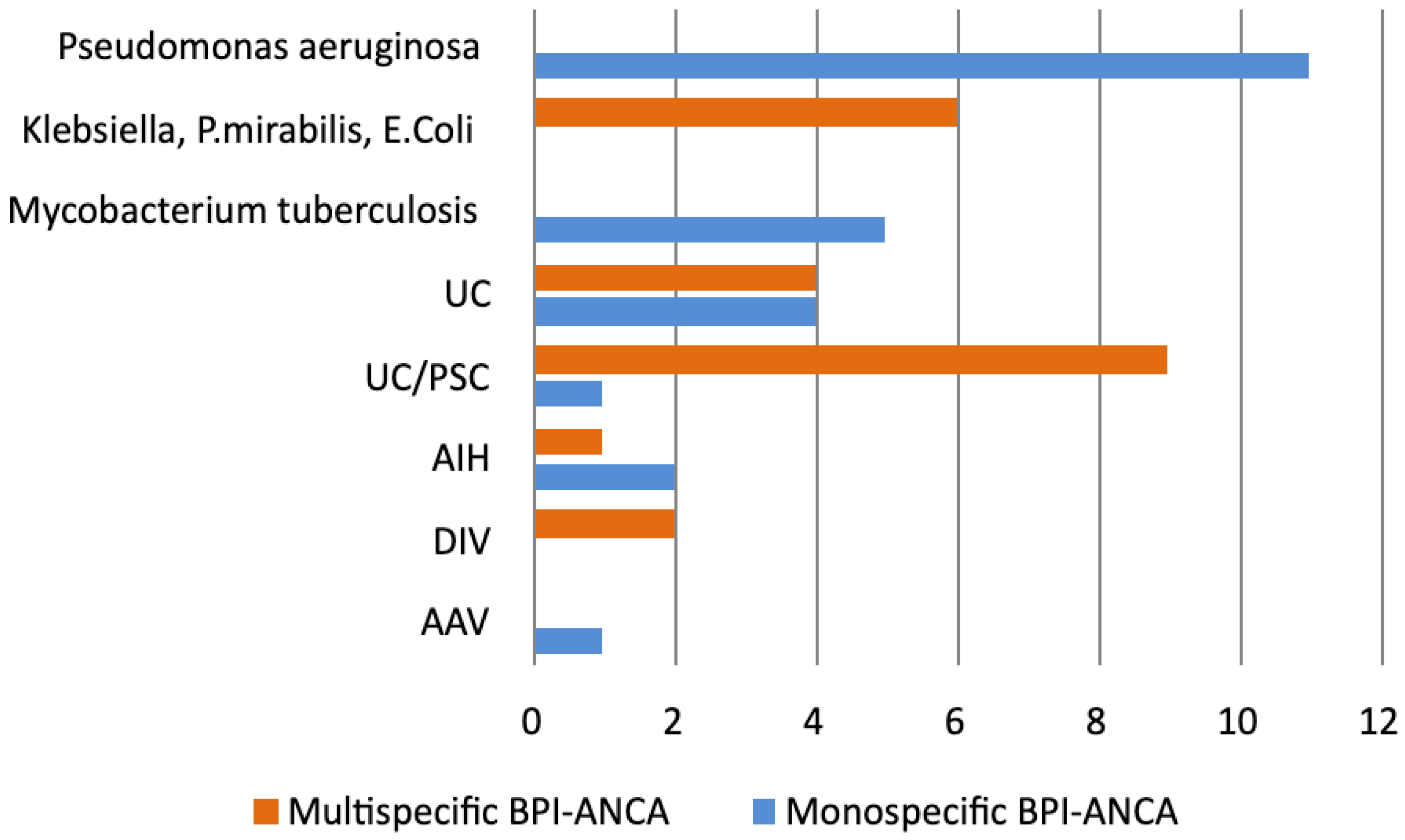
3.1.2. BPI-ANCA and ANCA Profile in Patients with Prolonged Infections
3.1.3. BPI-ANCA and ANCA Profile in IBD Patients
3.1.4. BPI-ANCA and ANCA Profile in Patients with AIH, DIV, AAV, and CTD
3.1.5. Sensitivity, Specificity, and Positive Predictive Value of Monospecific and Multispecific BPI-ANCA in Patients with Persistent Infections and UC/PSC
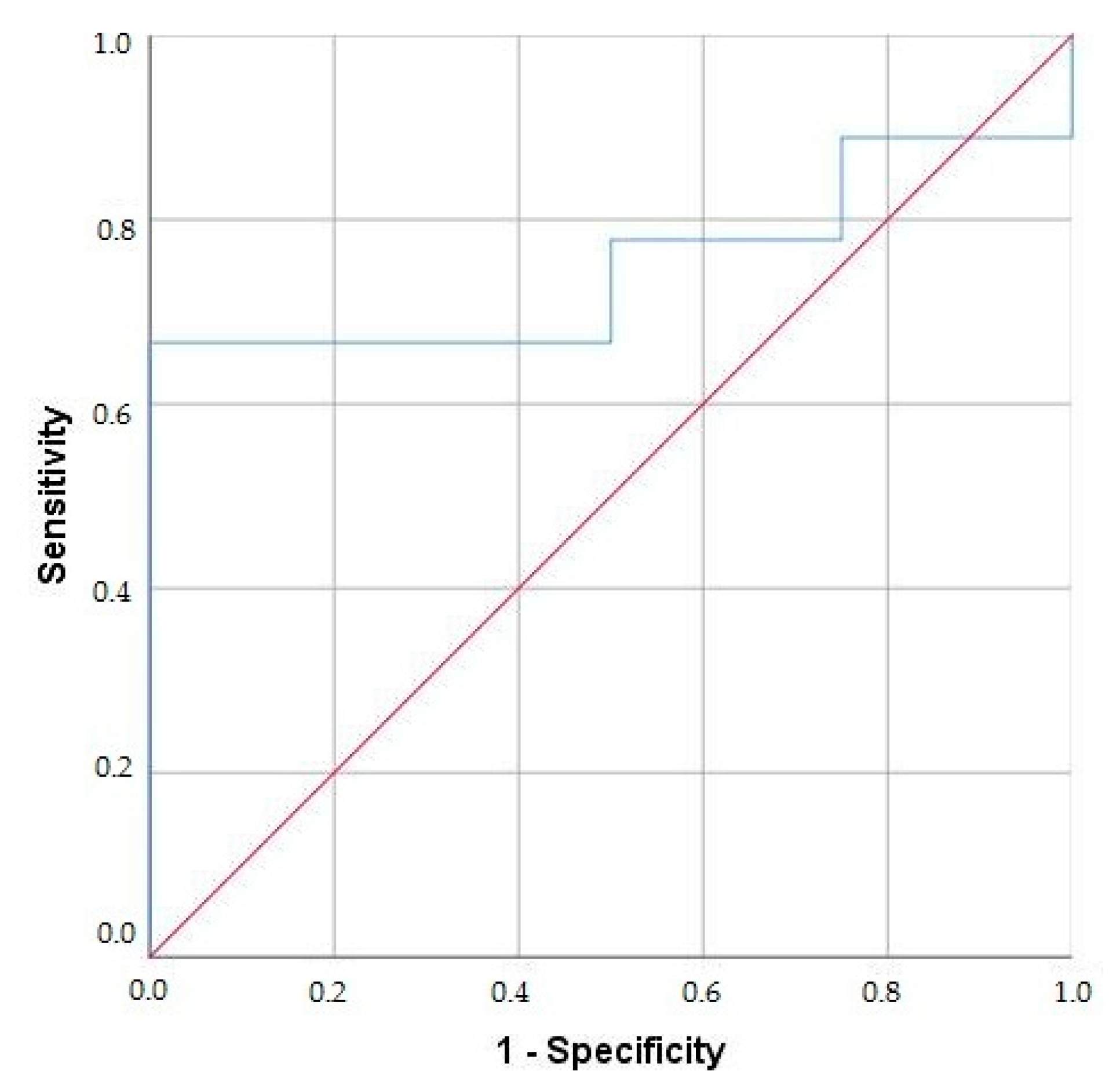
3.1.6. ROC Curve Analysis of Multispecific BPI-ANCA for Distinguishing UC/PSC from UC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCA | Antineutrophil cytoplasmic autoantibodies |
| AAV | ANCA-associated vasculitides |
| AIH | Autoimmune hepatitis |
| ASCA | Anti-Saccharomyces cerevisiae antibodies |
| BPI | Bactericidal/permeability-increasing protein |
| Cat-G | Catepsin G |
| CD | Crohn’s disease |
| CF | Cystic fibrosis |
| COPD | Chronic obstructive pulmonary disease |
| CTD | Connective tissue diseases |
| DIV | Drug-induced vasculitides |
| ELISA | Enzyme-linked immunosorbent assay |
| GN | Gram-negative |
| GP | Gram-positive |
| GPA | Granulomatosis with polyangiitis |
| HRP | Horseradish peroxidase |
| IBD | Inflammatory bowel diseases |
| IIF | Indirect immunofluorescence |
| IFN-γ | Interferon γ (IFN-γ) |
| Il-6 | Interleukin 6 |
| Lf | lactoferrin |
| LAM | Lipoarabinomannan |
| LE | Leucocyte elastase |
| LPB | Lipoprotein binding protein |
| LPS | Lipopolysaccharide |
| MD-2 | Myeloid differentiation factor |
| MPA | Microscopic polyangiitis |
| MPO | Myeloperoxidase |
| OD | Optical densities |
| OR | Odds Ratio |
| PR3 | Proteinase 3 |
| RPF | Retroperitoneal fibrosis |
| PSC | Primary sclerosing cholangitis |
| TBC | Tuberculosis |
| TMB | Tetramethylbenzidine |
| TNF-α | Tumor necrosis factor α |
| UC | Ulcerative colitis |
| UTI | Urinary tract infection |
References
- Theprungsirikul, J.; Skopelja-Gardner, S.; Rigby, W.F.C. Killing three birds with one BPI: Bactericidal, opsonic, and anti-inflammatory functions. J. Transl. Autoimmun. 2021, 28, 100105. [Google Scholar] [CrossRef]
- Schultz, H. From infection to autoimmunity: A new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI). Autoimmun. Rev. 2007, 6, 223–227. [Google Scholar] [CrossRef]
- Hovold, G.; Lindberg, U.; Ljungberg, J.K.; Shannon, O.; Påhlman, L.I. BPI-ANCA is expressed in the airways of cystic fibrosis patients and correlates to platelet numbers and Pseudomonas aeruginosa colonization. Respir. Med. 2020, 170, 105994. [Google Scholar] [CrossRef]
- Malhotra, S.; Hayes, D.; Wozniak, D.J. Cystic fibrosis and pseudomonas aeruginosa: The host-microbe interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, e1. [Google Scholar] [CrossRef]
- Girón, R.; Golpe, R.; Martínez-García, M.Á. Bronchiectasis not due to cystic fibrosis. Med. Clin. 2024, 163, 81–90. [Google Scholar] [CrossRef]
- Vaglio, A.; Maritati, F. Idiopathic retroperitoneal fibrosis. J. Am. Soc. Nephrol. 2016, 27, 1880–1889. [Google Scholar] [CrossRef]
- Kahnert, K.; Jörres, R.A.; Behr, J.; Welte, T. The Diagnosis and Treatment of COPD and Its Comorbidities. Dtsch. Arztebl. Int. 2023, 120, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, D.M.; Leonard, M.K.; Lobue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American thoracic society/Infectious diseases society of America/Centers for disease control and prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Mack, C.L.; Adams, D.; Assis, D.N.; Kerkar, N.; Manns, M.P.; Mayo, M.J.; Vierling, J.M.; Alsawas, M.; Murad, M.H.; Czaja, A.J. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology 2020, 72, 671–722. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Andrassy, K.; Bacon, P.A.; Churg, J.; Gross, W.L.; Hagen, E.C.; Hoffman, G.S.; Hunder, G.G.; Kallenberg, C.G.M.; et al. Nomenclature of Systemic Vasculitides. Arthritis Rheum. 1994, 37, 187–192. [Google Scholar] [CrossRef]
- Aringer, M.; Petri, M. New classification criteria for systemic lupus erythematosus. Curr. Opin. Rheumatol. 2020, 32, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Gross, W.L.; Trabandt, A.; Reinhold-Keller, E. Diagnosis and evaluation of vasculitis. Rheumatology 2000, 39, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Radice, A.; Yang, W.; Bentow, C.; Seaman, A.; Bianchi, L.; Sinico, R. Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies. Clin. Chim. Acta. 2012, 413, 719–726. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M. Etiopathogenesis of ANCA-Associated Vasculitis; Springer: Cham, Switzerland, 2020; pp. 33–45. [Google Scholar]
- Kim, J.M.; Choi, Y.M.; Jung, S.A.; Yang, H.R. Diagnostic utility, disease activity, and disease phenotype correlation of serum ASCA, pANCA, and PR3-ANCA in pediatric inflammatory bowel disease. J. Pediatr. 2024, 100, 204–211. [Google Scholar] [CrossRef]
- Bossuyt, X. Serologic markers in inflammatory bowel disease. Clin. Chem. 2006, 52, 171–181. [Google Scholar] [CrossRef]
- Dobric, S.; Popovic, D.; Nikolic, M.; Andrejevic, S.; Spuran, M.; Bonaci-Nikolic, B. Anti-neutrophil cytoplasmic antibodies (ANCA) specific for one or several antigens: Useful markers for subtypes of ulcerative colitis and associated primary sclerosing cholangitis. Clin. Chem. Lab. Med. 2012, 50, 503–509. [Google Scholar] [CrossRef]
- Sakurai, T.; Saruta, M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Niwa, M.; Takedatsu, H.; Yamasaki, H.; Kuwaki, K.; Yoshioka, S.; Yamauchi, R.; Fukunaga, S.; Torimura, T. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1304–1310. [Google Scholar] [CrossRef]
- Gao, X.; Hu, P.; He, Y.; Liao, S.; Peng, S.; Chen, M. Diagnostic role of anti-saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Zhonghua Nei Ke Za Zhi 2005, 44, 428–430. [Google Scholar]
- Ghouri, Y.A.; Tahan, V.; Shen, B. Secondary causes of inflammatory bowel diseases. World J. Gastroenterol. 2020, 26, 3998–4017. [Google Scholar] [CrossRef] [PubMed]
- Kontic, M.; Radovanovic, S.; Nikolic, M.; Bonaci-Nikolic, B. Concomitant drug-and infection-induced antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis with multispecific ANCA. Med. Princ. Pract. 2012, 21, 488–491. [Google Scholar] [CrossRef]
- Stinton, L.M.; Bentow, C.; Mahler, M.; Norman, G.L.; Eksteen, B.; Mason, A.L.; Kaplan, G.G.; Lindkvist, B.; Hirschfield, G.M.; Milkiewicz, P.; et al. PR3-ANCA: A promising biomarker in primary sclerosing cholangitis (PSC). PLoS ONE 2014, 9, e112877. [Google Scholar] [CrossRef]
- Beretta-Piccoli, B.T.; Mieli-Vergani, G.; Vergani, D. Autoimmune Hepatitis: Serum Autoantibodies in Clinical Practice. Clin. Rev. Allergy Immunol. 2022, 63, 124–137. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Zhao, M.H.; Hamilton, M.I.; Brownlee, A.; Chapman, P.; Pounder, R.E.; Wakefield, A.J.; Lockwood, C.M. Antineutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut 1997, 40, 105–109. [Google Scholar] [CrossRef]
- Mumtaz, S.; Valecha, J.; Hochwald, A.; Berianu, F.; Majithia, V.; Abril, A. Investigating the concomitance of anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitides and inflammatory bowel disease (IBD). Semin. Arthritis Rheum. 2024, 66, 152452. [Google Scholar] [CrossRef] [PubMed]
- Laass, M.W.; Ziesmann, J.; de Laffolie, J.; Röber, N.; Conrad, K. Anti-Proteinase 3 Antibodies as a Biomarker for Ulcerative Colitis and Primary Sclerosing Cholangitis in Children. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 463–470. [Google Scholar] [CrossRef]
- Lee, W.I.; Subramaniam, K.; Hawkins, C.A.; Randall, K.L. The significance of ANCA positivity in patients with inflammatory bowel disease. Pathology 2019, 51, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, J.; Ji, C.; Bao, X.; Yuan, C. Predicting bacterial infection risk in patients with ANCA-associated vasculitis in southwest China: Development of a new nomogram. Clin. Rheumatol. 2022, 41, 3451–3460. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L. Virus-induced systemic vasculitides: New therapeutic approaches. Clin. Dev. Immunol. 2004, 11, 227–231. [Google Scholar] [CrossRef]
- Zhao, M.H.; Jones, S.J.; Lockwood, C.M. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin. Exp. Immunol. 1995, 99, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, L.; Ren, Q.; Feng, H.; Tao, S.; Cheng, L.; Ma, L.; Gou, S.J.; Fu, P. Share Understanding the Gut-Kidney Axis in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: An Analysis of Gut Microbiota Composition. Front. Pharmacol. 2022, 24, 783679. [Google Scholar]
- Iwuji, K.; Kanu, A.; Stroever, S.; Nugent, K.; Hamood, A.; Scott, C.; Navarro, S. Clinical significance of BPI-ANCA in patients with cystic fibrosis: A single center prospective study. Sci. Rep. 2023, 13, 18138. [Google Scholar] [CrossRef]
- Munford, R.S. Detoxifying endotoxin: Time, place and person. J. Endotoxin Res. 2005, 11, 69–84. [Google Scholar] [CrossRef]
- Fötisch, K.; Vieths, S. N- and O-linked oligosaccharides of allergenic glycoproteins. Glycoconj. J. 2001, 18, 373–390. [Google Scholar] [CrossRef]
- Iwuji, K.; Larumbe-Zabala, E.; Bijlani, S.; Nugent, K.; Kanu, A.; Manning, E.; Solis, X. Prevalence of Bactericidal/Permeability-Increasing Protein Autoantibodies in Cystic Fibrosis Patients: Systematic Review and Meta-Analysis. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 45–51. [Google Scholar] [CrossRef]
- Adam, Z.; Čermák, A.; Adamová, Z.; Řehák, Z.; Koukalová, R.; Pour, L. Retroperitoneal fibrosis-diagnosis and treatment. Rozhl. Chir. 2022, 101, 265–272. [Google Scholar]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Characteristics and prognosis of ANCA-positive retroperitoneal fibrosis: A systematic literature review. Autoimmun. Rev. 2020, 19, 102642. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Valerio, J.A.; Flores-Suárez, L.F.; Rodríguez-Amado, J.; Garza-Elizondo, M.A.; Rendón, A.; Salinas-Carmona, M.C. Antineutrophil cytoplasm autoantibodies in patients with tuberculosis are directed against bactericidal/permeability increasing protein and are detected after treatment initiation. Clin. Exp. Rheumatol. 2010, 28, 35–39. [Google Scholar] [PubMed]
- Guzmán-Beltrán, S.; Juárez, E.; Cruz-Muñoz, B.L.; Páez-Cisneros, C.A.; Sarabia, C.; González, Y. Bactericidal Permeability-Increasing Protein (BPI) Inhibits Mycobacterium tuberculosis Growth. Biomolecules 2024, 14, 475. [Google Scholar] [CrossRef]
- Weiss, J.; Olsson, I. Cellular and subcellular localization of the bactericidal/permeability- increasing protein of neutrophils. Blood 1987, 69, 652–659. [Google Scholar] [CrossRef]
- Pugin, J.; Heumann, D.; Tomasz, A.; Kravchenko, V.V.; Akamatsu, Y.; Nishijima, M.; Glauser, M.P.; Tobias, P.S.; Ulevitch, R.J. CD14 Is a pattern recognition receptor. Immunity 1994, 1, 509–516. [Google Scholar] [CrossRef]
- Huan, G.; Yang, G.; Qu, X.; Xu, J.; Song, Y. Antineutrophil cytoplasmic antibodies in Chinese patients with tuberculosis. Rev. Soc. Bras. Med. Trop. 2018, 51, 475–478. [Google Scholar] [CrossRef]
- Bonaci-Nikolic, B.; Andrejevic, S.; Pavlovic, M.; Dimcic, Z.; Ivanovic, B.; Nikolic, M. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: Diagnostic and therapeutic challenge. Clin. Rheumatol. 2010, 29, 893–904. [Google Scholar] [CrossRef] [PubMed]
| ANCA-Positive (IIF) Patients n = 193 | |
|---|---|
| Age, years (MV ± SD) Years range | 45.76 ± 15.97 18–80 |
| Male sex, n (%) | 121 (62.7) |
| Female sex, n (%) | 72 (37.3) |
| Persistent bacterial and viral infections, n (%) | 41 (21.2) |
| Pseudomonas aeruginosa, n (%) | 14 (7.3) |
| - Cystic fibrosis | 3 |
| - Bronchiectasis | 5 |
| - Chronic obstructive pulmonary disease | 6 |
| Klebsiella pneumoniae, Proteus mirabilis or Echerichia coli, n (%) | 6 (3.1) |
| - Urinary tract infection (UTI) | 3 |
| - UTI complicated by retroperitoneal fibrosis | 3 |
| Mycobacterium tuberculosis, n (%) | 7 (3.6) |
| Streptococcus pyogenes, Staphylococcus aureus or Streptococcus pneumoniae, n (%) | 9 (4.7) |
| - Empyema | 2 |
| - Chronic obstructive pulmonary disease | 5 |
| - Chronic sinusitis | 2 |
| Hepatitis C Virus, n (%) | 5 (2.6) |
| Inflammatory bowel diseases, n (%) | 48 (24.9) |
| Ulcerative colitis, n (%) | 24 (12.4) |
| Ulcerative colitis with primary sclerosing cholangitis, n (%) | 14 (7.3) |
| Crohn’s disease, n (%) | 10 (5.2) |
| Autoimmune hepatitis, n (%) | 19 (9.8) |
| Vasculitides, n (%) | 57 (29.5) |
| Drug-induced vasculitides, n (%) | 17 (8.8) |
| ANCA-associated vasculitides, n (%) | 40 (20.7) |
| - Granulomatosis with polyangiitis | 16 (8.3) |
| - Microscopic polyangiitis | 20 (10.4) |
| - Eosinophilic granulomatosis with polyangiitis | 4 (2.1) |
| Connective tissue diseases, n (%) | 28 (14.5) |
| - Systemic lupus erythematosus, n (%) | 14 (7.3) |
| - Sjögren syndrome, n (%) | 10 (5.2) |
| - Systemic sclerosis, n (%) | 4 (2.1) |
| Monospecific BPI-ANCA + n = 24 | Multispecific BPI-ANCA + n = 22 | Negative BPI-ANCA n = 147 | |
|---|---|---|---|
| Age, years range (MV ± SD) | 49.6 ± 17.7 | 39.5 ± 13.9 | 45.5 ± 17.6 |
| Male sex, n = 121 | 9 | 8 | 55 |
| (%) | 50.7 ± 19.0 | 36.5 ± 13.4 | 45.6 ± 18.4 |
| Female sex, n = 72 | 15 | 14 | 92 |
| (%) | 48.7 ± 16.4 | 42.4 ± 14.7 | 45.3 ± 16.6 |
| Persistent infections, n = 41 | 16 (66.7) * | 6 (27.3) | 19 (12.9) |
| BPI-ANCA (MV ± SD) U/mL | 69.4 ± 23.4 ** | 28.1 ± 2.6 | 3.5 ± 2.9 |
| Gram-negative bacterial infections, n = 20 (%) | 11 (45.8) | 6 (27.3) | 3 (2.0) |
| Pseudomonas aeruginosa, n = 14 (%) | 11 (45.8) ** | 0 | 3 (2.0) |
| BPI-ANCA (MV ± SD) U/mL | 68.6 ± 28.1 ** | (3.0 ± 2.8) | |
| Klebsiella pneumoniae, Proteus mirabilis, or Echerichia coli, n = 6 (%) | 0 | 6 (27.3) ** | 0 |
| BPI-ANCA (MV ± SD)U/mL | 78.0 ± 25.7 ** | ||
| Mycobacterium tuberculosis, n = 7 (%) | 5 (20.8) * | 0 | 2 (1.7) |
| BPI-ANCA (MV ± SD) U/mL | 70.2 ± 18.8 ** | (2.8 ± 3.1) | |
| Gram-positive bacterial infections, n = 9 (%) | 0 | 0 | 9 (6.1) |
| Streptococcus pyogenes, Staphylococcus aureus or | |||
| Streptococcus pneumoniae, n = 9 (%) | 0 | 0 | 9 (6.1) |
| BPI-ANCA (MV ± SD) U/mL | (4.3 ± 3.1) | ||
| Viral infections, n = 5 | 0 | 0 | 5 (3.4) |
| Hepatitis C Virus, n = 5 (%) | 0 | 0 | 5 (3.4) |
| BPI-ANCA (MV ± SD) U/mL | (3.42 ± 2.9) | ||
| Inflammatory bowel diseases, n = 48 (%) | 5 (20.1) | 13 (59.1) * | 30(20.4) |
| BPI-ANCA (MV ± SD) U/mL | 47 ± 15.4 | 27.6 ± 15.2 | (3.1 ± 4.4) |
| UC, n = 24 (%) | 4 (16.7) | 4 (18.2) | 16 (10.9) |
| BPI-ANCA (MV ± SD) U/mL | 44.8 ± 15.9 ** | 19.5 ± 17.6 | (3.3 ± 3.0) |
| UC/PSC, n = 14 (%) | 1 (4.2) | 9 (40.9) ** | 4 (2.7) |
| BPI-ANCA (MV ± SD) U/mL | 49.2 ± 14.9 | 35.6 ± 12.7 | (3.8 ± 5.5) |
| CD, n = 10 (%) | 0 | 0 | 10 (11) |
| BPI-ANCA (MV ± SD) U/mL | (2.1 ± 4.4) | ||
| AIH, n = 19 (%) | 2 (8.3) | 1 (4.5) | 16 (6.8) |
| BPI-ANCA (MV ± SD) U/mL | 76.5 ± 18.8 ** | 15.6 ± 17.3 | (4.10 ± 3.9) |
| DIV (Propylthiouracil/methimazole), n = 17 (%) | 0 | 2 (4.4) | 15 (6.8) |
| BPI-ANCA (MV ± SD) U/mL | 13.8 ± 12.9 | (2.76 ± 3.2) | |
| AAV, n = 40 (%) | 1 (4.2) | 0 | 39 (26.5) |
| BPI-ANCA (MV ± SD) U/mL | 77.1 ± 16.5 ** | (4.21 ± 3.3) | |
| CTD, n = 28 (%) | 0 | 0 | 28 (19) |
| BPI-ANCA (MV ± SD) U/mL | (2.19 ± 3.6) | ||
| Healthy, n = 52 (%) | 0 | 0 | 52 (35.4) |
| BPI-ANCA (MV ± SD) U/mL | (3.88 ± 4.1) | ||
| All patients, n = 193 | 24 | 22 | 147 |
| BPI-ANCA (MV ± SD) U/mL | 64.3 ± 17.7 * | 32.4 ± 14.1 | (3.3 ± 4.1) |
| ANCA Profile | Klebsiella, Proteus mirabilis, or Echerichia coli (n = 6) | Pseudomonas Aeruginosa (n = 11) | TBC (n = 5) | UC (n = 8) | UC/PSC (n = 10) | AIH (n = 3) | DIV (n = 2) | AAV (n = 1) |
|---|---|---|---|---|---|---|---|---|
| BPI | ||||||||
| Patients, n (%) | 6 (100) | 11 (79) | 5 (71) | 8 (33) | 10 (71) | 3 (16) | 2 (12) | 1 (3) |
| Low, n (%) | 3 (50) | 1 (10) | 3 (43) | 5 (21) | 5 (36) | 2 (11) | 2 (12) | 1 (3) |
| Medium, n (%) | 3 (50) | 6 (54) | 0 | 1 (4) | 2 (14) | 0 | 0 | 0 |
| High, n (%) | 0 | 4 (36) | 2 (29) | 2 (8) | 3 (21) | 1 (5) | 0 | 0 |
| PR3 | ||||||||
| Patients, n (%) | 6 (100) | 0 | 0 | 3 (13) | 8 (57) | 1 (5) | 2 (12) | 0 |
| Low, n (%) | 3 (50) | 0 | 0 | 3 (13) | 7 (50) | 0 | 1 (6) | 0 |
| Medium, n (%) | 2 (20) | 0 | 0 | 0 | 1 (7) | 0 | 1 (6) | 0 |
| High, n (%) | 1 (10) | 0 | 0 | 0 | 0 | 1 (5) | 0 | 0 |
| MPO | ||||||||
| Patients, n (%) | 3 (50) | 0 | 0 | 0 | 0 | 1 (5) | 2 (12) | 0 |
| Low, n (%) | 1 (10) | 0 | 0 | 0 | 0 | 0 | 1 (6) | 0 |
| Medium, n (%) | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High, n (%) | 1 (10) | 0 | 0 | 0 | 0 | 1 (5) | 1 (6) | 0 |
| LE | ||||||||
| Patients, n (%) | 1 (10) | 0 | 0 | 2 (8) | 1 (7) | 0 | 2 (12) | 0 |
| Low, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medium, n (%) | 1 (10) | 0 | 0 | 0 | 1 (7) | 0 | 1 (6) | 0 |
| High, n (%) | 0 | 0 | 0 | 2 (8) | 0 | 0 | 1 (6) | 0 |
| Cat-G | ||||||||
| Patients, n (%) | 0 | 0 | 0 | 0 | 1 (7) | 0 | 2 (12) | 0 |
| Low, n (%) | 0 | 0 | 0 | 0 | 1 (7) | 0 | 2 (12) | 0 |
| Medium, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LF | ||||||||
| Patients, n (%) | 2 (20) | 0 | 0 | 0 | 1 (7) | 0 | 1 (6) | 0 |
| Low, n (%) | 1 (10) | 0 | 0 | 0 | 1 (7) | 0 | 1 (6) | 0 |
| Medium, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High, n (%) | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BPI-ANCA Positivity (ELISA) | Sensitivity, n (%) | Specificity, n (%) |
|---|---|---|
| Pseudomonas aeruginosa | 11/14 (79) | 144/179 (80.4) |
| Klebsiella pneumoniae, Proteus mirabilis or Echerichia coli | 6/6 (100) | 147/187 (78.6) |
| Mycobacterium tuberculosis | 5/7 (71.4) | 145/186 (77.9) |
| UC | 8/24 (33.3) | 131/169 (77.5) |
| UC/PSC | 10/14 (71.4) | 143/179 (79.9) |
| AIH | 3/19 (15.8) | 131/174 (75.3) |
| DIV | 2/17 (11.8) | 132/176 (75) |
| AAV | 1/40 (2.5) | 108/153 (71) |
| Monospecific BPI-ANCA positivity | Sensitivity, n (%) | Specificity, n (%) |
| Pseudomonas aeruginosa | 11/14 (79) | 144/179 (80.4) |
| Klebsiella pneumoniae, Proteus mirabilis, or Echerichia coli | 0 (0) | 163/187 (87.1) |
| Mycobacterium tuberculosis | 5/7 (71.4) | 145/186 (77.9) |
| UC | 4/24(16.7) | 131/169 (77.5) |
| UC/PSC | 1/14 (7.1) | 143/179 (79.9) |
| AIH | 2/19 (10.5) | 131/174 (75.3) |
| DIV | 0 (0) | 152/176 (86.3) |
| AAV | 1/40 (2.5) | 108/153 (71) |
| Multispecific BPI-ANCA positivity | Sensitivity, n (%) | Specificity, n (%) |
| Pseudomonas aeruginosa | 0 (0) | 160/182 (87.9) |
| Klebsiella pneumoniae, Proteus mirabilis, or Echerichia coli | 6/6 (100) | 147/187 (78.6) |
| Mycobacterium tuberculosis | 0 (0) | 164/186 (89.1) |
| UC | 4/24(16.7) | 131/169 (77.5) |
| UC/PSC | 9/14 (64.3) | 143/179 (79.9) |
| AIH | 1/19 (5.3) | 131/174 (75.3) |
| DIV | 2/17 (11.8) | 132/176 (75) |
| AAV | 0 (0) | 131/153 (85.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanovic, D.; Miskovic, R.; Plavsic, A.; Radovic, S.; Nagorni-Obradovic, L.; Popovic, D.; Nikolic, M.M.; Bonaci-Nikolic, B. Antineutrophil Cytoplasmic Autoantibodies Specific to Bactericidal/Permeability-Increasing Protein: A Cross-Road Between Prolonged Gram-Negative Bacterial Infections and Ulcerative Colitis/Primary Sclerosing Cholangitis. Diagnostics 2025, 15, 2309. https://doi.org/10.3390/diagnostics15182309
Jovanovic D, Miskovic R, Plavsic A, Radovic S, Nagorni-Obradovic L, Popovic D, Nikolic MM, Bonaci-Nikolic B. Antineutrophil Cytoplasmic Autoantibodies Specific to Bactericidal/Permeability-Increasing Protein: A Cross-Road Between Prolonged Gram-Negative Bacterial Infections and Ulcerative Colitis/Primary Sclerosing Cholangitis. Diagnostics. 2025; 15(18):2309. https://doi.org/10.3390/diagnostics15182309
Chicago/Turabian StyleJovanovic, Dragana, Rada Miskovic, Aleksandra Plavsic, Sara Radovic, Ljudmila Nagorni-Obradovic, Dragan Popovic, Milos M. Nikolic, and Branka Bonaci-Nikolic. 2025. "Antineutrophil Cytoplasmic Autoantibodies Specific to Bactericidal/Permeability-Increasing Protein: A Cross-Road Between Prolonged Gram-Negative Bacterial Infections and Ulcerative Colitis/Primary Sclerosing Cholangitis" Diagnostics 15, no. 18: 2309. https://doi.org/10.3390/diagnostics15182309
APA StyleJovanovic, D., Miskovic, R., Plavsic, A., Radovic, S., Nagorni-Obradovic, L., Popovic, D., Nikolic, M. M., & Bonaci-Nikolic, B. (2025). Antineutrophil Cytoplasmic Autoantibodies Specific to Bactericidal/Permeability-Increasing Protein: A Cross-Road Between Prolonged Gram-Negative Bacterial Infections and Ulcerative Colitis/Primary Sclerosing Cholangitis. Diagnostics, 15(18), 2309. https://doi.org/10.3390/diagnostics15182309







