Unsupervised Machine Learning to Identify Patient Clusters and Tailor Perioperative Care in Colorectal Surgery
Abstract
1. Introduction
2. Methods
2.1. Statistical Analysis
2.2. Outcomes
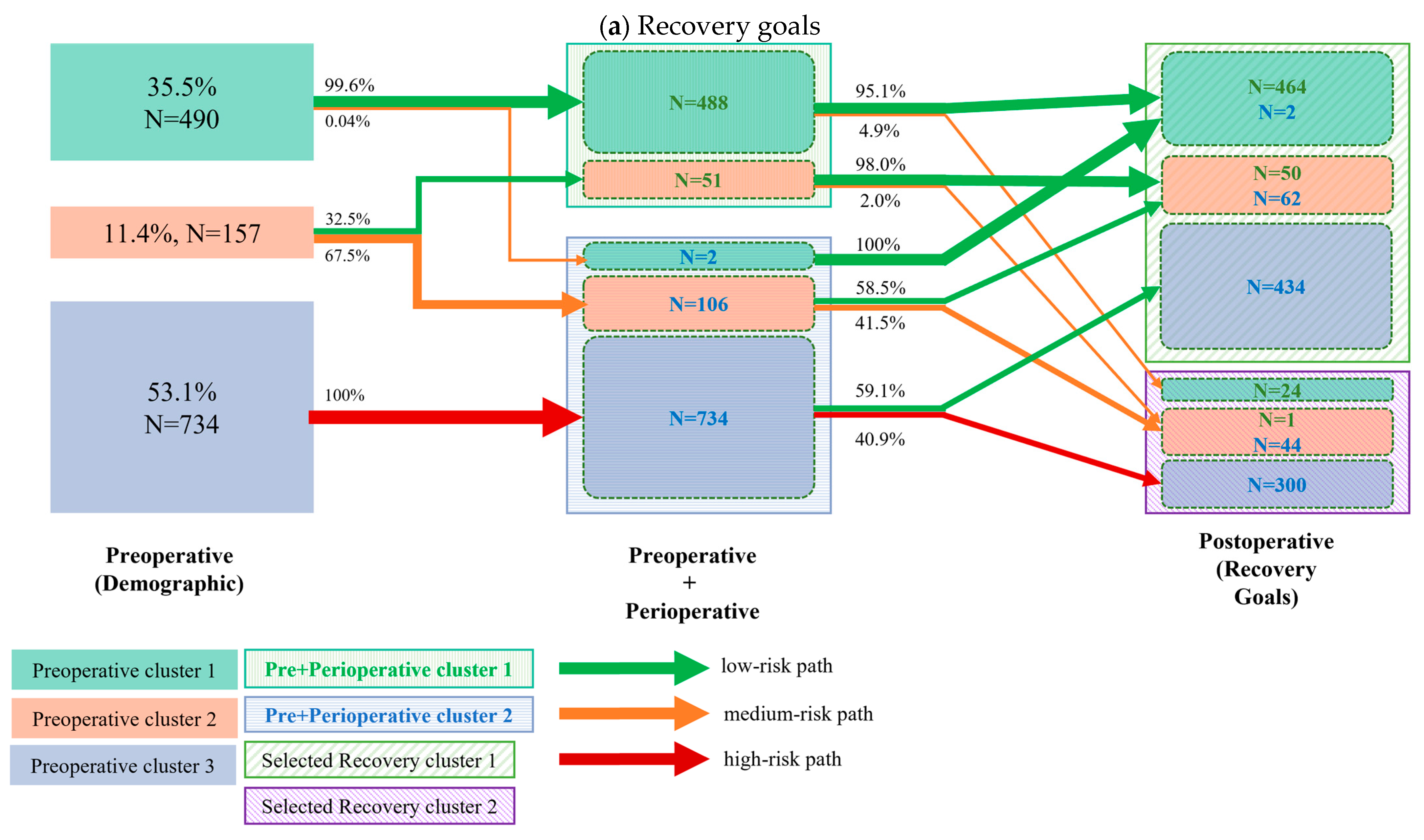
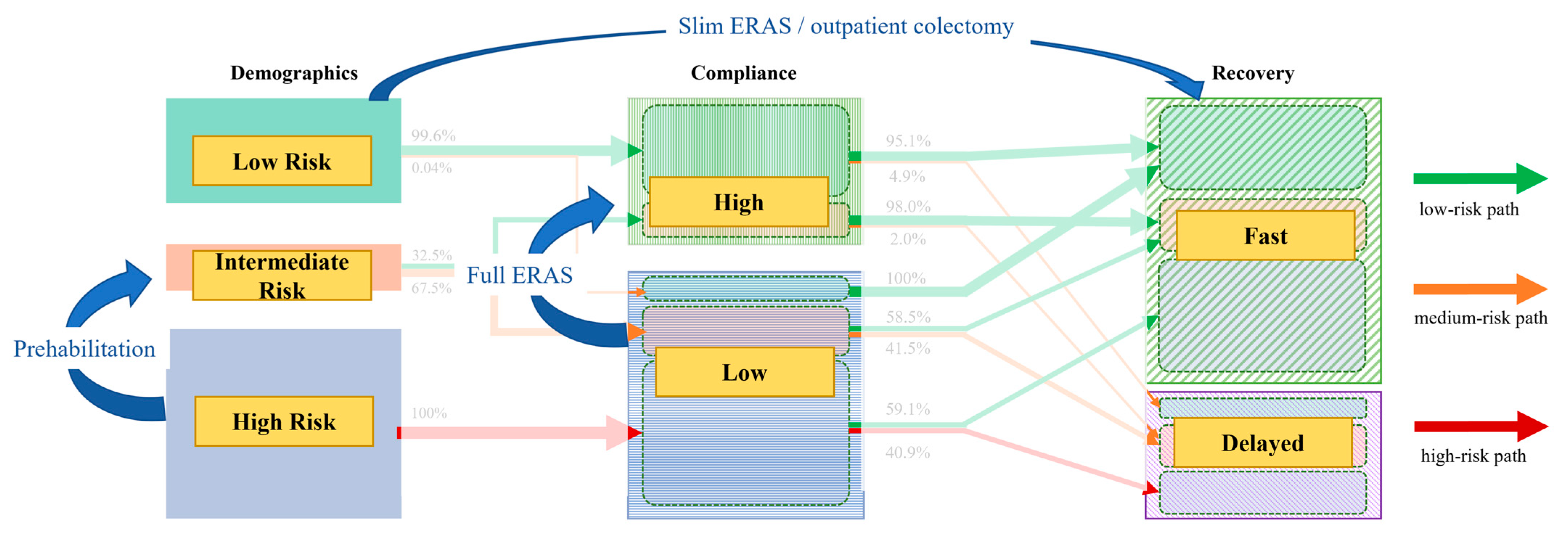
- Preoperative to perioperative transition: We first quantified patient transitions from the three initial demographic (preoperative) clusters to two summary clusters derived from a K-means analysis of combined demographic and perioperative variables.
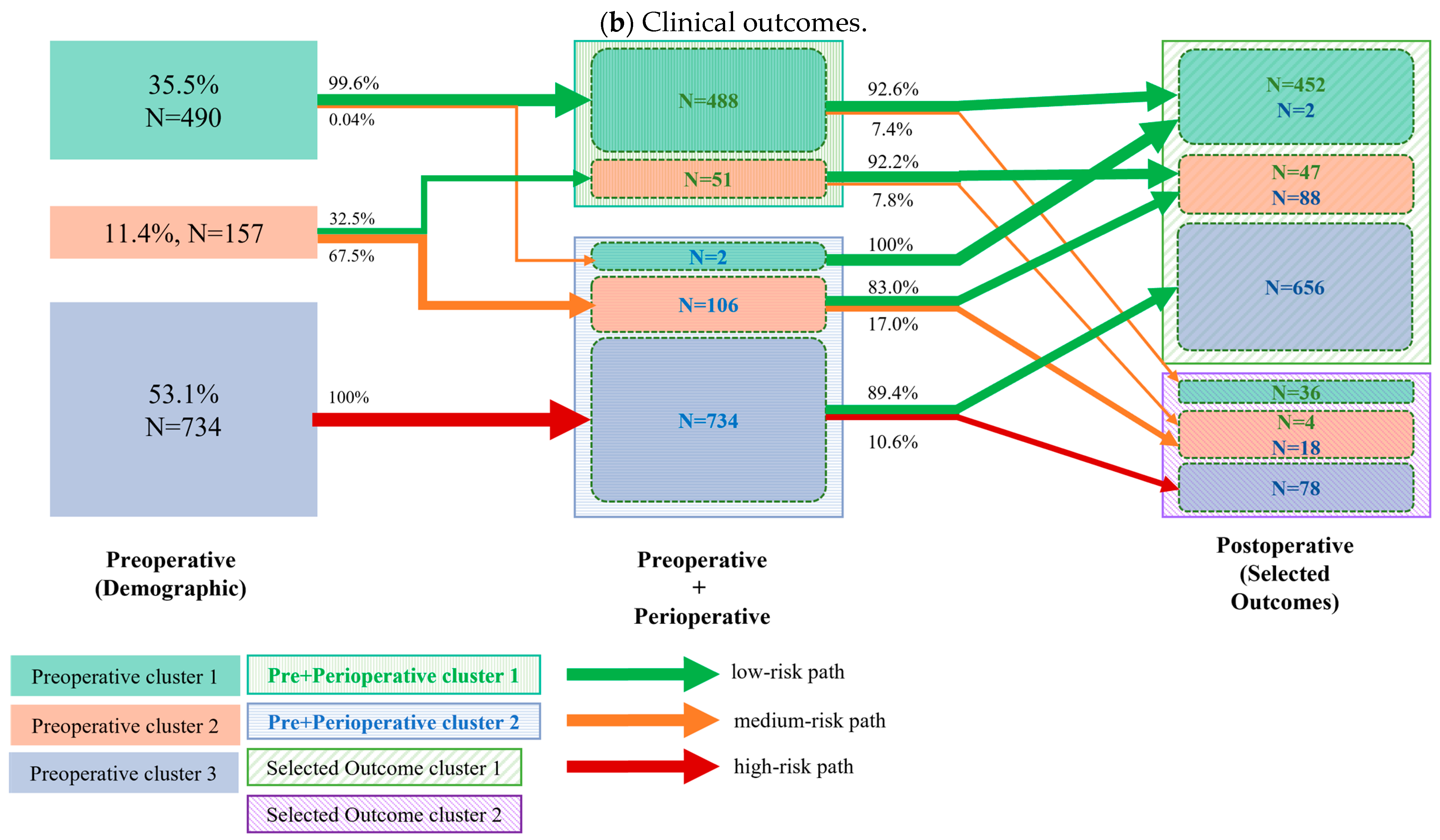
- Perioperative to postoperative transition: Next, we analyzed the transitions from the combined preoperative and perioperative clusters to the final postoperative clusters. This step was performed twice, as the postoperative clusters were independently generated based on two distinct sets of variables: (a) selected recovery goals and (b) clinical outcomes.
3. Results
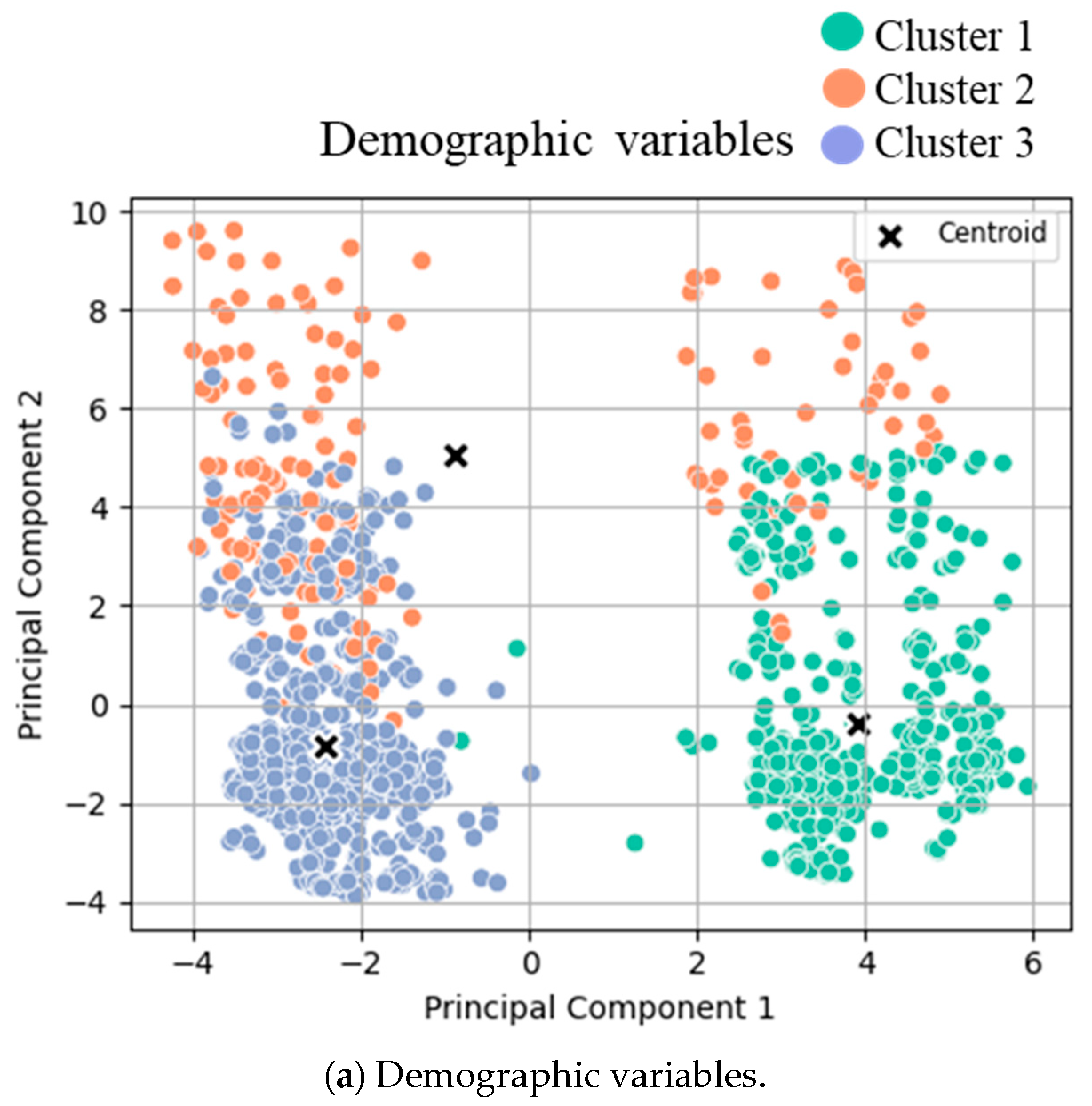
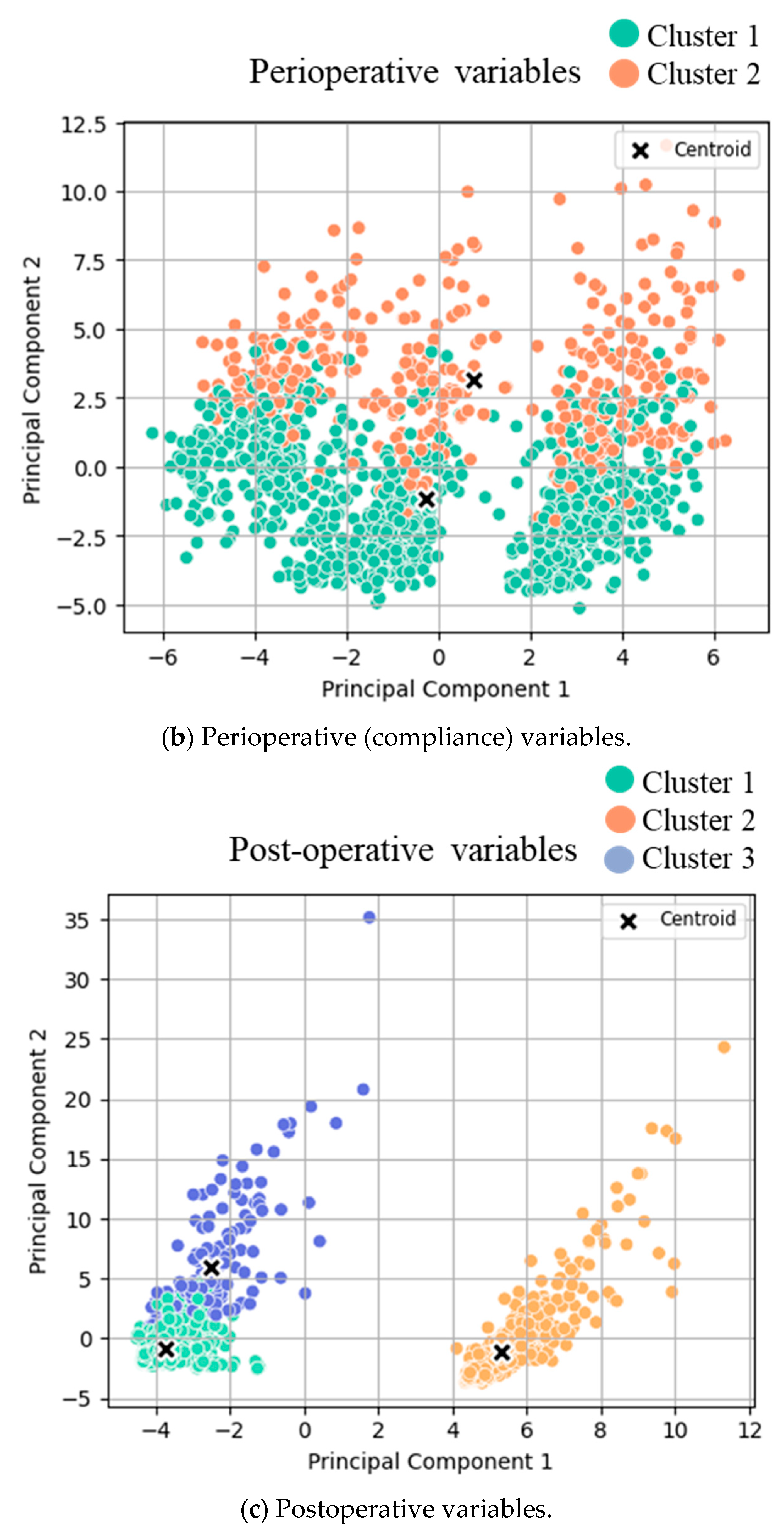
3.1. Cluster Analysis
- (a)
- Clusters for low- (green), intermediate- (orange), and high- (blue) risk groups.
- (b)
- Clusters for low (orange) and high (green) compliance.
- (c)
- Clusters for good (green) and poor (orange/blue) outcomes.
3.2. Cluster Transition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustafsson, U.O.; Oppelstrup, H.; Thorell, A.; Nygren, J.; Ljungqvist, O. Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study. World J. Surg. 2016, 40, 1741–1747. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Basse, L.; Hjort Jakobsen, D.; Billesbolle, P.; Werner, M.; Kehlet, H. A clinical pathway to accelerate recovery after colonic resection. Ann. Surg. 2000, 232, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Hausel, J.; Thorell, A.; Ljungqvist, O.; Soop, M.; Nygren, J.; Enhanced Recovery After Surgery Study Group. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch. Surg. 2011, 146, 571–577. [Google Scholar] [CrossRef]
- Larson, D.W.; Lovely, J.K.; Cima, R.R.; Dozois, E.J.; Chua, H.; Wolff, B.G.; Pemberton, J.H.; Devine, R.R.; Huebner, M. Outcomes after implementation of a multimodal standard care pathway for laparoscopic colorectal surgery. Br. J. Surg. 2014, 101, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Dosis, A.; Helliwell, J.; Syversen, A.; Tiernan, J.; Zhang, Z.; Jayne, D. Estimating postoperative mortality in colorectal surgery—A systematic review of risk prediction models. Int. J. Color. Dis. 2023, 38, 155. [Google Scholar] [CrossRef]
- Zain, Z.; Almadhoun, M.; Alsadoun, L.; Bokhari, S.F.H. Leveraging Artificial Intelligence and Machine Learning to Optimize Enhanced Recovery After Surgery (ERAS) Protocols. Cureus 2024, 16, e56668. [Google Scholar] [CrossRef]
- Roulin, D.; Donadini, A.; Gander, S.; Griesser, A.C.; Blanc, C.; Hubner, M.; Schafer, M.; Demartines, N. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br. J. Surg. 2013, 100, 1108–1114. [Google Scholar] [CrossRef]
- Joliat, G.R.; Ljungqvist, O.; Wasylak, T.; Peters, O.; Demartines, N. Beyond surgery: Clinical and economic impact of Enhanced Recovery After Surgery programs. BMC Health Serv. Res. 2018, 18, 1008. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; Macfie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef]
- Currie, A.; Soop, M.; Demartines, N.; Fearon, K.; Kennedy, R.; Ljungqvist, O. Enhanced Recovery After Surgery Interactive Audit System: 10 Years’ Experience with an International Web-Based Clinical and Research Perioperative Care Database. Clin. Colon. Rectal Surg. 2019, 32, 75–81. [Google Scholar] [CrossRef]
- Xu, Y.; Udumyan, R.; Fall, K.; Ljungqvist, O.; Montgomery, S.; Gustafsson, U.O. Validity of Routinely Collected Swedish Data in the International Enhanced Recovery After Surgery (ERAS) Database. World J. Surg. 2021, 45, 1622–1629. [Google Scholar] [CrossRef]
- Pache, B.; Martin, D.; Addor, V.; Demartines, N.; Hubner, M. Swiss Validation of the Enhanced Recovery After Surgery (ERAS) Database. World J. Surg. 2021, 45, 940–945. [Google Scholar] [CrossRef]
- Grass, F.; Slieker, J.; Jurt, J.; Kummer, A.; Sola, J.; Hahnloser, D.; Demartines, N.; Hubner, M. Postoperative ileus in an enhanced recovery pathway-a retrospective cohort study. Int. J. Color. Dis. 2017, 32, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Roulin, D.; Grass, F.; Addor, V.; Ljungqvist, O.; Demartines, N.; Hubner, M. A multicentre qualitative study assessing implementation of an Enhanced Recovery After Surgery program. Clin. Nutr. 2018, 37 Pt A, 2172–2177. [Google Scholar] [CrossRef]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 2014, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Pache, B.; Martin, D.; Addor, V.; Hahnloser, D.; Demartines, N.; Hubner, M. Feasibility of early postoperative mobilisation after colorectal surgery: A retrospective cohort study. Int. J. Surg. 2018, 56, 161–166. [Google Scholar] [CrossRef]
- Pedro, K.M.; Alvi, M.A.; Hejrati, N.; Quddusi, A.I.; Singh, A.; Fehlings, M.G. Machine learning-based cluster analysis identifies four unique phenotypes of patients with degenerative cervical myelopathy with distinct clinical profiles and long-term functional and neurological outcomes. EBioMedicine 2024, 106, 105226. [Google Scholar] [CrossRef]
- Khan, O.; Badhiwala, J.H.; Witiw, C.D.; Wilson, J.R.; Fehlings, M.G. Machine learning algorithms for prediction of health-related quality-of-life after surgery for mild degenerative cervical myelopathy. Spine J. 2021, 21, 1659–1669. [Google Scholar] [CrossRef]
- Gao, M.; Wang, M.; Zhou, S.; Hou, J.; He, W.; Shu, Y.; Wang, X. Machine learning-based prognostic model of lactylation-related genes for predicting prognosis and immune infiltration in patients with lung adenocarcinoma. Cancer Cell Int. 2024, 24, 400. [Google Scholar] [CrossRef] [PubMed]
- Jurt, J.; Hubner, M.; Pache, B.; Hahnloser, D.; Demartines, N.; Grass, F. Respiratory Complications After Colorectal Surgery: Avoidable or Fate? World J. Surg. 2018, 42, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Deslarzes, P.; Jurt, J.; Larson, D.W.; Blanc, C.; Hubner, M.; Grass, F. Perioperative Fluid Management in Colorectal Surgery: Institutional Approach to Standardized Practice. J. Clin. Med. 2024, 13, 801. [Google Scholar] [CrossRef]
- Grass, F.; Schafer, M.; Demartines, N.; Hubner, M. Normal Diet within Two Postoperative Days-Realistic or Too Ambitious? Nutrients 2017, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Coeckelberghs, E.; Vanhaecht, K.; Seys, D.; Cox, B.; Bislenghi, G.; Wolthuis, A.M.; D’Hoore, A.; on behalf of BIC4CRC Research Group. A Breakthrough Improvement Collaborative Significantly Reduces Hospital Stay After Elective Colectomy for Cancer Across a Healthcare System. Ann. Surg. 2022, 276, 890–896. [Google Scholar] [CrossRef]
- Agri, F.; Grass, F.; Kasmi, S.; Demartines, N.; Schafer, M.; Sauvain, M.O. Impact of Variations in the Nursing Care Supply-Demand Ratio on Postoperative Outcomes and Costs. J. Patient Saf. 2023, 19, 86–92. [Google Scholar] [CrossRef]
- Gignoux, B.; Pasquer, A.; Vulliez, A.; Lanz, T. Outpatient colectomy within an enhanced recovery program. J. Visc. Surg. 2015, 152, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Hubner, M.; Behm, K.T.; Mathis, K.L.; Hahnloser, D.; Day, C.N.; Harmsen, W.S.; Demartines, N.; Larson, D.W. Development and validation of a prediction score for safe outpatient colorectal resections. Surgery 2022, 171, 336–341. [Google Scholar] [CrossRef]
- Agri, F.; Moller, W.; Deslarzes, P.; Vogel, C.A.; Hahnloser, D.; Hubner, M.; Demartines, N.; Grass, F. Cost Analysis of Outpatient Colectomy in a Tertiary Center: A Projected Medico-Economic Evaluation. Health Serv. Insights 2024, 17, 11786329241284400. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, Y.; Wang, J.; Chen, G.; Chen, J.; Yu, S. Comparison of short-term surgical outcomes and long-term survival between emergency and elective surgery for colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2023, 38, 41. [Google Scholar] [CrossRef]
| Variable (Numeric) | Cluster 1 (n = 490) | Cluster 2 (n = 157) | Cluster 3 (n = 734) | p-Value | Sample Mean (n = 1381) |
|---|---|---|---|---|---|
| Age (years) | 61.2 | 63.2 | 60.8 | 0.24 | 61.2 |
| Weight 6 m prior to admission (kg) | 74 | 74.9 | 75 | 0.36 | 74.6 |
| Preoperative body weight (kg) | 73.3 | 73.2 | 74.4 | 0.46 | 73.8 |
| Preoperative weight change (kg) | −1.1 | −1.3 | −1.1 | 0.85 | −1.1 |
| Height (cm) | 168.7 | 168.2 | 169.2 | 0.46 | 168.9 |
| BMI (kg/m2) | 25.6 | 25.8 | 26 | 0.58 | 25.8 |
| Length of incision (cm) | 13.1 | 13.1 | 12.7 | 0.72 | 12.9 |
| Variable (Categorical) | Cluster 1 (n = 490) | Cluster 2 (n = 157) | Cluster 3 (n = 734) | p-Value | |
| Gender (male) | 57.9% | 50.9% | 58% | 0.24 | |
| Non-smoker or stopped | 81.3% | 76.4% | 75.2% | 0.13 | |
| Excess alcohol ingestion | 4.5% | 7.6% | 15.9% | <0.001 | |
| Diabetes mellitus | 8.9% | 15.3% | 11.8% | 0.13 | |
| Severe heart disease | 2% | 6.4% | 9.6% | <0.001 | |
| Severe pulmonary disease | 0.4% | 3.2% | 4.1% | <0.001 | |
| Variable | Cluster 1 (n = 1011) | Cluster 2 (n = 370) | p-Value |
|---|---|---|---|
| Core body temperature at end of operation (°C) | 36.3 | 36.4 | <0.0001 |
| IV volume of crystalloids, intraoperatively (mL) | 1310 | 2520 | <0.0001 |
| IV volume of colloids, intraoperatively (mL) | 80 | 400 | <0.0001 |
| Total IV volume of fluids, intraoperatively (mL) | 1380 | 2980 | <0.0001 |
| Total IV volume of fluids on day zero (mL) | 2160 | 4230 | <0.0001 |
| Morning weight (kg) | |||
| - On POD 1 | 74 | 78.8 | <0.0001 |
| Weight change POD 1 (kg) | 0.8 | 2.1 | <0.0001 |
| Morning weight (kg) | |||
| - On POD 2 | 73.1 | 80.4 | <0.0001 |
| Weight change POD 2 (kg) | 0.8 | 2.7 | <0.0001 |
| Morning weight (kg) | |||
| - On POD 3 | 72.6 | 80.5 | <0.0001 |
| Weight change POD 3 (kg) | 0.5 | 2.5 | <0.0001 |
| Oral fluids, total volume taken (mL) | |||
| - On POD 0 | 1010 | 660 | <0.0001 |
| - On POD 1 | 1620 | 1350 | <0.0001 |
| - On POD 2 | 1600 | 1350 | <0.0001 |
| Oral nutritional supplements, energy intake (kcal) | |||
| POD 0 | 130 | 50 | <0.0001 |
| POD 1 | 350 | 190 | <0.0001 |
| POD 2 | 310 | 210 | <0.0001 |
| POD 3 | 180 | 120 | 0.0001 |
| (a) Recovery goals | ||||
| Recovery Item | Cluster 1 (n = 1012) | Cluster 2 (n = 369) | p-Value | Sample Mean (n = 1381) |
| Time to tolerate solid food (nights) | 2.4 | 4 | <0.0001 | 2.8 |
| Total IV volume POD 0 | 2570 | 3110 | <0.0001 | 2710 |
| Mobilization > 6 h on POD 2 | 59.2% | 1.1% | <0.0001 | 603 |
| Weight change POD 2 (kg) | 1.36 | 1.27 | 0.45 | 1.34 |
| Weight change > 2.5 kg POD 2 | 21.7% | 59.1% | <0.0001 | 278 |
| (b) Clinical outcomes | ||||
| Type of Complication | Cluster 1 (n = 1245) | Cluster 2 (n = 136) | p-Value | Sample Mean (n = 1381) |
| Major complication | 1% | 62.5% | <0.0001 | 403 |
| Respiratory complication | 2.8% | 33.1% | <0.0001 | 80 |
| Infectious complication | 7.6% | 64.7% | <0.0001 | 183 |
| Renal dysfunction | 0.6% | 2.2% | <0.0001 | 72 |
| Anastomotic leak | 0.1% | 16.8% | <0.0001 | 88 |
| Delayed first passage of stool > POD 3 | 7.6% | 44.1% | <0.0001 | 155 |
| Death | 0.1% | 5.9% | <0.0001 | 9 |
| Reoperation | 1.2% | 80.1% | <0.0001 | 124 |
| Pain on POD 1 (VAS) | 3.8 | 4.2 | 0.08 | 3.82 |
| Ileus | 6.8% | 30.9% | <0.0001 | 127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deslarzes, P.; Xu, H.A.; Raisaro, J.L.; Hübner, M.; Grass, F. Unsupervised Machine Learning to Identify Patient Clusters and Tailor Perioperative Care in Colorectal Surgery. Diagnostics 2025, 15, 2124. https://doi.org/10.3390/diagnostics15172124
Deslarzes P, Xu HA, Raisaro JL, Hübner M, Grass F. Unsupervised Machine Learning to Identify Patient Clusters and Tailor Perioperative Care in Colorectal Surgery. Diagnostics. 2025; 15(17):2124. https://doi.org/10.3390/diagnostics15172124
Chicago/Turabian StyleDeslarzes, Philip, He Ayu Xu, Jean Louis Raisaro, Martin Hübner, and Fabian Grass. 2025. "Unsupervised Machine Learning to Identify Patient Clusters and Tailor Perioperative Care in Colorectal Surgery" Diagnostics 15, no. 17: 2124. https://doi.org/10.3390/diagnostics15172124
APA StyleDeslarzes, P., Xu, H. A., Raisaro, J. L., Hübner, M., & Grass, F. (2025). Unsupervised Machine Learning to Identify Patient Clusters and Tailor Perioperative Care in Colorectal Surgery. Diagnostics, 15(17), 2124. https://doi.org/10.3390/diagnostics15172124










