Motor Coordination Assessment in Autism Spectrum Disorder: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Source and Search Strategy
2.2. Study Eligibility Criteria
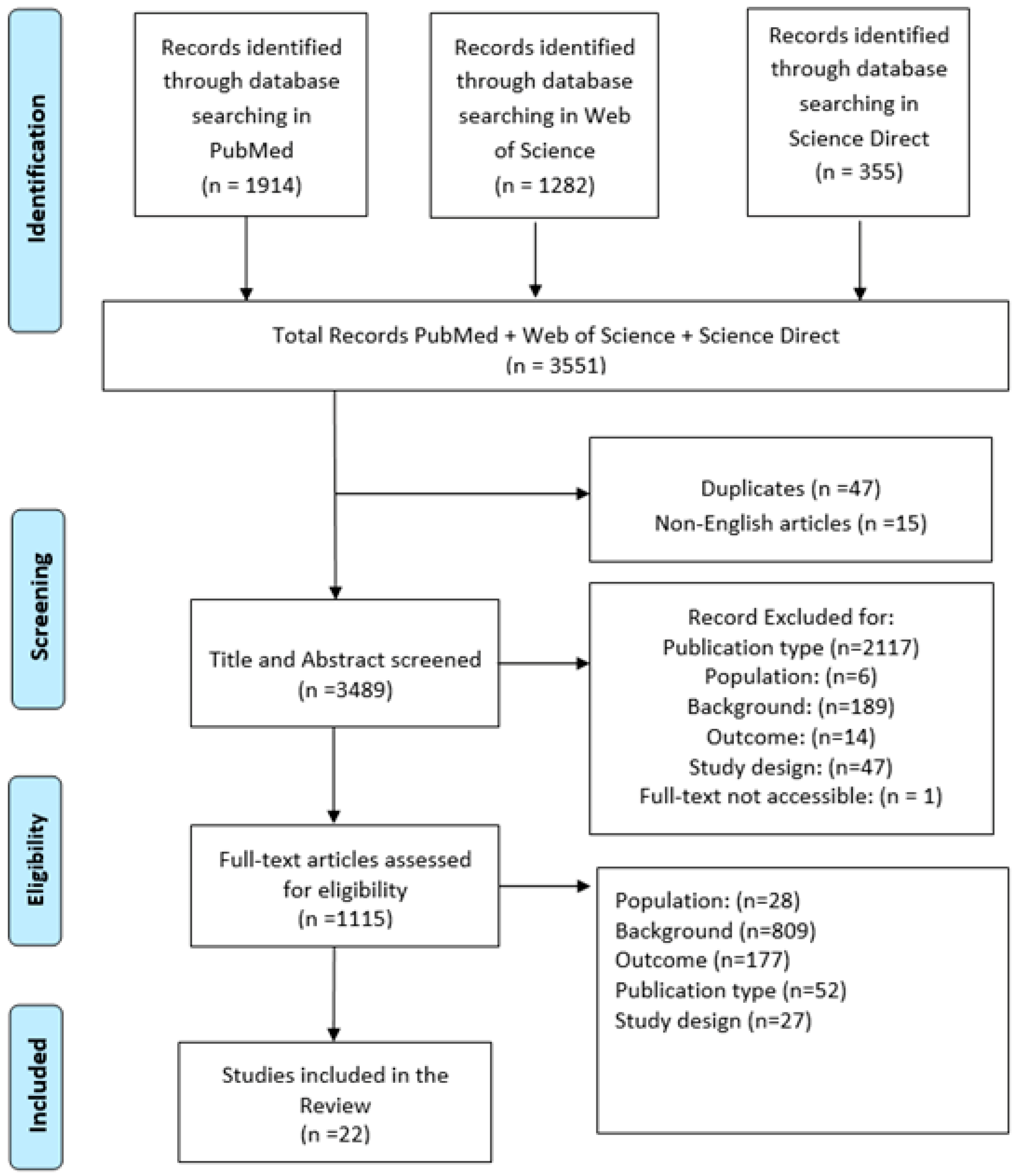
2.3. Selection Procedures
2.4. Data Extraction and Evaluation
2.5. Feasibility and Reliability
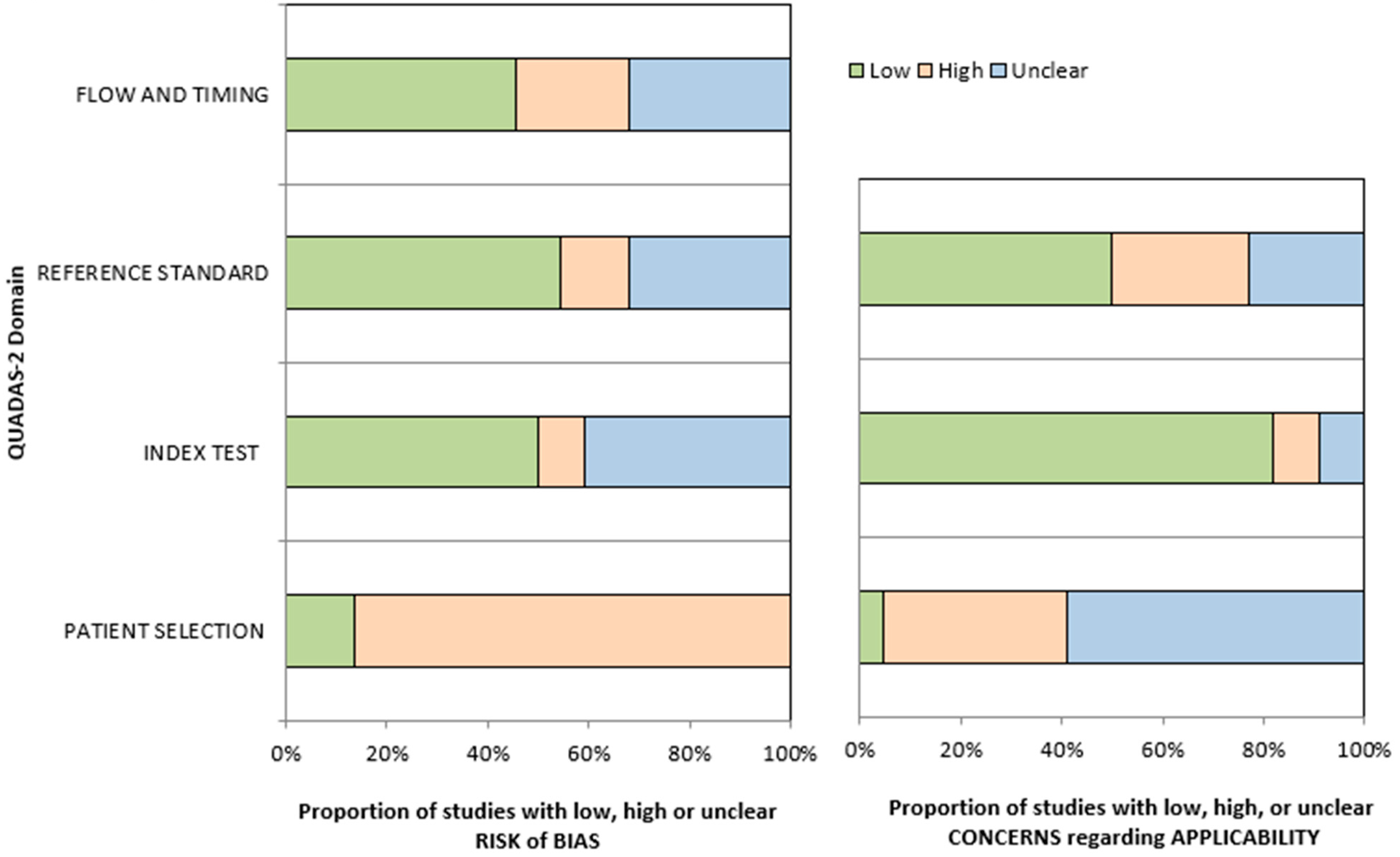
2.6. Risk of Bias Assessment
3. Results
3.1. Movement Assessment Battery for Children
3.2. Bruininks–Oseretsky Test of Motor Proficiency Second Edition and BOT-SF
3.3. Peabody Developmental Motor Scales
3.4. Test of Gross Motor Development
3.5. Alberta Infant Motor Scale
3.6. Feasibility and Reliability
3.7. Quality Assessment
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Doernberg, E.; Hollander, E. Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.L.; Pulvermüller, F. What can autism teach us about the role of sensorimotor systems in higher cognition? New clues from studies on language, action semantics, and abstract emotional concept processing. Cortex 2018, 100, 149–190. [Google Scholar] [CrossRef]
- Liu, T. Motor milestone development in young children with autism spectrum disorders: An exploratory study. Educ. Psychol. Pract. 2012, 28, 315–326. [Google Scholar] [CrossRef]
- Lim, Y.H.; Partridge, K.; Girdler, S.; Morris, S.L. Standing postural control in individuals with autism spectrum disorder: Systematic review and meta-analysis. J. Autism Dev. Disord. 2017, 47, 2238–2253. [Google Scholar] [CrossRef]
- Busti Ceccarelli, S.; Ferrante, C.; Gazzola, E.; Marzocchi, G.M.; Nobile, M.; Molteni, M.; Crippa, A. Fundamental motor skills intervention for children with autism spectrum disorder: A 10-year narrative review. Children 2020, 7, 250. [Google Scholar] [CrossRef]
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007, 29, 565–570. [Google Scholar] [CrossRef]
- Camia, M.; Sacco, R.; Boncoddo, M.; Bellomo, F.; Cucinotta, F.; Ricciardello, A.; Persico, A.M. Toe walking in children and adolescents with autism spectrum disorder: Relationship with sensory and motor functions, language, cognition, and autism severity. Res. Autism Spectr. Disord. 2024, 117, 102457. [Google Scholar] [CrossRef]
- Hallett, M.; Lebiedowska, M.K.; Thomas, S.L.; Stanhope, S.J.; Denckla, M.B.; Rumsey, J. Locomotion of autistic adults. Arch. Neurol. 1993, 50, 1304–1308. [Google Scholar] [CrossRef]
- Papadopoulos, N.; McGinley, J.; Tonge, B.; Bradshaw, J.; Saunders, K.; Murphy, A.; Rinehart, N. Motor proficiency and emotional/behavioural disturbance in autism and Asperger’s disorder: Another piece of the neurological puzzle? Autism Int. J. Res. Pract. 2012, 16, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.P.; Myers, S.M.; American Academy of Pediatrics Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007, 120, 1183–1215. [Google Scholar] [CrossRef] [PubMed]
- Kangarani-Farahani, M.; Malik, M.A.; Zwicker, J.G. Motor impairments in children with autism spectrum disorder: A systematic review and meta-analysis. J. Autism Dev. Disord. 2024, 54, 1977–1997. [Google Scholar] [CrossRef]
- Gallese, V.; Rochat, M.J.; Berchio, C. The mirror mechanism and its potential role in autism spectrum disorder. Dev. Med. Child Neurol. 2013, 55, 15–22. [Google Scholar] [CrossRef]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Jarus, T.; Lourie-Gelberg, Y.; Engel-Yeger, B.; Bart, O. Participation patterns of school-aged children with and without DCD. Res. Dev. Disabil. 2011, 32, 1323–1331. [Google Scholar] [CrossRef]
- Leonard, H.C.; Hill, E.L. Review: The impact of motor development on typical and atypical social cognition and language: A systematic review. Child Adolesc. Ment. Health 2014, 19, 163–170. [Google Scholar] [CrossRef]
- Wang, L.A.L.; Petrulla, V.; Zampella, C.J.; Waller, R.; Schultz, R.T. Gross motor impairment and its relationship to social skills in autism spectrum disorder: A systematic review and two meta-analyses. Psychol. Bull. 2022, 148, 273–300. [Google Scholar] [CrossRef]
- Battah, H.W.; Lotan, M.; Moran, D.S. The Need for a Motor Assessment Tool for Children with Autism-An Opinion Article. Diagnostics 2023, 13, 2095. [Google Scholar] [CrossRef]
- Anderson, D.K.; Lord, C.; Risi, S.; DiLavore, P.S.; Shulman, C.; Thurm, A.; Pickles, A. Patterns of growth in verbal abilities among children with autism spectrum disorder. J. Consult. Clin. Psychol. 2007, 75, 594–604. [Google Scholar] [CrossRef]
- Sadozai, A.K.; Sun, C.; Demetriou, E.A.; Lampit, A.; Munro, M.; Perry, N.; Boulton, K.A.; Guastella, A.J. Executive function in children with neurodevelopmental conditions: A systematic review and meta-analysis. Nat. Hum. Behav. 2024, 8, 2357–2366. [Google Scholar] [CrossRef]
- Taverna, E.C.; Huedo-Medina, T.B.; Fein, D.A.; Eigsti, I.M. The interaction of fine motor, gesture, and structural language skills: The case of autism spectrum disorder. Res. Autism Spectr. Disord. 2021, 86, 101824. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- DiSantostefano, J. International Classification of Diseases 10th Revision (ICD-10). J. Nurse Pract. 2009, 5, 56–57. [Google Scholar] [CrossRef]
- Slee, V. Disease classification: ICD-9 and beyond. PAS Rep. 1978, 16, 1–9. [Google Scholar]
- Beattie, M.; Murphy, D.J.; Atherton, I.; Lauder, W. Instruments to measure patient experience of healthcare quality in hospitals: A systematic review. Syst. Rev. 2015, 4, 97. [Google Scholar] [CrossRef]
- Klingberg, B.; Schranz, N.; Barnett, L.M.; Howard, S.J.; Cliff, D.P. The feasibility of fundamental movement skill assessments for pre-school aged children. J. Sports Sci. 2019, 37, 378–386. [Google Scholar] [CrossRef]
- Green, D.; Charman, T.; Pickles, A.; Chandler, S.; Loucas, T.; Simonoff, E.; Baird, G. Impairment in movement skills of children with autistic spectrum disorders. Dev. Med. Child Neurol. 2009, 51, 311–316. [Google Scholar] [CrossRef]
- Kaur, M.; Srinivasan, S.M.; Bhat, A.N. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism spectrum disorder (ASD). Res. Dev. Disabil. 2018, 72, 79–95. [Google Scholar] [CrossRef]
- Odeh, C.E.; Gladfelter, A.L.; Stoesser, C.; Roth, S. Comprehensive motor skills assessment in children with autism spectrum disorder yields global deficits. Int. J. Dev. Disabil. 2022, 68, 290–300. [Google Scholar] [CrossRef]
- Miller, H.L.; Sherrod, G.M.; Mauk, J.E.; Fears, N.E.; Hynan, L.S.; Tamplain, P.M. Shared features or co-occurrence? Evaluating symptoms of developmental coordination disorder in children and adolescents with autism spectrum disorder. J. Autism Dev. Disord. 2021, 52, 1782–1794. [Google Scholar] [CrossRef]
- Ament, K.; Mejia, A.; Buhlman, R.; Erklin, S.; Caffo, B.; Mostofsky, S.; Wodka, E. Evidence for specificity of motor impairments in catching and balance in children with autism. J. Autism Dev. Disord. 2015, 45, 742–751. [Google Scholar] [CrossRef]
- Faber, L.; van den Bos, N.; Houwen, S.; Schoemaker, M.M.; Rosenblum, S. Motor skills, visual perception, and visual–motor integration in children and youth with autism spectrum disorder. Res. Autism Spectr. Disord. 2022, 96, 101998. [Google Scholar] [CrossRef]
- Fulceri, F.; Grossi, E.; Contaldo, A.; Narzisi, A.; Apicella, F.; Parrini, I.; Tancredi, R.; Calderoni, S.; Muratori, F. Motor skills as moderators of core symptoms in autism spectrum disorders: Preliminary data from an exploratory analysis with artificial neural networks. Front. Psychol. 2019, 9, 2683. [Google Scholar] [CrossRef]
- Alsaedi, R.H. An assessment of the motor performance skills of children with autism spectrum disorder in the Gulf region. Brain Sci. 2020, 10, 607. [Google Scholar] [CrossRef]
- Bricout, V.A.; Pace, M.; Dumortier, L.; Miganeh, S.; Mahistre, Y.; Guinot, M. Motor capacities in boys with high functioning autism: Which evaluations to choose? J. Clin. Med. 2019, 8, 1521. [Google Scholar] [CrossRef]
- Liu, T.; Breslin, C.M. Fine and gross motor performance of the MABC-2 by children with autism spectrum disorder and typically developing children. Res. Autism Spectr. Disord. 2013, 7, 1244–1249. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lu, L.; Jeng, S.F.; Tsao, P.N.; Cheong, P.L.; Li, Y.J.; Wu, Y.T. Multidimensional developments and free-play movement tracking in 30- to 36-month-old toddlers with autism spectrum disorder who were full term. Phys. Ther. 2019, 99, 1535–1550. [Google Scholar] [CrossRef]
- Crippa, A.; Craig, F.; Busti Ceccarelli, S.; Mauri, M.; Grazioli, S.; Scionti, N.; Nobile, M. A multimethod approach to assessing motor skills in boys and girls with autism spectrum disorder. Autism 2021, 25, 1481–1491. [Google Scholar] [CrossRef]
- Vanvuchelen, M.; Roeyers, H.; De Weerdt, W. Nature of motor imitation problems in school-aged boys with autism: A motor or a cognitive problem? Autism 2007, 11, 225–240. [Google Scholar] [CrossRef]
- Provost, B.; Heimerl, S.; Lopez, B.R. Levels of gross and fine motor development in young children with autism spectrum disorder. Phys. Occup. Ther. Pediatr. 2007, 27, 21–36. [Google Scholar] [CrossRef]
- Biffi, E.; Costantini, C.; Ceccarelli, S.B.; Cesareo, A.; Marzocchi, G.M.; Nobile, M.; Crippa, A. Gait pattern and motor performance during discrete gait perturbation in children with autism spectrum disorders. Front. Psychol. 2018, 9, 2530. [Google Scholar] [CrossRef]
- De Francesco, S.; Morello, L.; Fioravanti, M.; Cassaro, C.; Grazioli, S.; Busti Ceccarelli, S.; Crippa, A. A multimodal approach can identify specific motor profiles in autism and attention-deficit/hyperactivity disorder. Autism Res. 2023, 16, 1550–1560. [Google Scholar] [CrossRef]
- Allen, K.A.; Bredero, B.; Van Damme, T.; Ulrich, D.A.; Simons, J. Test of Gross Motor Development–3 (TGMD-3) with the use of visual supports for children with autism spectrum disorder: Validity and reliability. J. Autism Dev. Disord. 2017, 47, 813–833. [Google Scholar] [CrossRef]
- Staples, K.L.; Reid, G. Fundamental movement skills and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 209–217. [Google Scholar] [CrossRef]
- Martel, M.; Finos, L.; Bahmad, S.; Koun, E.; Salemme, R.; Sonié, S.; Fourneret, P.; Schmitz, C.; Roy, A.C. Motor deficits in autism differ from that of developmental coordination disorder. Autism 2024, 28, 415–432. [Google Scholar] [CrossRef]
- Kalfiřt, L.; Su, C.T.; Fu, C.P.; Lee, S.D.; Yang, A.L. Motor skills, heart rate variability, and arterial stiffness in children with autism spectrum disorder. Healthcare 2023, 11, 1898. [Google Scholar] [CrossRef]
- Kochav-Lev, M.; Bennett-Back, O.; Lotan, M.; Stein-Zamir, C. The use of the Alberta Infant Motor Scale (AIMS) as a diagnostic scale for infants with autism. Diagnostics 2023, 13, 1045. [Google Scholar] [CrossRef]
- Martín-Díaz, P.; Cuesta-Gómez, A.; Fernádez-González, P.; Carratlá-Tejada, M. Balance and motor skills differences between children and teenagers with autism spectrum disorder and neurotypically developing. Autism Res. Off. J. Int. Soc. Autism Res. 2024, 17, 1545–1555. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Henderson, S.E.; Sugden, D.A.; Barnett, A.L. Movement Assessment Battery for Children—[Movement ABC-2], 2nd ed.; The Psychological Corporation: San Antonio, TX, USA, 2007. [Google Scholar]
- Bruininks, R.H.; Bruininks, B.D. Bruininks–Oseretsky Test of Motor Proficiency; American Guidance Service: Circle Pines, MN, USA, 1978. [Google Scholar]
- Folio, M.R. Peabody Developmental Motor Scales; DLM Teaching Resources: Lille, France, 1983. [Google Scholar]
- Ng, J.Y.Y.; Jiang, S.; Chan, C.H.S.; Ha, A.S. Assessing fundamental movement skills using the Test of Gross Motor Development (TGMD): Challenges and solutions to comparability and standardization. Sports Med. Health Sci. 2025, 7, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.C.; Pinnell, L.E.; Darrah, J.; Maguire, T.; Byrne, P.J. Construction and validation of the Alberta Infant Motor Scale (AIMS). Can. J. Public Health 1992, 83 (Suppl. 2), S46–S50. [Google Scholar]
- Lum, J.A.; Shandley, K.; Albein-Urios, N.; Kirkovski, M.; Papadopoulos, N.; Wilson, R.B.; Rinehart, N.J. Meta-analysis reveals gait anomalies in autism. Autism Res. 2021, 14, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.; Carment, L.; Lindberg, P.; Gaillard, R.; Krebs, M.O.; Amado, I. Sensorimotor aspects and manual dexterity in autism spectrum disorders: A literature review. L’Encéphale 2020, 46, 135–145. [Google Scholar] [CrossRef]
- Whyatt, C.; Craig, C.M. Interceptive skills in children aged 9–11 years, diagnosed with autism spectrum disorder. Res. Autism Spectr. Disord. 2013, 7, 613–623. [Google Scholar] [CrossRef]
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef] [PubMed]
- Ohara, R.; Kanejima, Y.; Kitamura, M.; Izawa, K.P. Association between social skills and motor skills in individuals with autism spectrum disorder: A systematic review. Eur. J. Investig. Health Psychol. Educ. 2019, 10, 276–296. [Google Scholar] [CrossRef] [PubMed]
- Fears, N.E.; Palmer, S.A.; Miller, H.L. Motor skills predict adaptive behavior in autistic children and adolescents. Autism Res. 2022, 15, 1083–1089. [Google Scholar] [CrossRef]
- Di Leva, A.; Filippini, M.; Zampi, R.; Verniti, S.; Nugnes, E.; Glorioso, A. Visual-W: Adattamento della WISC/WAIS a pazienti con sindrome dello spettro autistico. Phenom. J.—Int. J. Psychopathol. Neurosci. Psychother. 2022, 4, 109–116. [Google Scholar]
- Fournier, K.A.; Kimberg, C.I.; Radonovich, K.J.; Tillman, M.D.; Chow, J.W.; Lewis, M.H.; Bodfish, J.W.; Hass, C.J. Decreased static and dynamic postural control in children with autism spectrum disorders. Gait Posture 2010, 32, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Braconnier, M.L.; Siper, P.M. Neuropsychological assessment in autism spectrum disorder. Curr. Psychiatry Rep. 2021, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Fay, D.; Brock, E.; Peneton, S.; Simon, R.; Splan, M.; Sullivan, L.; Weiler, A. Physical therapists’ use and alteration of standardized assessments of motor function in children. Pediatr. Phys. Ther. 2018, 30, 318–325. [Google Scholar] [CrossRef] [PubMed]
| Assessment Measure | Time | Space | Equipment | Qualification | Training |
|---|---|---|---|---|---|
| MABC-2 | 3 | 3 | 2 | 1 | 2 |
| BOT-2 | 2 | 2 | 3 | NR | 2 |
| PDMS-2 | 1 | 3 | 3 | 1 | 2 |
| TGMD-2 | 3 | 2 | 3 | NR | 2 |
| TGMD-3 | 3 | 1 | 3 | NR | 2 |
| AIMS | 4 | 4 | 4 | 2 | 2 |
| References | Reliability Measurement | Result | Quality of Results | |
|---|---|---|---|---|
| [30] | Green, D. et al. (2009) | Not reported | Not reported | Not reported |
| [31] | Kaur, M. et al. (2018) | Inter-rater reliability Intra-rater reliability | BOT: (inter) Gross motor ICC = 0.99 Fine motor ICC = 0.98 (intra) Gross motor ICC = 0.99 Fine motor ICC = 0.98 | + + + + |
| SIPT-BMC: (inter) Rhythmicity ICC 0.96, Mirroring ICC 0.99, Overflow ICC0.80, Time ICC 0.80. (intra) Rhythmicity ICC = 0.97, Mirroring ICC = 0.97, Overflow ICC = 0.95, Time ICC = 0.99 | ||||
| [32] | Odeh, C.E., et al. (2022) | Intra-rater reliability | MABC-2: ICC = 0.988–0.994 BOT-2: ICC = 0.970–0.996 | + + |
| [33] | Miller, HL. et al. (2021) | Not reported | Not reported | Not reported |
| [34] | Ament, K. et al. (2015) | Not reported | Not reported | Not reported |
| [35] | Faber, L. et al. (2022) | Inter-rater reliability | VMI: ICC 0.941 | + |
| [36] | Fulceri, F. et al. (2019) | Not reported | Not reported | Not reported |
| [37] | Alsaedi, RH. et al. (2020) | Not reported | Not reported | Not reported |
| [38] | Bricout, VA. et al. (2019) | Not reported | Not reported | Not reported |
| [39] | Liu, T. et al. (2013) | Not reported | Not reported | Not reported |
| [40] | Yang, Y. C. et al. (2019) | Not reported | Not reported | Not reported |
| [41] | Crippa et al. (2021) | Not reported | Not reported | Not reported |
| [42] | Vanvuchelen (2007) | Not reported | Not reported | Not reported |
| [43] | Provost (2007) | Inter-rater reliability | PDMS-2: ICC = 0.98 | + |
| [44] | Biffi et al. (2018) | Not reported | Not reported | Not reported |
| [45] | De Francesco et al. (2023) | Not reported | Not reported | Not reported |
| [46] | Allen et al. (2017) | Intra-rater reliability | TGMD-3 Traditional: ICC = 0.95 TGDM-3 Visual: ICC = 0.95 | + + |
| [47] | Staples & Reid (2010) | Not reported | Not reported | Not reported |
| [48] | Martel et al. (2024) | Not reported | Not reported | Not reported |
| [49] | Kalfiřt et al. (2023) | Not reported | Not reported | Not reported |
| [50] | Kochav-Lev et al. (2023) | Not reported | Not reported | Not reported |
| [51] | Martín-Díaz et al. (2024) | Not reported | Not reported | Not reported |
| Assessment Name | Variable Assessed | Age Range | Assessment Context | Citation |
|---|---|---|---|---|
| Movement Assessment Battery for Children—Second Edition (MABC-2) | Manual dexterity, aiming and catching, balance | 3–16 | Structured probes | Bricout et al. (2019) [38] Green et al. (2009) [30]; Odeh et al. (2022) [32]; Miller et al. (2021) [33]; Ament et al. (2015) [34]; Faber et al. (2022) [35]; Liu et al. (2013) [39]; Crippa et al. (2021) [41]; Vanvuchelen et al. (2007) [42]; Biffi et al. (2018) [44]; De Francesco et al. (2023) [45]; Martel et al. (2024) [48] |
| Bruininks–Oseretsky Test of Motor Proficiency, Second Edition (BOT-2) | Fine motor precision, fine motor integration, manual dexterity, bilateral, coordination, balance, running speed and agility, upper-limb coordination, strength | 4–21 | Structured probes | Kaur et al. (2018) [31]; Odeh et al. (2022) [32]; Alsaedi et al. (2020) [37]; Kalfirt et al. (2023) [49]; Martín-Díaz et al. (2024) [51] |
| Peabody Developmental Motor Scales-Second Edition (PDMS-2) | Reflexes, stationary, locomotion, object manipulation, grasping, visual–motor integration. | Birth-5 | Structured probes | Fulceri et al. (2019) [36]; Yang et al. (2019) [40]; Vanvuchelen et al. (2007) [42]; Provost et al. (2007) [43] |
| Test of Gross Motor Development (TGMD) | Locomotor skills: running, jumping, walking on a line, crawling, hurdling. Object control skills: throwing, catching, kicking, bouncing, dribbling | 3–10 | Structured probes | Allen et al. (2017) [46]; Staples & Reid (2010) [47] |
| Alberta Infant Motor Scale (AIMS) | Gross motor development, postural control, movement quality | 0–18 months | Standardized observational assessment | Kochav-Lev et al. (2023) [50] |
| References | Study Design | Sample Size | M:F | Years Range (M; SD) | Outcome Measures | Aims | Results | |
|---|---|---|---|---|---|---|---|---|
| [30] | Green, D. et al. (2009) | observational study | 47 ASD 43 broader autistic spectrum disorder | 7.4:1 | 10–14 (M 11.33 ± 0.83) | MABC-2; DCDQ | Explore the degree of impairment in movement skills in autistic children and a wide IQ range. | Among autistic children, 79% showed marked motor deficits on the M-ABC test, and an additional 10% had borderline difficulties. Children diagnosed with infantile autism showed more motor difficulties than those with a broader autistic profile, and children with an IQ below 70 were more affected than those with an IQ above 70. |
| [31] | Kaur, M. et al. (2018) | qualitative observational study | 12 HASD 12 LASD 12 TD | 3:9 0:12 2:10 | 5–12 (M 7.44 ± 0.57) 5–12 (M 8.74 ± 0.59) 5–12 (M 7.75 ± 0.55) | BOT-2; SIPT | Study measures for the comprehensive profiling of the motor system in autistic children. | The BOT-2 revealed a significant group effect for the composite body coordination, composite fine manual, and manual dexterity scores. Both ASD groups scored lower than the TD group on all BOT-2 outcome measures. Compared with the differences between the two ASD groups, the LASD group scored lower than the HASD group on the BOT-SF and body coordination subtests. Both groups demonstrated similarly poor performance on the composite fine manual and manual dexterity subtests. |
| [32] | Odeh, C.E., et al. (2022) | observational study | 12 ASD 12 TD | 11:1 11:1 | 5–12 (M 8.71 ± 1.69) 5–11 (8.74 ± 2.42) | BOT-2, MABC-2 | The purpose of this preliminary study was to establish a robust motor profile in autistic children across a wider range of motor skills. | In this study, autistic children demonstrated deficits in the performance of tasks targeting strength, speed, agility, coordination, and both static and dynamic balance. Furthermore, differences were also found between the MABC-2 and BOT-2 scores for the same subtest and aiming and catching tasks. |
| [33] | Miller, HL. et al. (2021) | retrospective study | 43 ASD 18 DCD | 37:6 7:2 | 5–19 (M 11.05 ± 3.6) 8–14 (M 11.05 ± 3.6) | DCDQ; MABC-2; Beery VMI | The study aimed to determine whether motor problems in ASD represent the possible co-occurrence of DCD. | Over 97% of cases in our ASD group met DSM-5 Criterion A based on MABC-2 scores; over 92% met Criterion B based on DCD-Q scores. |
| [34] | Ament, K. et al. (2015) | observational study | 56 ASD 63 ADHD 81 TD | 8:1 6:1 23:4 | (M 10.27 ± 1,8) (M 9.98 ± 1.34) (M 10.31 ± 1.18) | MABC-2 | The study aims to compare motor functioning among three groups: autistic children, ADHD children, and TD children, in order to better define motor deficits in these clinical groups and understand whether motor deficits assist in distinguishing between clinical groups. | Comparing the typically developing group and the developmental disability (DD) group revealed that all three MABC subscale scores were significantly negatively associated with having a DD. For manual dexterity, the mean is 4.88; aiming/catching is 6.52; and balance is 4.82. Impairments in motor skills that require the coupling of visual and temporal feedback to regulate movement appear deficient in ASD. |
| [35] | Faber, L. et al. (2022) | observational study | 17 ASD 17 TD | 1:0.24 | 9.83–15.13 ASD; 10.51–14.80 TD | MABC-2, VMI, VP | The first aim of this study was to examine motor skills, visual perception, and visual–motor integration of autistic children and youth in comparison with age- and gender-matched individuals without ASD. The second aim was to determine if there was an association between motor skills, visual perception, and VMI among children and youth with and without ASD. | Autistic children and youth showed lower overall motor skills, particularly in aiming and catching and balance subscales of the MABC-2, compared with neurotypical peers. No significant associations were found between motor skills, visual perception, and visual–motor integration in the ASD group, suggesting these are not underlying mechanisms of motor deficits. |
| [36] | Fulceri, F. et al. (2019) | qualitative observational study | 32 ASD | 2.5–5 (M 48.5 ± 8.8) | PDMS-2; | This study aimed to explore the associations between motor skills and clinical/developmental features in a sample of ASD preschoolers through ANNs (artificial neural networks). | The findings revealed that poor motor skills were a common clinical feature of ASD preschoolers, relating both to the high level of repetitive behaviors and to the low level of expressive language. Total QM, gross QM, and fine QM mean scores were placed into the “Poor class”; any child that had an individual total QM score was in the Average class; one child and two children had, respectively, gross QM and fine QM in the Average class. Almost all subscale mean scores were in the “Below Average class” or the “Poor class.” | |
| [37] | Alsaedi, RH. et al. (2020) | observational study | 119 ASD 30 TD | 24:6 4:1 | 6–12 (M 8.7 ± NS) | BOT-2 | This study aims to determine the prevalence, severity, and nature of the motor abnormalities seen in ASD children as well as to elucidate the associated developmental profiles. | The results revealed the high prevalence of motor abnormalities in the ASD group compared with normative data derived from the BOT-2 manual. Furthermore, the findings suggest that the age variable may influence the overall motor performance of ASD children and that children’s motor abnormalities may decrease with maturation. |
| [38] | Bricout, VA. et al. (2019) | observational study | 22 ASD 20 TD | 22:0 20:0 | 8–12 (M 10.7 ± 1.3) 8–12 (M 10.0 ± 1.6) | EUROFIT; PANESS; M-ABC | The present study provides a physical fitness profile of autistic children. The second aim of this study was to identify which motor tests best discriminate between autistic and non-autistic children. | In the ASD group, flexibility, explosive power, and strength scores were significantly lower compared with the control group. The results also showed significant difficulties in ASD children regarding dexterity and ball skills. The principal component analysis and agglomerative hierarchical cluster analysis allowed for the classification of children based on motor tests, correctly distinguishing clusters between children with and without motor impairments. Autistic children showed significantly lower MABC scores compared with the control group. Major deficits were observed in manual dexterity and ball skills, while balance differences were not statistically significant. |
| [39] | Liu, T. et al. (2013) | retrospective study | 30 ASD 30 TD | 13:2 8:7 | 3–16 (M 7.96 ± 3.14) 3–16 (M 7.44 ± 2.36) | MABC-2 | The aim of this study was to investigate the fine and gross motor performance of autistic children and typically developing children of the same age. | According to the results obtained, autistic children presented delays in the performance of both fine and gross motor skills on the MABC-2 compared with typically developing children of the same age. A total of 77% of autistic children were in the red zone, 3% were in amber zone, and 20% were in the green zone. |
| [40] | Yang, Y. C. et al. (2019) | prospective observational study | 15 ASD 15 TD 15 VLBW-PT | 13:2 3:2 3:2 | 2.5–3 (M 2.5 ± NS) | PDMS-2; | This study aims to compare cognitive, motor, and behavioral developments and free-play movement performance in ASD toddlers who were full term (FT-ASD), toddlers who were full term and are typically developing (FT-TD), and toddlers who were born preterm and had a very low birth weight (VLBW-PT). | Children with FT-ASD showed lower cognitive and motor scores and a higher degree of behavioral problems than children with FT-TD or VLBW-PT. Furthermore, in free play, children with FTASD performed a higher degree of rotation speed, a higher movement time, and a higher frequency of movement towards the peripheral region than children with FT-TD or VLBW-PT. |
| [41] | Crippa et al. (2021) | observational study | 98 ASD 98 TD | 3,3:1 | 3–11 (M 7.21 ± 2.41) | MABC2-DCDQ | To investigate sex-related differences in the motor profiles of autistic boys and girls aged 3 to 11 years using a multi-method approach. | Autistic children showed significantly lower scores than typically developing peers on all subscales of the MABC-2: manual dexterity, aiming and grasping, and balance. No interaction between diagnosis and sex was found, suggesting similar motor profiles in autistic boys and girls. A sex-related difference was observed in one kinematic characteristic: females showed reduced motor anticipation compared with males. |
| [42] | Vanvuchelen (2007) | observational study | 8 low-functioning with autism (LFA) 13 with mental retardation (MR) 17 high-functioning with autism (HFA) 17 typically developing (TD) | 5.1–10.6 (M 8.04 ± 0.84) | MABC-PDMS | The aim was to investigate whether the difficulties in gestural imitation of children with autism depended on a cognitive–representational or perceptual–motor deficit through the manipulation of task variables in two groups of autistic subjects and one group with typical development. | The results revealed that all autistic children, with and without mental disorders, had more problems imitating non-significant gestures than significant gestures compared with non-autistic controls. LFA performed significantly worse than RM on the motor test and imitation tasks. The HFA performed significantly worse than the TD in the motor test, but not in the imitation tasks, with the exception of non-significant gestures. This study supports the idea that underlying the difficulties in gestural imitation of children with autism is a perceptual–motor impairment. | |
| [43] | Provost (2007) | observational study | 19 ASD 19 DD | 30:8 | 1–3 (M 2.53 ± 0.38) | PDMS | The aim of the study was to compare the levels of gross motor (GM) and fine motor (FM) development in toddler-aged autistic children with the GM and FM development levels of children with developmental delay (DD) who are not autistic. | The results showed that autistic children had generally similar levels of GM and FM development. The motor profiles of autistic children did not differ significantly from those of children with DD. |
| [44] | Biffi et al. (2018) | comparative experimental study | 15 ASD 16 TD | 14:1 15:1 | 7.4–11.6 (M 9.91 ± 1.44) | MABC2-DCDQ | The objective is to describe the gait pattern and motor performance of school-age autistic children, not treated with medication, compared with a sample of the same gender and age with TD. | The results indicated an abnormal gait pattern, in which autistic children tend to increase their locomotion stability. No difference between the groups regarding the spatio-temporal parameters. A significant difference was observed in walking speed and a slightly reduced stride length and an increase in the stance phase of the gait cycle. Motor skills measured by tests showed that the ASD group had lower scores on MABC2 manual pointing and grasping, balance and total score, and lower DCDQ scores than TD. |
| [45] | De Francesco et al. (2023) | observational study | 25 ASD 25 ADHD 25 TD | 21:4 21:4 21:4 | 7–13 (10.23 ± 1.22) | DCDQ; MABC2; NEPSY-II | The aim was to discriminate through multimodal assessment the motor skills of autistic children, ADHD children, and peers with typical development. | The results showed that the DCDQ questionnaire and motor imitation skills were the best predictors of autism when compared with TD children, with an accuracy of 87.2%. Differences between ADHD and TD children in the DCDQ and motor skills differentiated the two groups of participants with an accuracy level of 73.5%. The difference between the groups was observed in the MABC2 subscales of pointing and catching, which predicted the status of the autistic group with an accuracy of 70%. |
| [46] | Allen et al. (2017) | observational study | 14 ASD 21 TD | 12:9 5:2 | 4–10 (M 7.13 ± 1.96) | TGMD-3 | Evaluate the reliability and validity of the TGDM-3 in two conditions: with the traditional protocol and with the visual support protocol. | Results confirm the traditional TGMD-3 protocol and the TGMD-3 visual support protocol as reliable measures in measuring gross motor performance children with typical development and autistic children. The test has internal consistency and excellent levels of test–retest, inter-rater, and intra-rater reliability using both tests and both experimental groups. |
| [47] | Staples & Reid (2010) | observational study | 25 ASD 25 chronological age-matched 22 developmentally matched group 19 mental age-matched group | 21:4 21:4 18:4 16:3 | 9–18 (M 11.15 NS) | TGMD-2 | The study compares the motor skill performance of autistic children with three types of typically developing groups individually matched on specific developmental variables: chronological age, motor ability, and cognitive development. | Motor skill performance in ASD is significantly delayed in late childhood. |
| [48] | Martel et al. (2024) | observational study | 14 ASD 14 DCD 14 TD | 6:1 4:3 2.5:1 | 7–12 (M 10.4 ± 0.29) | MABC-2 | To compare motor deficits in autistic children with those in children with DCD and TD in order to identify distinctive features between the three groups. | ASD children demonstrate specific motor deficits, distinct from those of DCD. TD children showed significantly better scores on all motor measures compared with ASD and DCD groups. |
| [49] | Kalfirt et al. (2023) | observational study | 17 ASD 15 TD | 32:0 | 9–13 (ASD: M 11.1 ± 1.0; TD: M 11.0 ± 0.5) | BOT-2; Heart Rate Variability; Arterial Stiffness | To compare motor skills, heart rate variability, and arterial stiffness between autistic children and TD. | ASD children showed significant deficits in manual coordination, strength, agility, and total motor composite compared with TD. HRV: Significantly reduced in ASD, indicating autonomic dysfunction. Arterial stiffness: No significant differences between groups. |
| [50] | Kochav-Lev et al. (2023) | observational study | 15,185 MDD; 388 ASD and MDD | 3.3:1 | 0–2 | AIMS | To evaluate the use of AIMS for early detection of motor developmental delay (MDD) as an indicator for ASD in infants. | Odds ratio for ASD with MDD: 4.1 (95% CI: 3.6–4.6). A total of 87% of ASD infants showed MDD or suspected MDD on AIMS. A total of 98% of motor delay referrals confirmed as MDD or suspected MDD. Early referral (0–6 months): there was 39% MDD detection, increasing to 68% at older ages. |
| [51] | Martín-Díaz et al. (2024) | cross-sectional observational study | 50 ASD 50 TD | 6:1 6:1 | (ASD M 9.54 ± 3.09) (TD M 9.54 ± 3.09) | SFBOT-2; TUG | To compare static and dynamic balance, postural control, and motor skills between autistic children and adolescents and those with TD, using specific assessment tests. To examine the potential correlation between the scores of the assessment tools used in the study among children and adolescents with ASD. | The ASD group showed significantly worse performance in TUG, SFBOT-2, and PBS. Motor deficits were most evident in strength, manual dexterity, agility, and balance. Weak correlations were found between TUG and SFBOT-2/PBS; moderate correlation between SFBOT-2 and PBS. Monopodal support with hands on hips showed the largest difference in PBS. |
| Study | Risk of Bias | Applicability Concerns | |||||
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| [48] Martel, 2024 |  |  |  | ? |  |  |  |
| [51] Martín-Díaz, 2024 |  | ? | ? | ? | ? |  |  |
| [45] De Francesco, 2023 |  |  |  |  | ? |  |  |
| [49] Kalfirt, 2023 |  | ? |  | ? |  | ? |  |
| [50] Kochav-Lev, 2023 |  | ? | ? |  |  |  |  |
| [35] Faber, 2022 |  | ? |  |  | ? |  |  |
| [32] Odeh, 2022 |  |  | ? |  |  |  |  |
| [41] Crippa, 2021 |  |  |  |  | ? |  |  |
| [33] Miller, 2021 |  | ? |  |  | ? |  | ? |
| [37] Alsaedi, 2020 |  | ? |  | ? |  |  |  |
| [38] Bricout, 2019 |  |  | ? | ? | ? |  | ? |
| [36] Fulceri, 2019 |  |  |  |  | ? |  |  |
| [40] Yang, 2019 |  |  |  |  | ? |  |  |
| [44] Biffi, 2018 |  |  |  |  | ? |  |  |
| [31] Kaur, 2018 |  |  | ? |  |  |  |  |
| [46] Allen, 2017 |  |  |  |  |  |  |  |
| [34] Ament, 2015 |  | ? | ? | ? | ? |  | ? |
| [39] Liu, 2013 |  | ? | ? | ? | ? |  | ? |
| [30] Green, 2009 |  |  |  |  |  |  |  |
| [47] Staples, 2009 |  |  |  |  |  | ? | ? |
| [43] Provost, 2007 |  |  |  |  | ? |  |  |
| [42] Vanvuchelen, 2007 |  | ? |  |  | ? |  |  |
 Low risk;
Low risk;  high risk; ? unclear risk.
high risk; ? unclear risk.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, A.; Raciti, C.; Di Cara, M.; Portaro, S.; Muratore, R.; De Domenico, C.; Fulgenzi, A.; Settimo, C.; Quartarone, A.; Cucinotta, F.; et al. Motor Coordination Assessment in Autism Spectrum Disorder: A Systematic Review. Diagnostics 2025, 15, 2118. https://doi.org/10.3390/diagnostics15172118
Piccolo A, Raciti C, Di Cara M, Portaro S, Muratore R, De Domenico C, Fulgenzi A, Settimo C, Quartarone A, Cucinotta F, et al. Motor Coordination Assessment in Autism Spectrum Disorder: A Systematic Review. Diagnostics. 2025; 15(17):2118. https://doi.org/10.3390/diagnostics15172118
Chicago/Turabian StylePiccolo, Adriana, Chiara Raciti, Marcella Di Cara, Simona Portaro, Rosalia Muratore, Carmela De Domenico, Alessia Fulgenzi, Carmela Settimo, Angelo Quartarone, Francesca Cucinotta, and et al. 2025. "Motor Coordination Assessment in Autism Spectrum Disorder: A Systematic Review" Diagnostics 15, no. 17: 2118. https://doi.org/10.3390/diagnostics15172118
APA StylePiccolo, A., Raciti, C., Di Cara, M., Portaro, S., Muratore, R., De Domenico, C., Fulgenzi, A., Settimo, C., Quartarone, A., Cucinotta, F., & Alito, A. (2025). Motor Coordination Assessment in Autism Spectrum Disorder: A Systematic Review. Diagnostics, 15(17), 2118. https://doi.org/10.3390/diagnostics15172118






