Is GDF15 a Feasible Biomarker in Sepsis?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval, Study Design, and Patient Selection
2.2. Exclusion Criteria and Comorbidity Assessment
2.3. Blood Sample Collection and Measurement of Biochemical Parameters
2.4. Statistical Analysis
3. Results
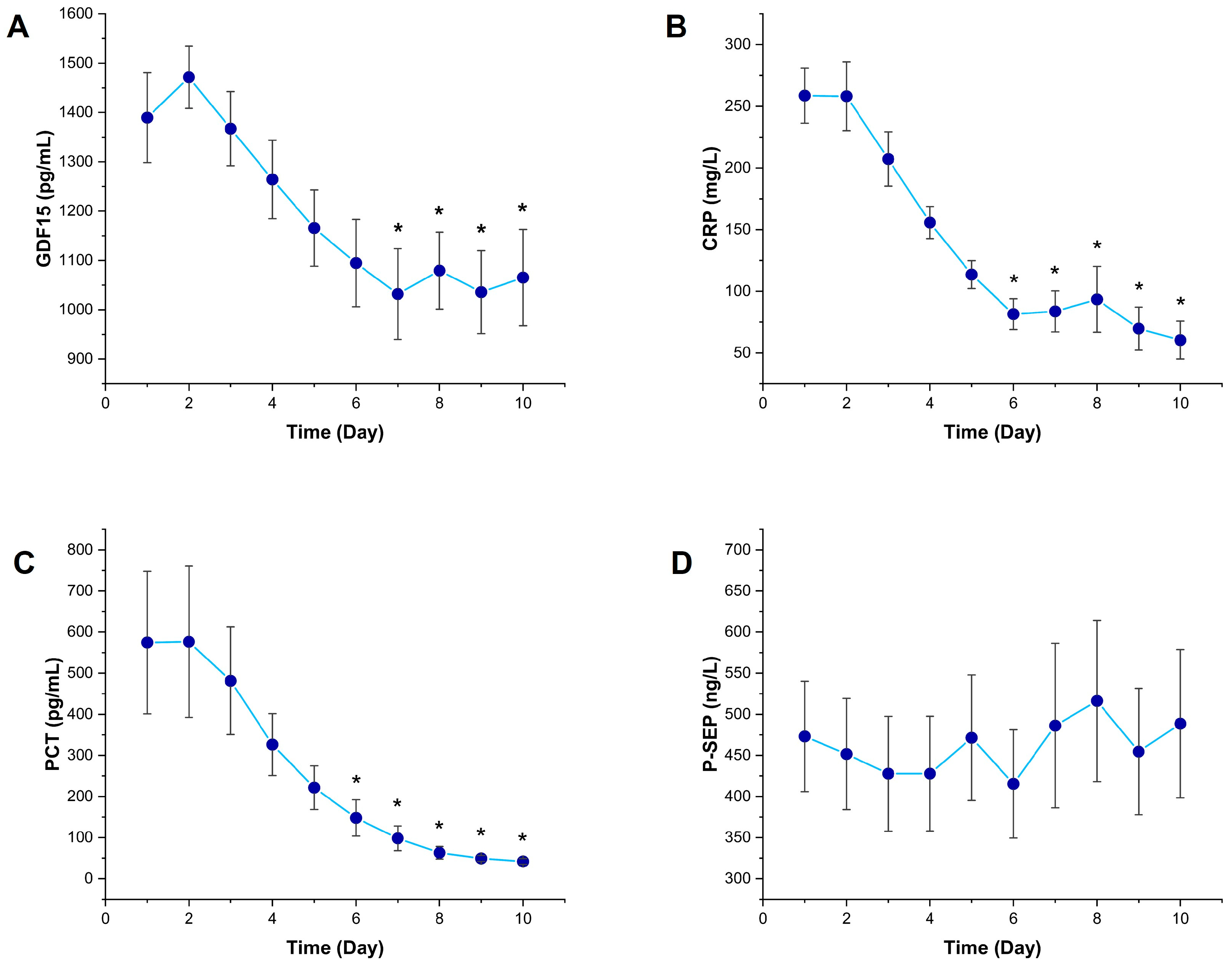
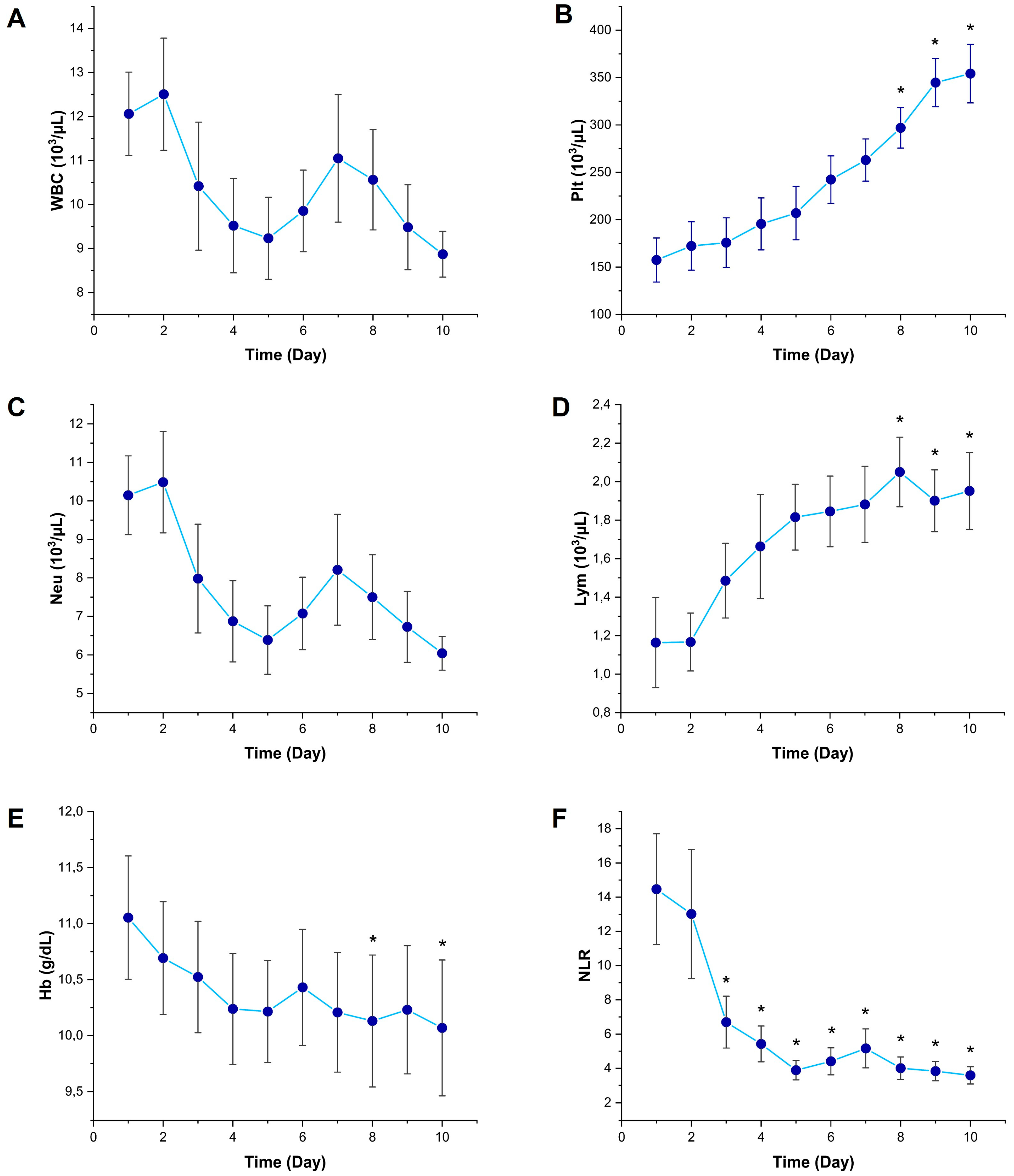
3.1. Temporal Changes in Inflammatory Biomarkers
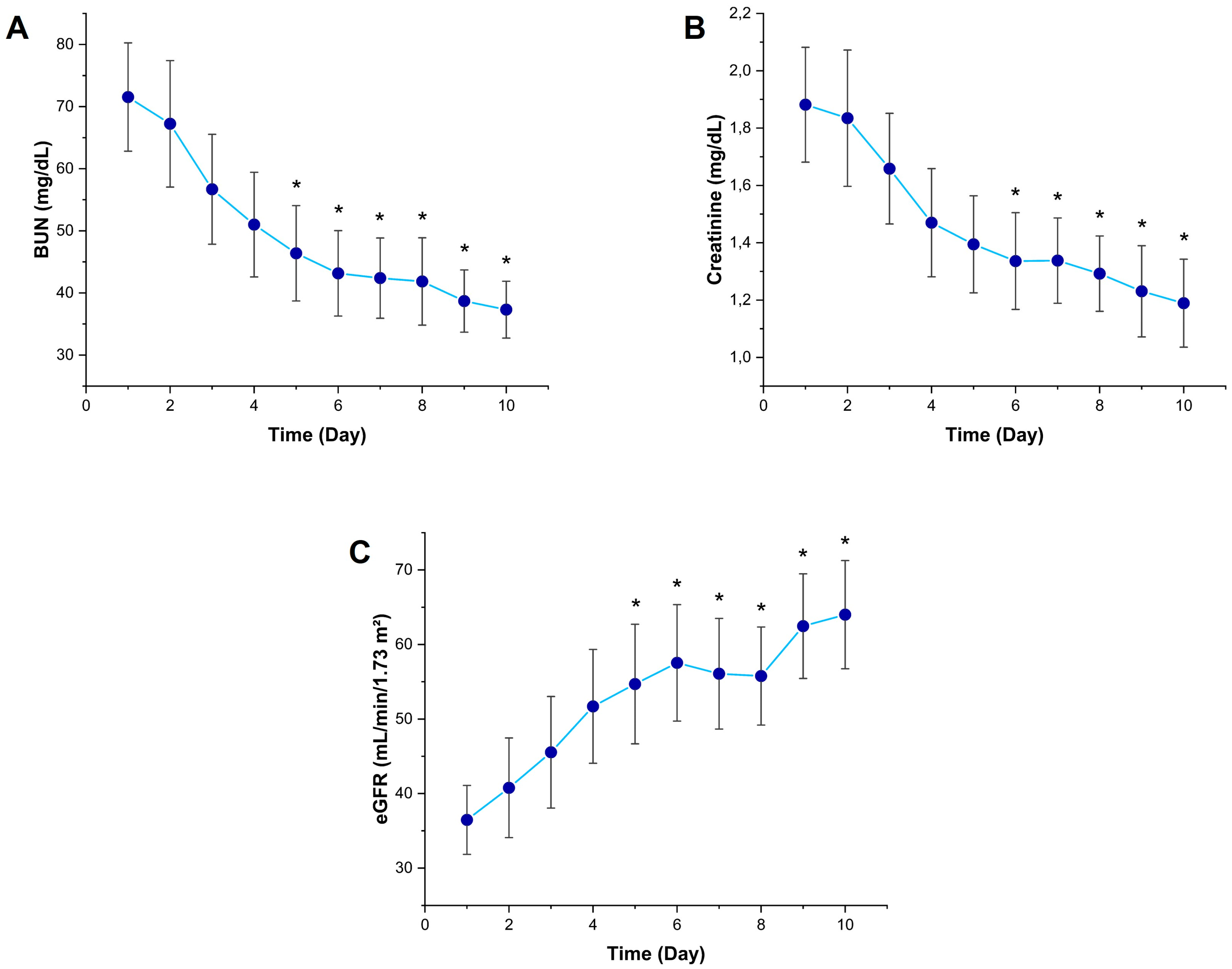
3.2. Temporal Changes in Kidney Function Markers
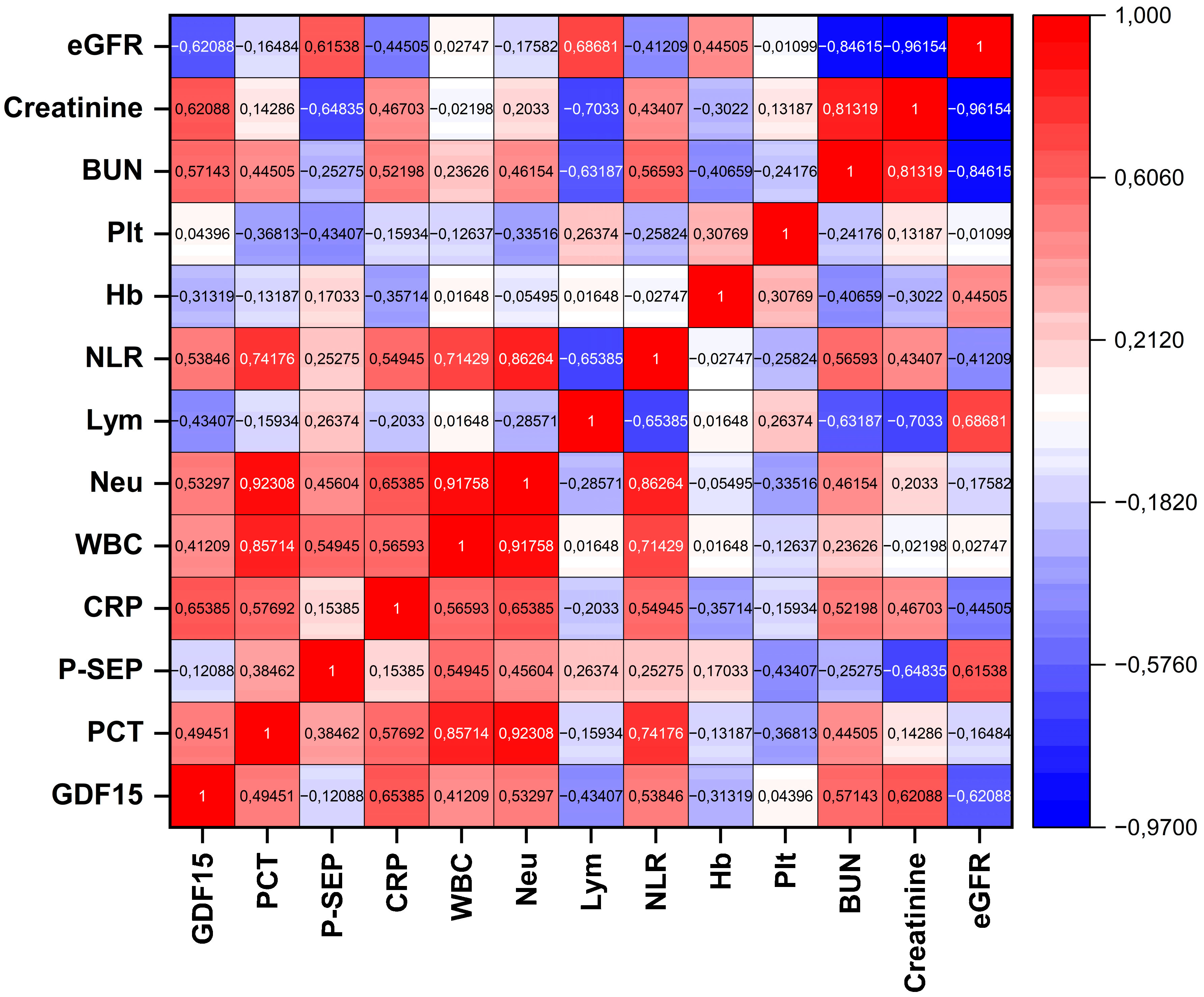
3.3. Correlation of GDF15 with Inflammatory and Renal Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Ning, B.T. Signaling Pathways and Intervention Therapies in Sepsis. Signal Transduct. Target. Ther. 2021, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Porat, A.; Bhutta, B.S.; Kesler, S. Urosepsis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tandogdu, Z.; Koves, B.; Ristovski, S.; Balci, M.B.C.; Rennesund, K.; Gravas, S.; Nale, D.; Medina-Polo, J.; Garabášová, M.K.; Costantini, E.; et al. Urosepsis 30-day mortality, morbidity, and their risk factors: SERPENS study, a prospective, observational multi-center study. World J. Urol. 2024, 42, 314. [Google Scholar] [CrossRef]
- Wu, C.C.; Lan, H.M.; Han, S.T.; Chaou, C.H.; Yeh, C.F.; Liu, S.H.; Li, C.H.; Blaney, G.N., 3rd; Liu, Z.Y.; Chen, K.F. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 91. [Google Scholar] [CrossRef]
- Velissaris, D.; Zareifopoulos, N.; Karamouzos, V.; Karanikolas, E.; Pierrakos, C.; Koniari, I.; Karanikolas, M. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus 2021, 13, e15019. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Han, K.S.; Ahn, S.; Shin, T.G.; Hwang, S.Y.; Chung, S.P.; Hwang, Y.J.; Park, Y.S.; Jo, Y.H.; Chang, H.L.; et al. The Usefulness of C-Reactive Protein and Procalcitonin to Predict Prognosis in Septic Shock Patients: A Multicenter Prospective Registry-Based Observational Study. Sci. Rep. 2019, 9, 6579. [Google Scholar] [CrossRef]
- Lee, S.; Song, J.; Park, D.W.; Seok, H.; Ahn, S.; Kim, J.; Park, J.; Cho, H.-J.; Moon, S. Diagnostic and Prognostic Value of Presepsin and Procalcitonin in Non-Infectious Organ Failure, Sepsis, and Septic Shock. BMC Infect. Dis. 2022, 22, 8. [Google Scholar] [CrossRef]
- Henriquez-Camacho, C.; Losa, J. Biomarkers for Sepsis. Biomed Res. Int. 2014, 2014, 547818. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.K.; Lan, H.M.; Han, S.T.; Wu, C.C.; Chen, K.F. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines 2020, 8, 494. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.; Liu, T.; Shuai, Z.; Song, Y.; Yang, Q.; Wang, Y.; Huang, H.; Luo, Y. Growth Differentiation Factor 15 Aggravates Sepsis-Induced Cognitive and Memory Impairments by Promoting Microglial Inflammatory Responses and Phagocytosis. J. Neuroinflamm. 2025, 22, 44. [Google Scholar] [CrossRef]
- Pence, B.D. Growth Differentiation Factor-15 in Immunity and Aging. Front. Aging 2022, 3, 837575. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Buendgens, L.; Yagmur, E.; Bruensing, J.; Herbers, U.; Baeck, C.; Trautwein, C.; Tacke, F. Growth Differentiation Factor-15 is a Predictor of Mortality in Critically Ill Patients with Sepsis. Dis. Markers 2017, 2017, 5271203. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, D.; Chen, J.; Hu, Y.; Cai, X.; Zhang, P. The Clinical Value of GDF15 and Its Prospective Mechanism in Sepsis. Front. Immunol. 2021, 12, 710977. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Domingues, P.; Furtado, T.; Valério, P.; Matias, J.; Cunha, L. Navigating the Cytokine Storm: A Comprehensive Review of Chemokines and Cytokines in Sepsis. Cureus 2024, 16, e35445. [Google Scholar]
- Atak, M.; Yigit, E.; Huner Yigit, M.; Topal Suzan, Z.; Yilmaz Kutlu, E.; Karabulut, S. Synthetic and Non-Synthetic Inhibition of ADAM10 and ADAM17 Reduces Inflammation and Oxidative Stress in LPS-Induced Acute Kidney Injury in Male and Female Mice. Eur. J. Pharmacol. 2024, 983, 176964. [Google Scholar] [CrossRef]
- Yigit, E.; Huner Yigit, M.; Atak, M.; Topal Suzan, Z.; Karabulut, S.; Yildiz, G.; Deger, O. Kaempferol Reduces Pyroptosis in Acute Lung Injury by Decreasing ADAM10 Activity through the NLRP3/GSDMD Pathway. Food Biosci. 2024, 62, 105140. [Google Scholar] [CrossRef]
- Ji, D.; Li, J.; Liu, A.; Ye, R.; Zhang, S.; Gao, L.; Huang, Z. Predictive Value of Combined Detection of Serum LGALS3BP and GDF-15 for the Prognosis of ICU Sepsis Patients. Infect. Drug Resist. 2024, 17, 4417–4426. [Google Scholar] [CrossRef]
- Lim, J.H.; Jeon, Y.; Ahn, J.S.; Kim, S.; Kim, D.K.; Lee, J.P.; Ryu, D.R.; Seong, E.Y.; Ahn, S.Y.; Baek, S.H.; et al. GDF-15 Predicts In-Hospital Mortality of Critically Ill Patients with Acute Kidney Injury Requiring Continuous Renal Replacement Therapy: A Multicenter Prospective Study. J. Clin. Med. 2021, 10, 3660. [Google Scholar] [CrossRef] [PubMed]
- Alipanah-Lechner, N.; Willmore, A.; Leroux, C.; Ringor, A.; Moss, M.; Rogers, A.; Matthay, M.A.; Calfee, C.S. Elevated Growth and Differentiation Factor 15 (GDF15) Levels in Subphenotypes of ARDS and Sepsis: A Marker of Mitochondrial Dysfunction and Mortality Risk. Am. J. Respir. Crit. Care Med. 2025, 211, A5363. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, S. Protective effect of growth differentiation factor 15 in sepsis by regulating macrophage polarization and its mechanism. Bioengineered 2022, 13, 9687–9707. [Google Scholar] [CrossRef]
- Li, M.; Song, K.; Huang, X.; Fu, S.; Zeng, Q. GDF-15 prevents LPS and D-galactosamine-induced inflammation and acute liver injury in mice. Int. J. Mol. Med. 2018, 42, 1756–1764. [Google Scholar] [CrossRef]
- Lu, S.; Li, R.; Deng, Y.; Bai, J.; Ji, B.; Chu, Y.; Xu, Y.; Qu, H.; Guo, X.; Li, P.; et al. GDF15 ameliorates sepsis-induced lung injury via AMPK-mediated inhibition of glycolysis in alveolar macrophages. Respir. Res. 2024, 25, 201. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Zhang, L.; Liang, H.; Zhang, B.; Yang, J.; Peng, X.; Sun, J.; Zhou, Y.; Zhai, M.; et al. GDF15 attenuates sepsis-induced myocardial dysfunction by inhibiting cardiomyocyte ferroptosis via the SOCS1/GPX4 signaling pathway. Eur. J. Pharmacol. 2024, 982, 176894. [Google Scholar] [CrossRef]
- Abulizi, P.; Loganathan, N.; Zhao, D.; Mele, T.; Zhang, Y.; Zwiep, T.; Liu, K.; Zheng, X. Growth Differentiation Factor-15 Deficiency Augments Inflammatory Response and Exacerbates Septic Heart and Renal Injury Induced by Lipopolysaccharide. Sci. Rep. 2017, 7, 1037. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Baroni, S.; Rozzi, G.; Belvederi, F.; Leggeri, S.; Spagnuolo, F.; Novelli, M.; Pignataro, G.; Candelli, M.; Covino, M.; et al. Evaluation of Presepsin for Early Diagnosis of Sepsis in the Emergency Department. J. Clin. Med. 2025, 14, 2480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yigit, E.; Simsek, M.A.; Huner Yigit, M.; Akca, G.; Sonmez, B.; Uzun, H. Is GDF15 a Feasible Biomarker in Sepsis? Diagnostics 2025, 15, 2224. https://doi.org/10.3390/diagnostics15172224
Yigit E, Simsek MA, Huner Yigit M, Akca G, Sonmez B, Uzun H. Is GDF15 a Feasible Biomarker in Sepsis? Diagnostics. 2025; 15(17):2224. https://doi.org/10.3390/diagnostics15172224
Chicago/Turabian StyleYigit, Ertugrul, Mehmet Akif Simsek, Merve Huner Yigit, Gorkem Akca, Berat Sonmez, and Hakki Uzun. 2025. "Is GDF15 a Feasible Biomarker in Sepsis?" Diagnostics 15, no. 17: 2224. https://doi.org/10.3390/diagnostics15172224
APA StyleYigit, E., Simsek, M. A., Huner Yigit, M., Akca, G., Sonmez, B., & Uzun, H. (2025). Is GDF15 a Feasible Biomarker in Sepsis? Diagnostics, 15(17), 2224. https://doi.org/10.3390/diagnostics15172224





