An Uncommon Cause of Angina
Abstract
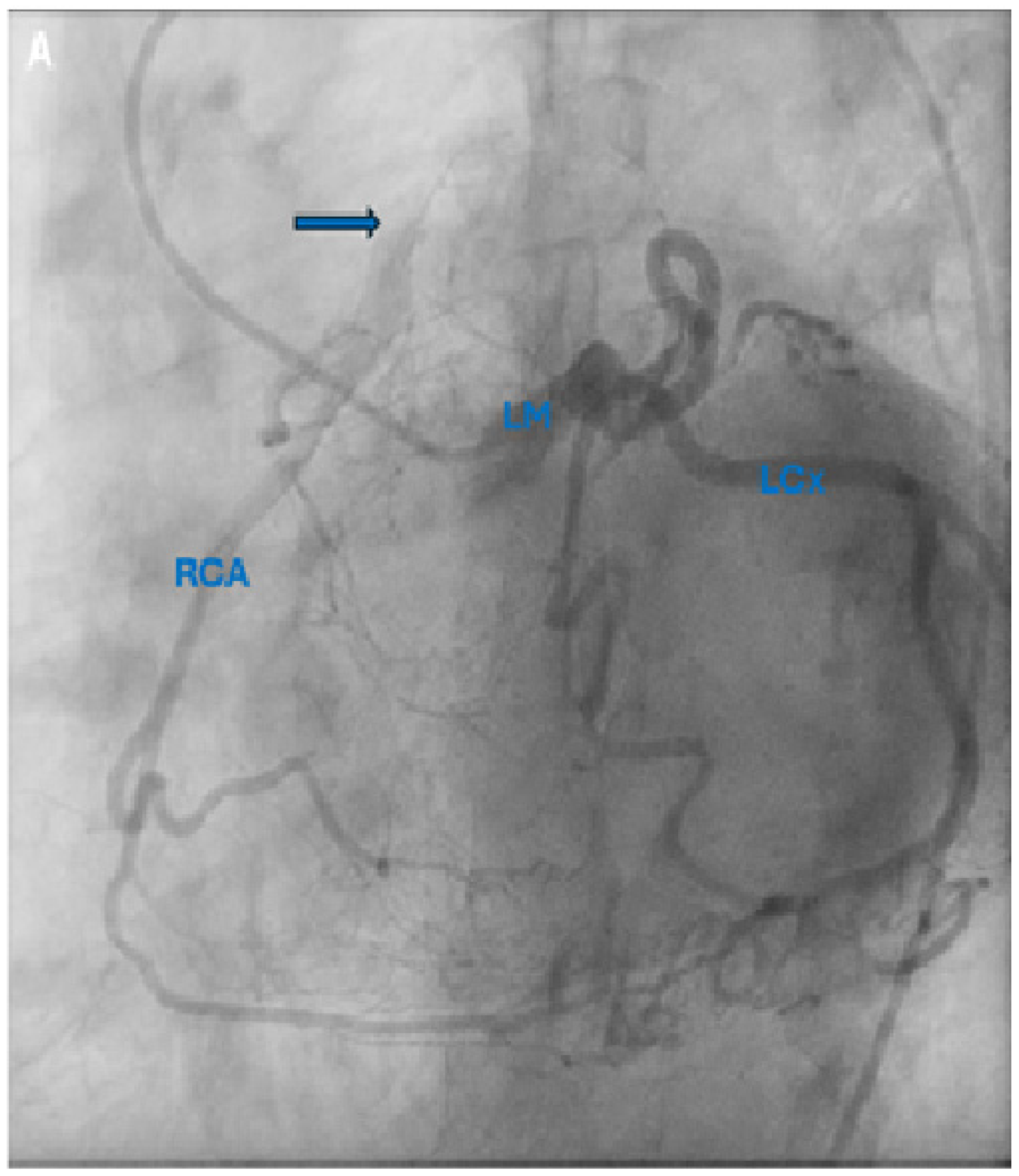

Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guenther, T.M.; Sherazee, E.A.; Wisneski, A.D.; Gustafson, J.D.; Wozniak, C.J.; Raff, G.W. Anomalous origin of the right coronary artery from the pulmonary artery: A systematic review. Ann. Thorac. Surg. 2020, 110, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Uzcategui, C.E.; das Neves, B.; Salinas, P.; Fernández-Ortiz, A.; Núñez-Gil, I.J.; Kunadian, V.; Kundi, H.; Rampat, R.; Milasinovic, D.; Sayers, M.; et al. Anomalous origin of coronary arteries from pulmonary artery in adults: A case series. Eur. Hear. J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rawala, M.S.; Shah Naqvi, S.H.; Farhan, K.; Yasin, M.; Rizvi, S.B. Anomalous origin of the right coronary artery from pulmonary artery. Case Rep. Cardiol. 2018, 2018, 2583918. [Google Scholar] [CrossRef] [PubMed]
- Blickenstaff, E.A.; Smith, S.D.; Cetta, F.; Connolly, H.M.; Majdalany, D.S. Anomalous left coronary artery from the pulmonary artery: How to diagnose and treat. J. Pers. Med. 2023, 13, 1561. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, H.; Fawcett, J.S.; Johnson, A.L. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 1968, 38, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e637–e697. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.A.; Gersony, W.M.; Hellenbrand, W.E. Anomalous right coronary artery arising from the pulmonary artery: A report of 7 cases and a review of the literature. Am. Heart J. 2006, 152, 1004.e9–1004.e17. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-Y.; Xu, Z.-H. Surgical treatment of anomalous origin of coronary artery from the pulmonary artery. Chin. Med. J. 2008, 121, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-P.; Guo, H.-W.; Hu, S.-S.; Sun, L.-Z.; Song, Y.-H.; Sun, H.-S. Results of surgical correction in patients with anomalous origin of the coronary artery from the pulmonary artery. Zhonghua Wai Ke Za Zhi 2006, 44, 1525–1528. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majdalany, D.S.; Blickenstaff, E.A.; Marcotte, F.; Anderson, J.H. An Uncommon Cause of Angina. Diagnostics 2025, 15, 2241. https://doi.org/10.3390/diagnostics15172241
Majdalany DS, Blickenstaff EA, Marcotte F, Anderson JH. An Uncommon Cause of Angina. Diagnostics. 2025; 15(17):2241. https://doi.org/10.3390/diagnostics15172241
Chicago/Turabian StyleMajdalany, David S., Elaina A. Blickenstaff, Francois Marcotte, and Jason H. Anderson. 2025. "An Uncommon Cause of Angina" Diagnostics 15, no. 17: 2241. https://doi.org/10.3390/diagnostics15172241
APA StyleMajdalany, D. S., Blickenstaff, E. A., Marcotte, F., & Anderson, J. H. (2025). An Uncommon Cause of Angina. Diagnostics, 15(17), 2241. https://doi.org/10.3390/diagnostics15172241





