First-Trimester Uterine Artery Doppler Indices and Pregnancy Outcomes in Naturally Conceived and Frozen–Thawed Embryo Transfer Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Participant Selection
2.3. Data Collection and Clinical Assessments
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assisted Reproductive Technology |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| FET | Frozen–Thawed Embryo Transfer |
| GDM | Gestational Diabetes Mellitus |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| HRT | Hormone-Replacement Therapy |
| IQR | Interquartile Range |
| IVF | In Vitro Fertilization |
| LPS | Luteal Phase Support |
| OR | Odds Ratio |
| PI | Pulsatility Index |
| PCOS | Polycystic Ovary Syndrome |
| OGTT | Oral Glucose Tolerance Test |
| TSH | Thyroid-Stimulating Hormone |
References
- Pereira, N.; Rosenwaks, Z. A fresh(er) perspective on frozen embryo transfers. Fertil. Steril. 2016, 106, 257–258. [Google Scholar] [CrossRef]
- Ong, J.; Mathew, J.; Choolani, M.; Wong, P.C. Oocytes on Ice: Exploring the Advancements in Elective Egg Freezing for Women. Ann. Acad. Med. Singap. 2024, 53, 34–42. [Google Scholar] [CrossRef]
- Martinez-Rodero, I.; Gallardo, M.; Pisaturo, V.; Scarica, C.; Conaghan, J.; Liebermann, J.; Cuevas-Saiz, I. Shorter Protocols for Vitrification and Post-Warming Dilution of Human Oocytes and Embryos: A Narrative Review. Reprod. Biomed. Online 2025, 51, 104857. [Google Scholar] [CrossRef] [PubMed]
- Pritts, E.A.; Atwood, A.K. Luteal Phase Support in Infertility Treatment: A Meta-Analysis of the Randomized Trials. Hum. Reprod. 2002, 17, 2287–2299. [Google Scholar] [CrossRef]
- Nosarka, S.; Kruger, T.; Siebert, I.; Grové, D. Luteal Phase Support in In Vitro Fertilization: Meta-Analysis of Randomized Trials. Gynecol. Obstet. Investig. 2005, 60, 67–74. [Google Scholar] [CrossRef]
- Dashti, S.; Eftekhar, M. Luteal-Phase Support in Assisted Reproductive Technology: An Ongoing Challenge. Int. J. Reprod. Biomed. 2021, 19, 761–772. [Google Scholar] [CrossRef]
- Stavridis, K.; Balafoutas, D.; Kalampokas, T.; Benetou, V.; Samoli, E.; Vlahos, N.; Kasdagli, M.I. Oral Dydrogesterone Versus Vaginal Progesterone for Luteal Phase Support in Frozen-Thawed Embryo Transfer Cycles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2025, 14, 3238. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, S.; Tamimi, M.; Saharkhiz, N. Comparison of Oral Dydrogesterone with Suppository Vaginal Progesterone for Luteal-Phase Support in In Vitro Fertilization (IVF): A Randomized Clinical Trial. Iran J. Reprod. Med. 2013, 11, 913–918. [Google Scholar] [PubMed]
- Tank, J.; Gupte, S.; Mahapatra, P.C.; Reddy, J.; Mittal, P.; Mukhopadhyay, A.K.; Vyas, L.; Batra, A.; Gupta, M.; Tandulwadkar, S.; et al. Real-World Utilization Pattern of Dydrogesterone in 7287 Indian Women with Obstetric and Gynecological Conditions: Data from Multicentric, Retrospective Study. Rev. Bras. Ginecol. Obstet. 2024, 46, e-rbgo18. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, G.; Blockeel, C.; Kahler, E.; Pexman-Fieth, C.; Olofsson, J.I.; Driessen, S.; Tournaye, H. Dydrogesterone as an Oral Alternative to Vaginal Progesterone for IVF Luteal Phase Support: A Systematic Review and Individual Participant Data Meta-Analysis. PLoS ONE 2020, 15, e0241044. [Google Scholar] [CrossRef]
- Lang, U.; Baker, R.S.; Braems, G.; Zygmunt, M.; Künzel, W.; Clark, K.E. Uterine Blood Flow—A Determinant of Fetal Growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110 (Suppl. S1), S55–S61. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, X. A Review of Roles of Uterine Artery Doppler in Pregnancy Complications. Front. Med. 2022, 9, 813343. [Google Scholar] [CrossRef]
- Campbell, S.; Pearce, J.M.F.; Hackett, G.; Cohen-Overbeek, T.A.; Hernandez. Qualitative Assessment of Uteroplacental Blood Flow: Early Screening Test for High-Risk Pregnancies. Obstet. Gynecol. 1986, 68, 649–653. [Google Scholar]
- Lees, C.; Parra, M.; Missfelder-Lobos, H.; Morgans, A.; Fletcher, O.; Nicolaides, K.H. Individualized Risk Assessment for Adverse Pregnancy Outcome by Uterine Artery Doppler at 23 Weeks. Obstet. Gynecol. 2001, 98, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, M.A.; Palmer, K.R.; Hodges, R.J.; Costa, F.D.S.; Rolnik, D.L. Uterine Artery Doppler in Screening for Preeclampsia and Fetal Growth Restriction. Rev. Bras. Ginecol. Obstet. 2018, 40, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, H.M.; Durnwald, C.P.; Catalano, P.; Mercer, B.M. The Influence of Obesity and Diabetes on the Risk of Cesarean Delivery. Am. J. Obstet. Gynecol. 2004, 191, 969–974. [Google Scholar] [CrossRef]
- Fuchs, F.; Senat, M.V.; Rey, E.; Balayla, J.; Chaillet, N.; Bouyer, J.; Audibert, F. Impact of Maternal Obesity on the Incidence of Pregnancy Complications in France and Canada. Sci. Rep. 2017, 7, 10859. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.E.; Ernst, A.; Brix, N.; Lauridsen, L.L.B.B.; Roos, L.; Ramlau-Hansen, C.H.M.; Ekelund, C.K. Risk of Adverse Pregnancy Outcomes at Advanced Maternal Age. Obstet. Gynecol. 2018, 131, 457–463. [Google Scholar] [CrossRef]
- Pinheiro, R.L.; Areia, A.L.; Mota Pinto, A.; Donato, H. Advanced Maternal Age: Adverse Outcomes of Pregnancy, A Meta-Analysis. Acta Med. Port. 2019, 32, 219–226. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Farina, A.; Gaeta, G.; Sigismondi, C.; Spinillo, S.; Casiero, D.; Pozzoni, M.; Vigano, P.; Papaleo, E.; Candiani, M. Uterine Artery Doppler in Singleton Pregnancies Conceived after In-Vitro Fertilization or Intracytoplasmic Sperm Injection with Fresh vs Frozen Blastocyst Transfer: Longitudinal Cohort Study. Ultrasound Obstet. Gynecol. 2020, 56, 603–610. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Farina, A.; Miglio, R.; Zamagni, G.; Girardelli, S.; Vanni, V.S.; Morano, D.; Spinillo, S.; Sartor, F.; Candiani, M. Prospective Longitudinal Cohort Study of Uterine Arteries Doppler in Singleton Pregnancies Obtained by IVF/ICSI with Oocyte Donation or Natural Conception. Hum. Reprod. 2020, 35, 2428–2438. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries in Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Osol, G.; Mandala, M. Maternal Uterine Vascular Remodeling during Pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Gebb, J.; Dar, P. Colour Doppler Ultrasound of Spiral Artery Blood Flow in the Prediction of Pre-Eclampsia and Intrauterine Growth Restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 355–366. [Google Scholar] [CrossRef]
- Duan, J.; Chabot-Lecoanet, A.C.; Perdriolle-Galet, E.; Christov, C.; Hossu, G.; Cherifi, A.; Morel, O. Utero-Placental Vascularisation in Normal and Preeclamptic and Intra-Uterine Growth Restriction Pregnancies: Third Trimester Quantification Using 3D Power Doppler with Comparison to Placental Vascular Morphology (EVUPA): A Prospective Controlled Study. BMJ Open 2016, 6, e009909. [Google Scholar] [CrossRef]
- Schiffer, V.; Evers, L.; de Haas, S.; Ghossein-Doha, C.; Al-Nasiry, S.; Spaanderman, M. Spiral Artery Blood Flow during Pregnancy: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2020, 20, 680. [Google Scholar] [CrossRef] [PubMed]
- Siargkas, A.; Tsakiridis, I.; Kappou, D.; Mamopoulos, A.; Papastefanou, I.; Dagklis, T. The Association Between Uterine Artery Pulsatility Index at Mid-Gestation and the Method of Conception: A Cohort Study. Medicina 2025, 61, 1093. [Google Scholar] [CrossRef]
- Zaat, T.R.; Kostova, E.B.; Korsen, P.; Showell, M.G.; Mol, F.; van Wely, M. Obstetric and Neonatal Outcomes after Natural Versus Artificial Cycle Frozen Embryo Transfer and the Role of Luteal Phase Support: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2023, 29, 634–654. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Aiello, E.; Pietrolucci, M.E.; Arduini, D. Placental Volume and Uterine Artery Doppler Evaluation at 11 + 0 to 13 + 6 Weeks’ Gestation in Pregnancies Conceived with In-Vitro Fertilization: Comparison between Autologous and Donor Oocyte Recipients. Ultrasound Obstet. Gynecol. 2016, 47, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Choux, C.; Ginod, P.; Barberet, J.; Rousseau, T.; Bruno, C.; Sagot, P.; Astruc, K.; Fauque, P. Placental Volume and Other First-Trimester Outcomes: Are There Differences between Fresh Embryo Transfer, Frozen-Thawed Embryo Transfer and Natural Conception? Reprod. Biomed. Online 2019, 38, 538–548. [Google Scholar] [CrossRef]
- Gullo, G.; Basile, G.; Cucinella, G.; Greco, M.E.; Perino, A.; Chiantera, V.; Marinelli, S. Fresh vs. Frozen Embryo Transfer in Assisted Reproductive Techniques: A Single Center Retrospective Cohort Study and Ethical-Legal Implications. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6809–6823. [Google Scholar] [CrossRef]
- Donno, V.; Prats, P.; Rodriguez, I.; Polyzos, N.P. First-Trimester Uterine Artery Pulsatility Index and Preeclampsia Risk in Pregnancies after Artificial Frozen Embryo Transfer: Analysis of over 27,000 Pregnancies. Am. J. Obstet. Gynecol. 2025, 232, 464.e1–464.e9. [Google Scholar] [CrossRef]
- Naghshineh, E.; Ghasemi Tehrani, H.; Sharifian, F.; Haghighat, S. A Comparison of Oral Dydrogesterone with Vaginal Progesterone for Luteal-Phase Support in In Vitro Fertilization: A Randomized Controlled Trial. Adv. Biomed. Res. 2023, 12, 132. [Google Scholar] [CrossRef]
- de Macedo, L.C.G.M.; Cavagna Neto, M.; Dzik, A.; do Rosário Rocha, A.; Rosa Lima, S.M.R. Randomized Clinical Trial Comparing Oral Dydrogesterone to Micronized Vaginal Progesterone for Endometrial Preparation in Frozen-Thawed Embryo Transfer Cycle. Clin. Exp. Obstet. Gynecol. 2023, 50, 8. [Google Scholar] [CrossRef]
- Almamou, M.A.A.; Kurjak, A. Pregnancy Success Predictive Value of Endometrial and Uterine Doppler Markers When Using Oral Ovarian Induction Medication in Subfertile Patients. J. Res. Med. Dent. Sci. 2020, 8, 232–240. [Google Scholar]
- Vidal, A.; Dhakal, C.; Werth, N.; Weiss, J.M.; Lehnick, D.; Kohl Schwartz, A.S. Supplementary Dydrogesterone Is Beneficial as Luteal Phase Support in Artificial Frozen-Thawed Embryo Transfer Cycles Compared to Micronized Progesterone Alone. Front. Endocrinol. 2023, 14, 1128564. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, K.; Sienko, J.; Mogilinski, M.; Bros, M.; Szczecina, R.; Czajkowska, A. Uteroplacental Circulation in Early Pregnancy Complicated by Threatened Abortion Supplemented with Vaginal Micronized Progesterone or Oral Dydrogesterone. Fertil. Steril. 2007, 87, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chattopadhyay, R.; Goswami, S.; Chaudhury, K.; Chakravarty, B.; Ganesh, A. Assessment of Sub-Endometrial Blood Flow Parameters Following Dydrogesterone and Micronized Vaginal Progesterone Administration in Women with Idiopathic Recurrent Miscarriage: A Pilot Study. J. Obstet. Gynaecol. Res. 2014, 40, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Szekeres-Bartho, J. Dydrogesterone and the Immunology of Pregnancy. Horm. Mol. Biol. Clin. Investig. 2016, 27, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J. The Role of Progesterone in Feto-Maternal Immunological Cross Talk. Med. Princ. Pract. 2018, 27, 301–307. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Raghupathy, R.; Saito, S.; Szekeres-Bartho, J. Cytokines, Hormones and Cellular Regulatory Mechanisms Favoring Successful Reproduction. Front. Immunol. 2021, 12, 717808. [Google Scholar] [CrossRef]
- Sarmiento, A.; Casasbuenas, A.; Rodriguez, N.; Angarita, A.M.; Sarmiento, P.; Sepulveda, W. First-Trimester Uterine Artery Doppler Velocimetry in the Prediction of Birth Weight in a Low-Risk Population. Prenat. Diagn. 2013, 33, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Prado, R.; Bugatto, F.; Sánchez-Martín, P.; Fajardo-Expósito, M.A.; Torrejón, R.; Bartha, J.L. The Influence of Placental Perfusion on Birthweight in Women with Gestational Diabetes. J. Matern. Fetal Neonatal Med. 2016, 29, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Gupta, S. Prediction of Adverse Pregnancy Outcomes Using Uterine Artery Doppler Imaging at 22–24 Weeks of Pregnancy: A North Indian Experience. Turk. J. Obstet. Gynecol. 2016, 13, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, E.; Del Barco, E.; Farràs, A.; Dalmau, A.; Garcia, E.; Gleeson-Vallbona, L.; Serrano, B.; Armengol-Alsina, M.; Catalan, S.; Hernadez, A.; et al. Role of Routine Uterine Artery Doppler at 18–22 and 24–28 Weeks’ Gestation Following Routine First-Trimester Screening for Pre-Eclampsia. Ultrasound Obstet. Gynecol. 2025, 65, 63–70. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, H.J.; Shin, J.E.; Lee, Y.; Shin, J.C.; Park, T.C.; Park, I.Y. The Predictive Value of the Uterine Artery Pulsatility Index during the Early Third Trimester for the Occurrence of Adverse Pregnancy Outcomes Depending on the Maternal Obesity. Obes. Res. Clin. Pract. 2015, 9, 374–381. [Google Scholar] [CrossRef]
- Teulings, N.E.W.D.; Wood, A.M.; Sovio, U.; Ozanne, S.E.; Smith, G.C.S.; Aiken, C.E. Independent Influences of Maternal Obesity and Fetal Sex on Maternal Cardiovascular Adaptation to Pregnancy: A Prospective Cohort Study. Int. J. Obes. 2020, 44, 2246–2255. [Google Scholar] [CrossRef]
- Yogev, Y.; Langer, O.; Xenakis, E.M.; Rosenn, B. The Association between Glucose Challenge Test, Obesity and Pregnancy Outcome in 6390 Non-Diabetic Women. J. Matern. Fetal Neonatal Med. 2005, 17, 29–34. [Google Scholar] [CrossRef]
- Mission, J.F.; Marshall, N.E.; Caughey, A.B. Obesity in Pregnancy: A Big Problem and Getting Bigger. Obstet. Gynecol. Surv. 2013, 68, 389–399. [Google Scholar] [CrossRef]
- Delhaes, F.; Giza, S.A.; Koreman, T.; Eastabrook, G.; McKenzie, C.A.; Bedell, S.; Regnault, T.R.H.; de Vrijer, B. Altered Maternal and Placental Lipid Metabolism and Fetal Fat Development in Obesity: Current Knowledge and Advances in Non-Invasive Assessment. Placenta 2018, 69, 118–124. [Google Scholar] [CrossRef]
- Chanthasenanont, A.; Pongrojpaw, D.; Chittithavorn, R. Relationship between Maternal Age and Uterine Artery Doppler Velocimetry. Thai J. Obstet. Gynaecol. 2012, 20, 113–118. [Google Scholar]
- Tayyar, A.; Guerra, L.; Wright, A.; Wright, D.; Nicolaides, K.H. Uterine Artery Pulsatility Index in the Three Trimesters of Pregnancy: Effects of Maternal Characteristics and Medical History. Ultrasound Obstet. Gynecol. 2015, 45, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cordero, M.; Sepúlveda-Martínez, A.; Rencoret, G.; Valdés, E.; Pedraza, D.; Muñoz, H. Is There a Role for Cervical Assessment and Uterine Artery Doppler in the First Trimester of Pregnancy as a Screening Test for Spontaneous Preterm Delivery? Ultrasound Obstet. Gynecol. 2014, 43, 291–296. [Google Scholar] [CrossRef] [PubMed]
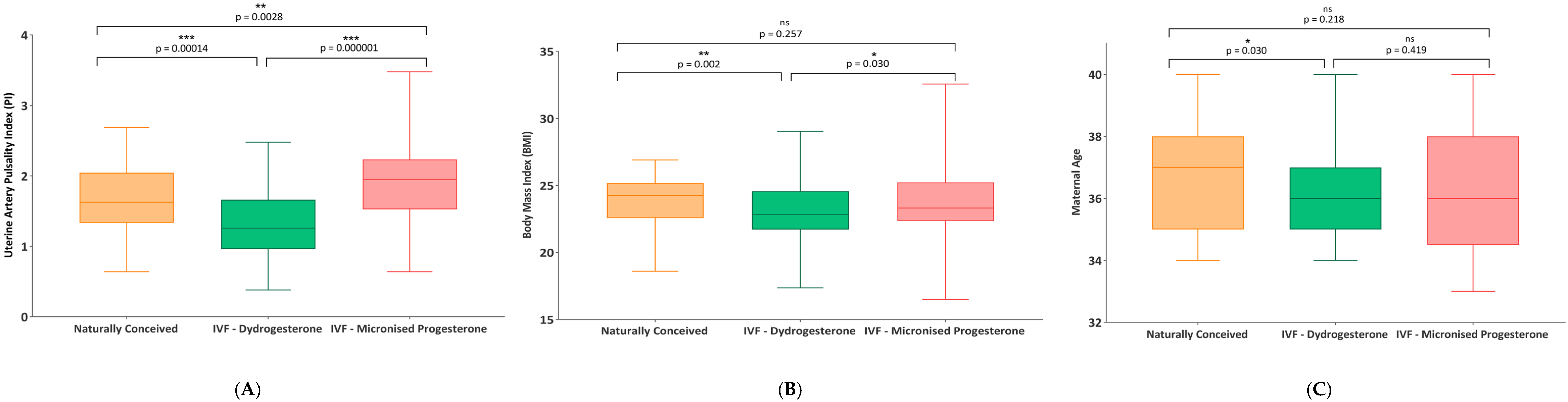

| DEMOGRAPHIC CHARACTERISTICS | ||||||||
| N | % | N | % | N | % | |||
| Agent | Birth week (week) | GDM | ||||||
| Naturally Conceived group | 72 | 24.90% | 36 | 22 | 7.60% | Present | 48 | 16.60% |
| IVF-Dydrogesterone group | 92 | 31.80% | 37 | 34 | 11.80% | Absent | 241 | 83.40% |
| IVF-Micronised Progesterone group | 125 | 43.30% | 38 | 122 | 42.20% | |||
| 39 | 101 | 34.90% | ||||||
| 40 | 10 | 3.50% | ||||||
| PREGNANCY MEASUREMENTS AND BIRTH OUTCOMES BY TREATMENT GROUP | ||||||||
| Naturally Conceived group | IVF-Dydrogesterone group | IVF-Micronised Progesterone group | ||||||
| Mean (SD) | Median (Min–Max) | Mean (SD) | Median (Min–Max) | Mean (SD) | Median (Min–Max) | |||
| Age (year) | 36.68 (1.93) | 37.0 (34–40) | 36.03 (1.75) | 36.0 (34–40) | 36.34 (2.08) | 36.0 (33–40) | ||
| BMI (kg/m2) | 23.92 (1.91) | 24.26 (18.6–26.9) | 22.97 (2.2) | 22.84 (17.37–29.04) | 23.93 (2.71) | 23.31 (16.49–32.56) | ||
| TSH level (mIU/L) | 1.56 (0.84) | 1.35 (0.4–3.75) | 1.82 (1.02) | 1.72 (0.45–6.2) | 1.79 (0.82) | 1.75 (0.45–4.52) | ||
| Cervical length (mm) | 36.81 (3.87) | 36.2 (30.2–51.4) | 36.02 (2.81) | 35.7 (30.4–44.1) | 36.52 (3.14) | 36.2 (29.8–46.3) | ||
| Haematocrit level (%) | 35.45 (3.36) | 36.1 (24.4–43.0) | 34.88 (3.12) | 34.85 (28.6–42.2) | 34.92 (3.16) | 35.35 (27.2–42.4) | ||
| Ultrasound day (days) | 38.15 (1.06) | 38.0 (36–40) | 38.25 (0.79) | 38.0 (36–40) | 38.07 (0.98) | 38.0 (36–40) | ||
| Haemoglobin level (g/dL) | 11.86 (1.19) | 12.05 (8.9–14.1) | 11.79 (1.15) | 11.7 (9.0–14.1) | 11.76 (1.21) | 11.75 (9.0–14.2) | ||
| Platelet count × 103 (cell/mL) | 231.64 (62.65) | 221.5 (123.0–435.0) | 232.82 (65.24) | 225.5 (119.0–433.0) | 226.19 (74.12) | 211.0 (109.0–458.0) | ||
| Average PI | 1.64 (0.5) | 1.62 (0.64–2.69) | 1.33 (0.5) | 1.26 (0.38–2.48) | 1.86 (0.52) | 1.95 (0.64–3.48) | ||
| Placenta weight (g) | 481.74 (49.91) | 488.0 (375.0–578.0) | 486.38 (69.36) | 483.0 (357.0–850.0) | 479.13 (57.63) | 487.0 (317.0–612.0) | ||
| Explanatory Variable | β Coefficients | 95% CI | p Value | Significance |
|---|---|---|---|---|
| BMI | −0.035 | −0.061 to −0.009 | 0.0090 | ** |
| Maternal Age | −0.018 | −0.042 to 0.006 | 0.1310 | NS |
| Micronised Progesterone (vs. Dydrogesterone) | 0.553 | 0.401 to 0.705 | 0.0001 | *** |
| Naturally Conceived (vs. Dydrogesterone) | 0.307 | 0.195 to 0.419 | 0.0001 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aygün, E.G.; Kahraman, E. First-Trimester Uterine Artery Doppler Indices and Pregnancy Outcomes in Naturally Conceived and Frozen–Thawed Embryo Transfer Cycles. Diagnostics 2025, 15, 2223. https://doi.org/10.3390/diagnostics15172223
Aygün EG, Kahraman E. First-Trimester Uterine Artery Doppler Indices and Pregnancy Outcomes in Naturally Conceived and Frozen–Thawed Embryo Transfer Cycles. Diagnostics. 2025; 15(17):2223. https://doi.org/10.3390/diagnostics15172223
Chicago/Turabian StyleAygün, Elif Ganime, and Edis Kahraman. 2025. "First-Trimester Uterine Artery Doppler Indices and Pregnancy Outcomes in Naturally Conceived and Frozen–Thawed Embryo Transfer Cycles" Diagnostics 15, no. 17: 2223. https://doi.org/10.3390/diagnostics15172223
APA StyleAygün, E. G., & Kahraman, E. (2025). First-Trimester Uterine Artery Doppler Indices and Pregnancy Outcomes in Naturally Conceived and Frozen–Thawed Embryo Transfer Cycles. Diagnostics, 15(17), 2223. https://doi.org/10.3390/diagnostics15172223









