Abstract
The L1 molecule is a cell adhesion molecule (L1CAM) that was originally implicated in neuronal development. In recent years, studies of several large cohorts of patients with endometrial cancer have revealed that L1CAM acts as a poor prognostic factor, in most cases independent of other parameters. It seems to be an important factor, especially in the non-specific molecular profile subgroup (p53 normal expression, MMR proficient, POLE not mutated) of endometrial cancer, and a factor predictive of the response to chemotherapy. This review aims to gather most of the current knowledge regarding this promising prognostic factor.
1. Introduction
L1CAM was discovered over 40 years ago as a neural cell adhesion molecule; however, it has only recently gained attention in gynecological pathology for two reasons: as a surrogate marker of TRAF7 mutations in the diagnosis of adenomatoid tumors and well-differentiated papillary mesothelial tumors, and as a prognostic factor in endometrial carcinoma. Several recent studies have shown that L1CAM expression is associated with high-risk endometrial cancer, retains its prognostic significance in multivariate analysis, and might predict response to chemotherapy. This review aims to gather information regarding the pathophysiology of this molecule and its prognostic role in endometrial cancer by presenting the studies that have led to the current state of knowledge.
2. L1CAM Physiology and Pathways
In the early 1980s, a cell line called PC12, cloned from a rat adrenal pheochromocytoma, was extensively used to study neuronal differentiation [1]. When this cell line was treated with nerve growth factor (NGF), a marked induction of a large membranous glycoprotein was observed, which, on the basis of these properties, was named NGF-inducible large external (NILE) glycoprotein (GP) [1]. This NILE GP was found in almost all kinds of neuron cultures from the central and peripheral nervous systems [1]. At that time, two well-known cell adhesion molecules were implicated in interactions during nervous system development: the neural cell adhesion molecule (N-CAM), which is expressed at early developmental stages, and the L1 cell adhesion molecule, which appears later in development [2]. Further studies revealed that the L1 molecule and NILE GP were actually the same molecule [2] and that the L1 molecule was implicated in neuron-neuron interaction but not in neuron-astrocyte adhesion, as N-CAM did [3]. Later, it was shown that L1 is a member of the immunoglobulin (Ig)-domain superfamilies, which, along with integrin, cadherin, and selectin families, play important roles in cell–cell interactions during development [4]. This Ig-domain cell adhesion molecule (CAM) superfamily contains several subgroups, such as the L1 and N-CAM families, according to the number and arrangement of their domains [4]. The L1 adhesion molecule, also called CD171, is a 200–220 kDa type I membrane glycoprotein of the immunoglobulin (Ig) family, consisting of six Ig-like domains and five fibronectin-type III repeats, a transmembrane region, and a cytoplasmic tail [5]. Members of the L1 subgroup of CAMs have been implicated in neurons migration, myelination, axonal growth, and pathfinding, and mutations in L1 genes cause severe neurological defects [4]. Despite this major role in the development of the neural system, further studied revealed that L1CAM is involved in many other tissues, such as in kidney morphogenesis [6], that it is present in granulocytes and lymphocytes [7], and in the development of several tumors, such as melanoma [5,8], renal cell cancer [9,10], colorectal cancer [11], gallbladder cancer [12], pancreatic cancer [13], and even gastrointestinal stromal tumors [14]. The monoclonal antibody UJ127, which binds to the extracellular domain of L1CAM, was used in the latter case, showing expression in 74% of the 72 tumors studies [14]. Furthermore, the expression of two monoclonal antibodies was studied by immunohistochemistry in a variety of normal and neoplastic tissues; among normal tissues, only the renal collecting tubules and peripheral nerve bundles showed L1CAM expression [15]. Regarding neoplastic ones, gynecological tumors, some neuroendocrine and neural tumors, as well as melanomas, showed L1CAM expression, whereas most carcinomas of other sites did not express L1CAM [15].
Before presenting the studies that revealed the importance of L1CAM in endometrial cancer, it is worth reiterating that L1CAM is used in the diagnostic pathology of two other neoplasms often encountered in gynecological pathology: the adenomatoid tumor and the well-differentiated papillary mesothelial tumor [16,17,18,19]. These tumors harbor tumor necrosis factor receptor-associated factor 7 (TRAF7) mutations, which are thought to activate nuclear factor-kappa B (NF-κB) signaling and, thus, the expression of L1CAM (clone UJ127.11, dilution 1:1800) by the neoplastic mesothelial cells of these tumors, since L1CAM is considered a transcriptional target of NF-κB [16,17]. However, no further studies exist on the link between TRAF7 and L1CAM expression [18]. In any case, how exactly L1CAM controls its cellular effects—especially in the case of cancer cells, where it seems to offer them increased motility and invasiveness, as well as an epithelial-to-mesenchymal phenotype—remains unclear and warrants further research. Apart from adhesion between cells, L1CAM, when cleaved by several proteases and by further post-translational modifications of its fragments, acquires new and heterogeneous functions [20] that contribute to these effects.
3. L1CAM in Endometrial Cancer
Potential Mechanisms of L1CAM (Figure 1)
The mechanism of how L1CAM is activated/overexpressed and the molecular pathways it activates downstream remain unclear. As previously mentioned, L1CAM “offers” an epithelial-to-mesenchymal transition phenotype to tumor cells, which seems to be regulated by TGFb1 in a Slug-dependent manner [21]. L1CAM at the protein and mRNA levels was also found to be expressed in higher levels in endometriosis tissues than in healthy controls [22]. Despite all this evidence for an implication of the L1CAM molecule in endometrial tissue pathophysiology, very limited information is still available on L1CAM gene regulation in endometrial tissue or its subsequent molecular pathway. In 2010, Pfeifer et al. showed that the L1CAM gene harbors two promoters, both of which are present in endometrial cell lines, that appear to be activated in a cell-type-specific manner [23]. The two promoters were activated by Slug, and one of them by the overexpression of b-catenin [23]. It has been previously shown that b-catenin indeed plays a role in the regulation of L1CAM expression in colon cancer [24], and that in pancreatic cells, the upregulation of L1CAM is Slug-dependent and TGFb1-dependent and promotes tumor cell migration and chemoresistance [25]. In addition, epigenetic mechanisms can regulate the expression of the L1CAM gene, which is localized at the Xq28 chromosome; however, no differences in promoter methylation were found between L1CAM-negative and -positive tumors [26]. Given that a negative prognostic impact of L1CAM expression was also reported in patients with ovarian cancer, single nucleotide polymorphisms (SNPs) of the L1CAM gene were sought in 103 ovarian cancer patients and 104 age-matched controls, finding that the genotype AA of one SNP in intron 1 was associated with ovarian cancer presence [27]. In cancer cell lines, L1CAM promotes not only epithelial-to-mesenchymal transition but also chemotherapy and anoikis resistance [28]. Interestingly, in endometrial cancer cell lines, the presence of FBXW7 mutations affects the protein levels of L1CAM [29]. FBXW7 mutations were found to affect L1CAM gene expression using bioinformatics in The Cancer Genome Atlas (TCGA), confirming a relationship between these two targets [30]. In line with the probable role of L1CAM in the epithelial-to-mesenchymal transition, the expression profile data of 1169 epithelial-to-mesenchymal transition-related genes in endometrial cancer from the TCGA were analyzed in comparison to overall survival and revealed that L1CAM is indeed one of the genes implicated [31]. One of the hallmarks of cancer pathophysiology is the immune response to tumors; it has been found that the so-called tertiary lymphoid structures, specialized ectopic lymphoid formations harboring high endothelial venules, are associated with good prognosis and response to immunomodulatory treatment in several forms of cancer [32,33]. A study of these structures in endometrial cancer revealed that L1CAM is expressed inside the lymphoid structures from follicular dendritic cells independently from its tumor expression, and that this L1CAM-expressing lymphoid structure is an independent prognostic factor [34]. This study used the same previously stained slides of the PORTEC-3 trial, which used the clone 14.10 to define the L1CAM-positive lymphoid structures [35]. Of interest, L1CAM gene expression is associated with RNA methylation, and its expression controls the immunological tumor response [36]. In a bioinformatics analysis of endometrial cancers from the TCGA database, the regulators of RNA methylation and their relationship with prognosis and the immune microenvironment were studied, revealing that L1CAM acts as an RNA methylation-related gene and is implicated in the immune response [36]. In a more recent analysis of endometrial cancer cells, the expression of L1CAM promoted the expression of focal adhesion kinase (FAK) and activation of the FAK–GRB2 (growth factor receptor-bound protein 2)–SOS (Son of Sevenless)–RAS (Rat Sarcoma) pathway [37]; FAK is encoded by the protein tyrosine kinase 2 gene (PTK2), which in glioma cells interacts with L1CAM to allow FAK production [37].
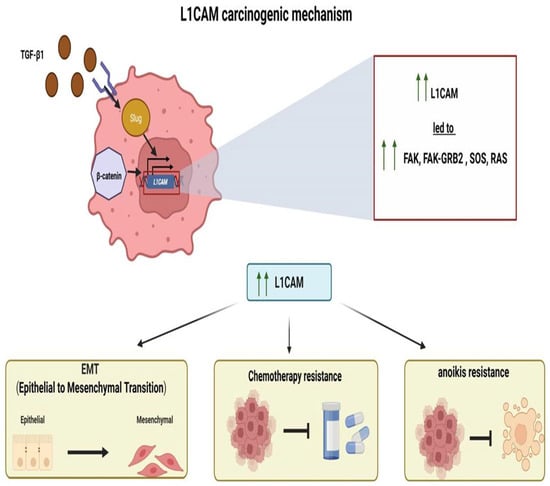
Figure 1.
Potential carcinogenic mechanism of L1CAM.
4. Diagnostic Role of LICAM
Regarding the diagnostic value of L1CAM, it has been proposed that L1CAM expression, together with IMP3 (insulin-like growth factor-II mRNA-binding protein 3) expression, can accurately distinguish low-grade from high-grade endometrial carcinomas [38]. In line with this notion, another study using all three—L1CAM, IMP3, and progesterone receptor (PR)—improved the diagnostic accuracy in preoperative endometrial samples compared to morphological assessment alone in detecting high-grade carcinomas [39]. Despite these findings, at this moment, this marker has no diagnostic utility in endometrial cancer.
5. The Prognostic Role of L1CAM
5.1. L1CAM Expression and Endometrial Cancer Histology (Table 1)
One of the first studies regarding L1CAM in gynecological carcinomas was published by Fogel et al. in The Lancet in 2003 [40]. The authors used a monoclonal antibody against the ectodomain of L1 (UJ127.11) and two monoclonal and polyclonal antibodies against the cytoplasmic portion of L1 (polyclonal antibody pcytL1 and monoclonal antibody 745H7) in 72 endometrial carcinomas and 10 non-cancerous hysterectomies [40]. Differences in positivity regarding different antibodies are not reported. They found 20 positive uterine carcinomas, corresponding to 16% of endometrioid adenocarcinomas, 75% of serous carcinomas, and 71% of “mixed” (not further specified) carcinomas; all non-cancerous controls were negative [40]. Higher-stage tumors were all L1CAM positive, compared with 16% of low-stage ones (all histologies included). Soluble L1CAM was also detected in the blood of some patients with L1CAM-positive cancers compared with healthy controls or patients with L1CAM-negative tumors [40]. The authors reported a shorter survival time for L1CAM-positive tumors, but other prognostic factors were not considered in the form of a multivariate analysis [40]. Continuing this research, the authors showed that overexpression of L1CAM in ovarian cell lines enhanced the migration of tumor cells and resulted in better tumor growth in mice [41]. Later, they used two monoclonal antibodies to the ectodomain of L1CAM (L1-11A and L1-14.10) and two to the C-terminal part of L1CAM (2C2 and 745H7), but neither the expression of every different antibody nor the cutoff value for positivity are explained—in 10 normal endometria and in 296 endometrial carcinomas, corresponding to 272 endometrioid adenocarcinomas, 20 serous carcinomas, and 4 clear cell carcinomas [21]. In serous and clear cell carcinomas, the authors described L1CAM expression and ER/PR/E-cadherin negativity (percentages not given) [21]. In the endometrioid group of adenocarcinomas, 29% expressed L1CAM [21]. Patients with L1CAM-positive endometrioid carcinomas showed shorter recurrence-free survival in the univariate analysis, and despite a multivariate analysis mentioned in the discussion [21], these data are not presented to further understand the independent or non-independent role of L1CAM expression. Further techniques in cell lines revealed that TGFb1 enhances L1CAM expression in a Slug-dependent manner and that this upregulation led to a phenotype of epithelial-to-mesenchymal transition, which would be in accordance with their results in the endometrial cancer tissue specimens, where more L1CAM expression was associated with less estrogen receptor (ER), PR, and E-cadherin expression [21].
In 2013, one of the first numerous studies on L1CAM expression in endometrial cancer appeared, corresponding to a retrospective multicenter cohort of 1021 endometrial cancer tissues from stage I endometrioid adenocarcinomas [42]. The study revealed L1CAM expression (clone L1-40.10, cutoff 10%) in 17.7% of cases, and a poorer disease-free and overall survival for these patients [42]. This time, the authors performed a multivariate analysis, where L1CAM expression retained prognostic significance [42]. Slightly later, the Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC)-1 and -2 trials cohort was used to test L1CAM’s prognostic role [43]. These trials were initially designed to randomize patients with stage I endometrial cancer to receive external beam radiotherapy versus no adjuvant treatment or to receive external beam radiotherapy versus vaginal brachytherapy [43]. Regarding L1CAM, tumor samples of 865 patients were tested with the clone 14.10 (1:500 dilution), and a 10% tumor cell positivity was used as the cutoff value [43]. L1CAM tumor expression was associated with the risk of distant but not vaginal recurrence [43]. It was also associated with overall survival [43]. Most importantly, in multivariate analysis including age, depth of invasion, grade, lymphovascular invasion, and treatment, L1CAM was an independent factor of distant recurrence—a significance retained after excluding non-endometrioid histology [43]. In another study including 86 endometrioid and 30 non-endometrioid carcinomas, when the previously proposed cutoff value of 10% was applied, 44% of the tumors were L1CAM positive (clone 14.10, dilution 1:500) but showed no association with distant metastasis [35]. When the 50% cutoff value was applied, 24% of the tumors were positive and showed a significant association with distant metastasis [35]. Apart from immunohistochemistry, only a few other methods of studying L1CAM in endometrial cancer have been applied. Using the RNA sequencing expression data of 545 uterine carcinomas from TCGA, including all histological types, grades, and stages, Dellinger et al. found that high L1CAM expression (cutoff being the median value) was associated with advanced stage, high grade, serous carcinomas, positive lymph nodes, and poor survival [44]. The prognostic significance of RNA expression was retained in the multivariate analysis [44].
The 10% cutoff value and the 14.10 clone (dilution 1:300) were used in another study of stage I endometrial cancers diagnosed in 388 patients; a 9% positivity was noted, which was not associated with relapse or survival but was associated with relapse in patients not treated with chemotherapy [45]. One of the largest series considering L1CAM expression used the 14.10 antibody (dilution 1:100) and the 10% cutoff value to examine 1199 endometrial carcinomas [46]. The authors found 10% L1CAM expression in stage I endometrioid adenocarcinomas, 18% in 160 advanced-stage endometrioid adenocarcinomas, and 75% expression in non-endometrioid carcinomas [46]. L1CAM expression was significantly associated with advanced stage, positive lymph nodes, high grade and non-endometrioid histology, lymphovascular invasion, and distant recurrences [46]. It was associated with poor survival in endometrioid carcinomas but not in non-endometrioid carcinomas [46]. Another large series of 805 patients was studied for L1CAM expression (tissue microarrays, clone 14.10, dilution 1:300, cutoff 10%) and revealed positivity in 15% of the cases [47]. Similarly to the previous cohorts, L1CAM expression was associated with non-endometrioid histology, advanced stage, positive lymph nodes, lymphovascular invasion, and older age [47]. L1CAM expression was a poor prognostic factor in endometrioid but not non-endometrioid histology—a significance also retained in multivariate analysis [47]. Kommoss et al. reported L1CAM positivity (clone 14.1, dilution 1:50, cutoff 10%) in 8.4% of 344 endometrial carcinomas [48]. In contrast to previous studies, this positivity was not associated with lymphovascular invasion. However, similar to the findings of previous studies, L1CAM expression was an independent poor prognostic factor in the survival analysis [48]. The long-term results of the PORTEC-2 trial, consisting of the 10-year survival of 427 patients treated with external beam radiotherapy with vaginal brachytherapy, revealed L1CAM expression as one of the risk factors associated with pelvic and distant recurrence; multivariate analysis showed that L1CAM was a significant prognostic factor for distant recurrence and overall survival, but not for pelvic recurrence [49]. In another retrospective study of 312 endometrial carcinoma samples, almost 30% of the samples expressed L1CAM, and this expression was associated with distant metastasis but not with disease-free or overall survival [50]. A study of 162 patients with endometrial cancer revealed L1CAM (tissue microarrays, monoclonal antibody, clone UJ127.11, dilution 1:10,000, 10% cutoff) as an independent prognostic factor [51]. In 183 patients with early-stage endometrial cancer, L1CAM expression (clone 14.10, dilution 1:50, 10% cutoff) showed approximately 10% positive cases, and L1CAM was an independent prognostic factor in multivariate analysis [52]. No independent prognostic significance was noted for L1CAM (tissue microarrays, clone 14.10, dilution 1:100, 10% cutoff) in another study of 335 endometrial cancer patients [53].
Given the frequent association of L1CAM with lymph node metastasis, one could wonder if preoperative L1CAM expression can predict lymph node metastasis and prevent unnecessary morbidity of lymph node excision. The study by Zeiter et al. examined the expression of L1CAM in 212 patients and found positivity in 19.3% of the cases with the cutoff value of 10%, but without any association with lymph node metastasis [54]. This study also showed that treatment with radiotherapy improved survival for L1CAM-positive tumors [54]. The expression of L1CAM (tissue microarrays, clone UJ127, dilution 1:30, cutoff 5%) was also studied in the endometrial carcinomas of 34 diabetic patients compared with 34 endometrial carcinomas of matched non-diabetic patients, since the first group is more frequently associated with lymph node metastasis [55]. Despite no difference in L1CAM expression between the two groups, L1CAM expression in endometrial cancer of diabetic patients was associated with pelvic lymph node metastasis [55].
Another large series of L1CAM expression studies considered 1134 curettage specimens, 795 hysterectomy specimens, and the preoperative levels of L1CAM in the blood of 372 patients [56]. The authors found that the level of L1CAM expression in the curettage sample was correlated with that of the hysterectomy one, and that L1CAM expression predicted a poor outcome [56]. L1CAM levels in the blood were also associated with lymph node metastasis and poor outcome [56]. At almost the same time, a similar study compared the L1CAM in 241 endometrial biopsies to that of paired hysterectomy specimens of 75 patients; the serum levels of L1CAM were also measured in 40 patients with endometrial carcinoma [57]. They also showed a concordance between the preoperative biopsies and the hysterectomy specimens, but no association was found between L1CAM serum levels in L1CAM-positive and L1CAM-negative carcinomas [57].

Table 1.
L1CAM immunohistochemistry in the endometrial carcinoma.
Table 1.
L1CAM immunohistochemistry in the endometrial carcinoma.
| Author, Year | Antibody | Sample Size (n) | Positivity | Significance |
|---|---|---|---|---|
| Fogel et al. (2003) [40] |
|
|
|
|
| Huszar M et al. (2010) [21] |
|
|
|
|
| Zeimet AG et al. (2013) [42] |
|
|
|
|
| Bosse T et al., 2014 [43] |
|
|
|
|
| Van Gool IC et al. (2016) [35] |
|
|
|
|
| Smogeli E et al. (2016) [45] |
|
|
|
|
| van der Putten LJ et al. (2016) [46] |
|
|
|
|
| Pasanen A et al. (2016) [47] |
|
|
|
|
| Kommoss et al. (2017) [48] |
|
|
|
|
| Wortman BG, Creutzberg CL et al., 2018 [49] |
|
|
|
|
| Klat J et al. 2019 [50] |
|
|
|
|
| Kim J et al. (2023) [51] |
|
|
|
|
| Joe S et al. (2023) [52] |
|
|
|
|
| Yoon H et al. (2024) [53] |
|
|
|
|
| Zeiter D et al. (2021) [54] |
|
|
|
|
| Suh DH et al. 2014 [55] |
|
|
|
|
| Tangen IL et al. (2017) [56] |
|
|
|
|
| Pasanen A et al. (2017) [57] |
|
|
|
|
L1CAM: L1 cell adhesion molecule.
Conclusion: These studies reveal L1CAM expression level in endometrial cancer ranging from 7% to 44%, despite the same two antibody clones and 10% cutoff value almost always being used, raising questions about the preanalytical conditions and the pathologist’s interpretation, which probably impact results. According to the author’s personal experience, finding the optimal immunohistochemical protocol for this antibody is not easy and requires obtaining experience with it. Yet, most of these studies, despite the differences in protocol used or the positivity found, show that higher L1CAM immunohistochemical expression is observed in high-grade histology and advanced stages. Most importantly, the vast majority reveal the poor prognostic impact of L1CAM after adjusting for other factors in multivariate analysis, and they repeatedly show its prognostic significance for stage I endometrioid adenocarcinomas, the most heterogenous group of endometrial tumors lacking sufficient prognostic tools. Thus, despite the observed discrepancies, the fact that higher protein and RNA levels of L1CAM seem to be a poor prognostic factor in most studies highlights this factor as a potentially interesting tool in endometrial pathology.
5.2. L1CAM Expression and Molecular Subtype of Endometrial Carcinoma (Table 2)
When the four principal molecular subtypes of endometrial carcinomas started to appear as important prognosticators of this disease, the question of whether L1CAM is just a consequence of one of these subtypes—and therefore not an independent prognostic factor—began to be raised. A study of 947 endometrial carcinomas from patients with early-stage disease originally included in the PORTEC-1 and PORTEC-2 trials was classified according to the four major molecular subtypes: p53-mutant tumors, microsatellite instable tumors, POLE-mutant tumors, and tumors of a non-specific molecular profile. L1CAM expression (clone 14.10, dilution 1:500, 10% cutoff) was associated with distant recurrence and overall survival, and retained significance in multivariate analysis [58]. Karnezis et al. examined 413 endometrial carcinomas previously characterized for their molecular classification and found that 16% expressed L1CAM (tissue microarrays, cutoff 10%) [59]. Its expression was associated with aggressive factors such as advanced stage, non-endometrioid histology, grade 3 endometrioid adenocarcinomas, lymphovascular invasion, and negative ER and PR status [59]. L1CAM expression was associated with a poor outcome [59]. Importantly, it was associated with the p53-mutant tumor group [59]. In the multivariate analysis, L1CAM did not remain a significant prognostic factor [59]. Kommoss et al. further published data on 452 molecularly classified endometrial carcinomas and L1CAM expression (clone 14.10, dilution 1:50, cutoff 10%); they also showed that L1CAM expression was most frequent in p53-mutant tumors [60]. Interestingly, for tumors with no specific molecular profile, L1CAM predicted a poor outcome [60]. Similarly, in a cohort of 94 patients with endometrial cancer, L1CAM expression was of prognostic value only in the non-specific molecular profile subgroup, and in these patients, its expression was associated with early relapse after platinum-based chemotherapy [61]. Pasanen et al. classified 682 endometrioid endometrial adenocarcinomas according to their MMR protein expression (tissue microarrays) and their methylation status [62]. MMR deficiency was associated with a negative L1CAM (clone 14.10, 10% cutoff value for positivity) status. Survival was associated with L1CAM expression in the univariate analysis; however, this was not retained in the multivariate analysis [62]. In a study of 763 patients with endometrial cancer, tumors with abnormal expression of p53, L1CAM (cutoff 10%), ER, and PR showed the worst outcome, but in multivariate analysis, L1CAM retained only marginal significance [63]. The study of prognostic factors in 648 patients with high-risk endometrial cancer failed to show the independent prognostic significance of L1CAM (10% cutoff, clone 14.10, dilution 1:800) [64]. Another large cohort of 1110 non-specific molecular subgroup endometrial carcinomas, gathering data from previously reported cohorts (tissue microarrays, clone 14.10, dilutions 1:25–1:50), showed that L1CAM was a poor prognostic factor in univariate analysis but not in further analyses incorporating the grade of the endometrioid carcinomas and the stage of the disease [65]. L1CAM (clone 14.10, dilution 1:200, cutoff 10%) was studied in 626 stage I endometrioid carcinoma patients, finding expression in 8% of them and no association with survival in the multivariate analysis, but its expression in the non-specific molecular subgroup was associated with poor outcome [66]. In a cohort of 1044 patients with molecularly classified endometrial cancer [67], L1CAM expression (tissue microarrays, clone 14.10, dilution 1:300—according to the previous study by the authors [47], cutoff 10%) was found in almost 15% of the cases, and it was a prognostic factor only in the non-specific molecular subgroup. However, when controlling for further parameters in the multivariate analysis of this subgroup, it did not retain significance [67]. In 486 patients with endometrial cancer whose tumor samples were tested for L1CAM (clone 14.10, dilution 1:200, cutoff 10%), 53% of tumors expressed L1CAM—a higher percentage than most previous studies mentioned above, probably explained by including high-risk patients in this study [68]. L1CAM expression was a poor prognostic factor in both univariate and multivariate analysis, even when including the molecular subtypes [68]. However, in this study, L1CAM did not affect the chemotherapy effect (see discussion below) [68]. In a study of 61 patients with advanced-stage endometrial cancer with wild-type p53 expression and proficient MMR expression, L1CAM expression (clone UJ127.11, dilution 1:1000, cutoff 10%) was a poor prognostic factor [69]. Another study regarding L1CAM expression (technical details not provided) was conducted in 392 low-grade (endometrioid adenocarcinoma, grade 1 and 2) and 183 high-grade (grade 3 endometrioid adenocarcinoma, serous and clear cell carcinoma) cases, showing the independent significance of L1CAM expression in predicting recurrence only in the high-grade group in the multivariate analysis integrating several parameters but not the molecular classification [70]. Endometrioid adenocarcinoma specimens from 142 patients were tested for L1CAM and HER2 expression, revealing 27% and 12% positive cases, respectively [71]. The shortest disease-free survival was noted for patients expressing both HER2 and L1CAM [71]. Van der Putten et al. examined 293 endometrial carcinomas for ER, PR, and L1CAM expression to determine whether their combined study can be of prognostic value [72]. A 10% positivity cutoff value was used for all three markers [72]. They found 18% positivity for L1CAM [72]. L1CAM positivity and ER and PR negativity were associated with advanced stage, non-endometrioid histology, high grade, lymphovascular invasion, and shorter disease-free survival [72]. L1CAM did not retain prognostic significance in the multivariate model, whereas loss of PR did [72]. In a large retrospective study of prognostic algorithms in patients with endometrial cancer, L1CAM was an important risk factor in the p53-mutated subgroup [73].
Conclusion: These studies in molecularly classified endometrial carcinomas show even more variable results than previously noted in non-molecularly classified tumors. Some show that L1CAM remains a poor prognostic factor when adjusting for molecular subtypes, while others fail to find prognostic significance in multivariate analysis. They often show an association with the p53 mutated subtype which is in accordance with the previous finding of association with high grade histology. However, the results are conflicting in the nonspecific subtype; some studies found L1CAM’s poor prognostic role in this subtype, while others did not. The reasons for this discrepancy are not clear but the retrospective nature of these studies gathering tissues from different centers spanning several years, as well as the same issues of different protocols and preanalytical conditions, as previously mentioned, could contribute to the observed differences.

Table 2.
L1CAM immunohistochemistry in molecularly classified endometrial carcinoma in association with other predictive biomarkers (ER, PR, HER2).
Table 2.
L1CAM immunohistochemistry in molecularly classified endometrial carcinoma in association with other predictive biomarkers (ER, PR, HER2).
| Author, Year | Antibody | Sample Size (n) | Positivity | Significance |
|---|---|---|---|---|
| Stelloo E et al. (2016) [58] | Clone 14.10, dilution 1:500, cutoff 10% | 947 early-stage endometrial carcinomas classified molecularly | 5.6% |
|
| Karnezis et al. (2017) [59] | Anti-CD171 (L1) Antibody Clone 14.10, cutoff 10% | 413 endometrial carcinomas classified molecularly | 16% |
|
| Kommoss FK et al. (2018) [60] | Clone, 14.10; dilution, 1:50; cutoff, 10% | 452 endometrial carcinomas classified molecularly | 21.5% |
|
| Ravaggi A et al. (2022) [61] | Clone 14.10, 1:100 dilution, 10% cutoff | 94 patients with endometrial cancers | 30% |
|
| Pasanen et al. (2020) [62] | Clone 14.10, 10% cutoff | 682 endometrial endometroid adenocarcinomas classified according to their MMR protein expression (tissue microarrays) and methylation status | 10.77% |
|
| Vrede SW et al. (2021) [63] | Anti-CD171, clone 14.10, dilution 1:100, cutoff 10% | 763 patients with endometrial cancer, tumors with abnormal expression of p53, ER, and PR | 10.4% |
|
| Vermij L. et al. (2023) [64] | Clone 14.10, dilution 1:800, 10% cutoff, | 648 patients with high-risk endometrial cancer | 27.8% |
|
| Jamieson A et al. (2023) [65] | Clone 14.10, dilutions 1:25–1:50 | 1110 non-specific molecular subgroup of endometrial carcinomas | 10.6% |
|
| Lindemann K et al. (2024) [66] | Clone, 14.10; dilution, 1:200; cutoff, 10% | 626 patients with stage I endometrial endometrioid carcinoma | 8% |
|
| Aro K et al. (2024) [67] | Clone 14.10, dilution 1:300, cutoff 10% | 1044 patients with molecularly classified endometrial cancer | 15% |
|
| Kleppe A et al. (2025) [68] | Clone, 14.10; dilution, 1:200; cutoff, 10% | 486 patients with endometrial cancers | 53% |
|
| Kim JC et al. (2024) [69] | Clone UJ127.11, dilution 1:1000, cutoff 10% | 62 patients with advanced-stage endometrial cancer with wild-type p53 expression and proficient MMR expression | 32.26% |
|
| Li Y et al. (2025) [70] | N/A |
|
|
|
| Abdel Azim S et al. (2017) [71] | Anti-L1 (clone 14.10), cutoff not provided | 142 endometrial endometrioid adenocarcinoma tested for L1CAM and HER2 |
|
|
| van der Putten LJM et al. (2018) [72] | Anti-CD171 [L1] antibody clone 14.10, dilution 1:100, cutoff 10% | 293 endometrial carcinomas for ER, PR, and L1CAM expression | 18% |
|
L1CAM: L1 cell adhesion molecule, ER: Estrogen receptor, PR: Progesterone receptor, MMR: Mismatch repair, HER2: Human Epidermal Growth Factor Receptor 2, N/A: Not available.
5.3. L1CAM Expression and Rare Endometrial Cancer Types (Table 3)
As for rarer histologic types, L1CAM expression was also studied in 90 uterine carcinosarcomas, revealing that, using the 10% cutoff value, 65.4% of the cases were positive—in the epithelial component—much higher than in previous cohorts. In this study, no association between L1CAM and prognosis was found [74]. In 52 endometrial clear cell carcinomas, tissue microarrays were stained for L1CAM (clone not provided, cutoff 50%), showing overexpression in 60% of tumors but with no significant correlation with other factors studied or prognosis [75].

Table 3.
L1CAM immunohistochemistry in rare histological endometrial cancer subtypes.
Table 3.
L1CAM immunohistochemistry in rare histological endometrial cancer subtypes.
| Author, Year | Antibody | Sample Size (n) | Positivity | Significance |
|---|---|---|---|---|
| Versluis M et al. (2018) [74] | Monoclonal antibodies CD171, clone 14.10, 1:500 dilution, cutoff 10% | 90 cases of uterine carcinosarcomas | 65.4% | No association between L1CAM level and prognosis |
| Kim SR et al. 2020 [75] | Clone not provided, cutoff 50% | 52 endometrial clear cell carcinomas | 60% | No important correlation with other factors or prognosis |
L1CAM: L1 cell adhesion molecule.
5.4. L1CAM Expression in Endometrial Cancer Patients Undergoing Surgery and Adjuvant Chemotherapy
Asano et al. examined the possible prognostic role of L1CAM in 161 patients with endometrial cancer undergoing surgery and adjuvant chemotherapy; they found L1CAM expression (tissue microarrays, clone 14.10, dilution 1:50, H-score with the cutoff set at 35) in almost 30% of the cases [76]. Even in this group, L1CAM was associated with non-endometrioid histology and lymphovascular invasion and was a significant predictor of poor survival—a significance retained in the multivariate analysis [76]. L1CAM seems to predict the response of endometrial cancer to chemotherapy [77]. The authors studied two cohorts of 55 and 93 patients with endometrial cancer treated with surgery and adjuvant platinum-based chemotherapy [77]. Almost half of the patients also received radiotherapy [77]. Tumor samples were tested with the 14.10 clone (dilution 1:100), and the cutoff value was set at 10% [77]. In addition, fresh tissue obtained during surgery from 55 patients was used for RNA extraction; an endometrial carcinoma cell line was further used to test platinum sensitivity [77]. The expression of L1CAM at the gene and protein levels was found to be an independent factor of platinum resistance, and experiments on cell lines confirmed this resistance [77]. This is in concordance with the aforementioned study, which showed that despite L1CAM expression not being associated with relapse or survival, it was indeed associated with relapse only in patients not treated with chemotherapy [45].
6. Role of L1CAM in Immunomodulation−Serum L1CAM
Given the frequent reports of L1CAM association with endometrial cancer and more aggressive tumors, as well as the known susceptibility of L1CAM to protease activity, it is worth wondering if a soluble form of L1CAM exists. In a study using ELISA to detect the serum L1CAM level in a few patients with endometrial and ovarian cancer compared to non-cancerous patients, the serum L1CAM level was found to be lower in cancer-bearing patients than in healthy women [78]. On the contrary, another study comparing the serum levels of L1CAM between endometrial cancer patients and non-cancerous patients showed significantly higher levels for the first group [79]. Another study showed that the levels of L1CAM in the blood were associated with lymph node metastasis and poor outcome [56], whereas another one showed no difference in L1CAM serum levels between L1CAM-positive and L1CAM-negative carcinomas [57]. No significant difference in the L1CAM serum levels of endometrial cancer patients compared to healthy controls was found in a study of 45 endometrial carcinoma patients [80]. In 65 patients with endometrial carcinoma, serum L1CAM levels were higher at diagnosis than during follow-up, but no statistically significant augmentation was found in patients upon disease recurrence [81].
These results show that serum levels of L1CAM could be an interesting, minimally interventional method; however, the few studies available show variable results, correlation with tissue expression is often discordant or not studied, and methods or cutoff for this kind of sample are not well defined. The number of studies examining serum L1CAM levels is very poor compared with the numerous studies of L1CAM expression in tissue, but the latter ones are retrospective, while a prospective design would be probably required to adequately study serum L1CAM.
7. L1CAM as a Therapeutic Target
The prognostic importance of L1CAM in endometrial cancer suggests that it could be used as a therapeutic target. In small cell lung cancer cell lines, a toxin conjugate with an L1CAM monoclonal antibody successfully inhibited tumor progression [82], suggesting that this pathway could be druggable. Similar results were achieved in carcinoma cell lines and in tumor growth in mice in an earlier study [83]. An anti-L1CAM monoclonal antibody used in mice to treat endometriosis showed that this molecule indeed suppressed endometriosis growth [84]. These preliminary results are promising; however, data are scarce, and more translational studies in endometrial cancer are necessary.
8. Conclusions
With almost a decade of research on L1CAM and endometrial carcinoma, the results of its expression in thousands of patients have been published. In almost all these studies, the same 10% cutoff value of tumor positivity and the 14.10 clone, followed by the UJ127.11 one, was used. Most studies revealed an association of L1CAM expression with non-endometrioid, high-grade histology, advanced stages, lymphovascular invasion, and poor prognosis; this prognostic significance is often, but not always, retained in multivariate analyses. In the era of the molecular classification of endometrial carcinoma, L1CAM appears to be expressed more often by p53-mutant tumors and to have an independent prognostic role only in heterogeneous groups with no specific molecular subtype. The underlying mechanisms leading to its overexpression in some tumors and how it induces a more aggressive cancer cell phenotype downstream remain largely unknown, warranting further studies. Whether targeting L1CAM is a therapeutic option also remains unknown, but it is worth investigating in endometrial cancer.
It is worth reiterating that the current review is not a systematic one or a metanalysis, so strict selection criteria or quality grading of the cited studies is applied. Furthermore, as highlighted earlier, there is substantial heterogeneity in the findings across studies, which limits the ability to define the prognostic, predictive, or diagnostic role of LICAM with high confidence.
To conclude, most L1CAM studies in endometrial cancer are retrospective, often spanning long periods with different treatments, and they are based on different immunohistochemical techniques—different automated systems, different antibodies, different cutoffs, and probably pathologist interpretation-based expected biases. These findings could explain the observed discrepancies and highlight the need for prospective, multicenter studies with well-defined criteria to identify the role of L1CAM, in tissue or blood samples. In practice, there are no guidelines on how to use L1CAM in routine practice, but familiarizing oneself with this antibody could be useful in the future.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Salton, S.R.; Richter-Landsberg, C.; Greene, L.A.; Shelanski, M.L. Nerve growth factor-inducible large external (NILE) glycoprotein: Studies of a central and peripheral neuronal marker. J. Neurosci. 1983, 3, 441–454. [Google Scholar] [CrossRef]
- Bock, E.; Richter-Landsberg, C.; Faissner, A.; Schachner, M. Demonstration of immunochemical identity between the nerve growth factor-inducible large external (NILE) glycoprotein and the cell adhesion molecule L1. EMBO J. 1985, 4, 2765–2768. [Google Scholar] [CrossRef] [PubMed]
- Keilhauer, G.; Faissner, A.; Schachner, M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature 1985, 316, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Hortsch, M. Structural and functional evolution of the L1 family: Are four adhesion molecules better than one? Mol. Cell. Neurosci. 2000, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.; Mechtersheimer, S.; Huszar, M.; Smirnov, A.; Abu-Dahi, A.; Tilgen, W.; Reichrath, J.; Georg, T.; Altevogt, P.; Gutwein, P. L1 adhesion molecule (CD 171) in development and progression of human malignant melanoma. Cancer Lett. 2003, 189, 237–247. [Google Scholar] [CrossRef]
- Debiec, H.; Christensen, E.I.; Ronco, P.M. The cell adhesion molecule L1 is developmentally regulated in the renal epithelium and is involved in kidney branching morphogenesis. J. Cell Biol. 1998, 143, 2067–2079. [Google Scholar] [CrossRef]
- Kowitz, A.; Kadmon, G.; Eckert, M.; Schirrmacher, V.; Schachner, M.; Altevogt, P. Expression and function of the neural cell adhesion molecule L1 in mouse leukocytes. Eur. J. Immunol. 1992, 22, 1199–1205. [Google Scholar] [CrossRef]
- Thies, A.; Schachner, M.; Moll, I.; Berger, J.; Schulze, H.J.; Brunner, G.; Schumacher, U. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur. J. Cancer 2002, 38, 1708–1716. [Google Scholar] [CrossRef]
- Allory, Y.; Matsuoka, Y.; Bazille, C.; Christensen, E.I.; Ronco, P.; Debiec, H. The L1 cell adhesion molecule is induced in renal cancer cells and correlates with metastasis in clear cell carcinomas. Clin. Cancer Res. 2005, 11, 1190–1197. [Google Scholar] [CrossRef]
- Doberstein, K.; Wieland, A.; Lee, S.B.B.; Blaheta, R.A.A.; Wedel, S.; Moch, H.; Schraml, P.; Pfeilschifter, J.; Kristiansen, G.; Gutwein, P. L1-CAM expression in ccRCC correlates with shorter patients survival times and confers chemoresistance in renal cell carcinoma cells. Carcinogenesis 2011, 32, 262–270. [Google Scholar] [CrossRef]
- Boo, Y.J.; Park, J.M.; Kim, J.; Chae, Y.S.; Min, B.W.; Um, J.W.; Moon, H.Y. L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann. Surg. Oncol. 2007, 14, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Jo, Y.S.; Huang, S.M.; Liang, Z.L.; Min, J.K.; Hong, H.J.; Kim, J.M. L1 cell adhesion molecule as a novel independent poor prognostic factor in gallbladder carcinoma. Hum. Pathol. 2011, 42, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Morohashi, S.; Kudo, Y.; Akasaka, H.; Ogasawara, H.; Ono, M.; Takasugi, K.; Ishido, K.; Hakamada, K.; Kijima, H. L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2011, 103, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Kaifi, J.T.; Strelow, A.; Schurr, P.G.; Reichelt, U.; Yekebas, E.F.; Wachowiak, R.; Quaas, A.; Strate, T.; Schaefer, H.; Sauter, G.; et al. L1 (CD171) is highly expressed in gastrointestinal stromal tumors. Mod. Pathol. 2006, 19, 399–406. [Google Scholar] [CrossRef]
- Huszar, M.; Moldenhauer, G.; Gschwend, V.; Ben-Arie, A.; Altevogt, P.; Fogel, M. Expression profile analysis in multiple human tumors identifies L1 (CD171) as a molecular marker for differential diagnosis and targeted therapy. Hum. Pathol. 2006, 37, 1000–1008. [Google Scholar] [CrossRef]
- Stevers, M.; Rabban, J.T.; Garg, K.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Yeh, I.; Bastian, B.C.; Zaloudek, C.; Solomon, D.A. Well-differentiated papillary mesothelioma of the peritoneum is genetically defined by mutually exclusive mutations in TRAF7 and CDC42. Mod. Pathol. 2019, 32, 88–99. [Google Scholar] [CrossRef]
- Goode, B.; Joseph, N.M.; Stevers, M.; Van Ziffle, J.; Onodera, C.; Talevich, E.; Grenert, J.P.; Yeh, I.; Bastian, B.C.; Phillips, J.J.; et al. Adenomatoid tumors of the male and female genital tract are defined by TRAF7 mutations that drive aberrant NF-kB pathway activation. Mod. Pathol. 2018, 31, 660–673. [Google Scholar] [CrossRef]
- Karpathiou, G.; Casteillo, F.; Dridi, M.; Papoudou-Bai, A.; Dumollard, J.M.; Peoc’h, M. L1CAM expression in cystic mesothelial lesions: A comparison with adenomatoid tumours, well-differentiated papillary mesothelial tumours and malignant mesotheliomas. Histopathology 2021, 79, 272–274. [Google Scholar] [CrossRef]
- Itami, H.; Fujii, T.; Nakai, T.; Takeda, M.; Kishi, Y.; Taniguchi, F.; Terada, C.; Okada, F.; Nitta, Y.; Matsuoka, M.; et al. TRAF7 mutations and immunohistochemical study of uterine adenomatoid tumor compared with malignant mesothelioma. Hum. Pathol. 2021, 111, 59–66. [Google Scholar] [CrossRef]
- Stoyanova, I.I.; Lutz, D. Functional Diversity of Neuronal Cell Adhesion and Recognition Molecule L1CAM through Proteolytic Cleavage. Cells 2022, 11, 3085. [Google Scholar] [CrossRef]
- Huszar, M.; Pfeifer, M.; Schirmer, U.; Kiefel, H.; Konecny, G.E.; Ben-Arie, A.; Edler, L.; Münch, M.; Müller-Holzner, E.; Jerabek-Klestil, S.; et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J. Pathol. 2010, 220, 551–561. [Google Scholar] [CrossRef]
- Finas, D.; Huszar, M.; Agic, A.; Dogan, S.; Kiefel, H.; Riedle, S.; Gast, D.; Marcovich, R.; Noack, F.; Altevogt, P.; et al. L1 cell adhesion molecule (L1CAM) as a pathogenetic factor in endometriosis. Hum. Reprod. 2008, 23, 1053–1062. [Google Scholar] [CrossRef]
- Pfeifer, M.; Schirmer, U.; Geismann, C.; Schafer, H.; Sebens, S.; Altevogt, P. L1CAM expression in endometrial carcinomas is regulated by usage of two different promoter regions. BMC Mol. Biol. 2010, 11, 64. [Google Scholar] [CrossRef]
- Gavert, N.; Conacci-Sorrell, M.; Gast, D.; Schneider, A.; Altevogt, P.; Brabletz, T.; Ben-Ze’ev, A. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J. Cell Biol. 2005, 168, 633–642. [Google Scholar] [CrossRef]
- Geismann, C.; Morscheck, M.; Koch, D.; Bergmann, F.; Ungefroren, H.; Arlt, A.; Tsao, M.S.; Bachem, M.G.; Altevogt, P.; Sipos, B.; et al. Up-regulation of L1CAM in pancreatic duct cells is transforming growth factor beta1- and slug-dependent: Role in malignant transformation of pancreatic cancer. Cancer Res. 2009, 69, 4517–4526. [Google Scholar] [CrossRef]
- Schirmer, U.; Fiegl, H.; Pfeifer, M.; Zeimet, A.G.; Müller-Holzner, E.; Bode, P.K.; Tischler, V.; Altevogt, P. Epigenetic regulation of L1CAM in endometrial carcinoma: Comparison to cancer-testis (CT-X) antigens. BMC Cancer 2013, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Heubner, M.; Wimberger, P.; Kasimir-Bauer, S.; Otterbach, F.; Kimmig, R.; Siffert, W. The AA genotype of a L1C G842A polymorphism is associated with an increased risk for ovarian cancer. Anticancer. Res. 2009, 29, 3449–3452. [Google Scholar]
- Chen, J.; Gao, F.; Liu, N. L1CAM promotes epithelial to mesenchymal transition and formation of cancer initiating cells in human endometrial cancer. Exp. Ther. Med. 2018, 15, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Urick, M.E.; Yu, E.J.; Bell, D.W. High-risk endometrial cancer proteomic profiling reveals that FBXW7 mutation alters L1CAM and TGM2 protein levels. Cancer 2021, 127, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Rheinstein, P.H. Druggable genetic targets in endometrial cancer✰,✰✰. Cancer Treat. Res. Commun. 2022, 30, 100502. [Google Scholar] [CrossRef]
- Ye, L.; Wang, X.; Li, B. Expression profile of epithelial-mesenchymal transition-related genes as a prognostic biomarker for endometrial cancer. J. Cancer 2021, 12, 6484–6496. [Google Scholar] [CrossRef]
- Karpathiou, G.; Sramek, V.; Dagher, S.; Mobarki, M.; Dridi, M.; Picot, T.; Chauleur, C.; Peoc’h, M. Peripheral node addressin, a ligand for L-selectin is found in tumor cells and in high endothelial venules in endometrial cancer. Pathol. Res. Pract. 2022, 233, 153888. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Dumollard, J.M.; Gavid, M.; Casteillo, F.; Vieville, M.; Prades, J.M.; Froudarakis, M.; Peoc’h, M. High endothelial venules are present in pharyngeal and laryngeal carcinomas and they are associated with better prognosis. Pathol. Res. Pract. 2021, 220, 153392. [Google Scholar] [CrossRef] [PubMed]
- Horeweg, N.; Workel, H.H.; Loiero, D.; Church, D.N.; Vermij, L.; Léon-Castillo, A.; Krog, R.T.; de Boer, S.M.; Nout, R.A.; Powell, M.E.; et al. Tertiary lymphoid structures critical for prognosis in endometrial cancer patients. Nat. Commun. 2022, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, I.C.; Stelloo, E.; Nout, R.A.; Nijman, H.W.; Edmondson, R.J.; Church, D.N.; MacKay, H.J.; Leary, A.; Powell, M.E.; Mileshkin, L.; et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod. Pathol. 2016, 29, 174–181. [Google Scholar] [CrossRef]
- Fang, F.; Wang, P.; Huang, H.; Ye, M.; Liu, X.; Li, Q. m(6)A RNA methylation regulator-based signature for prognostic prediction and its potential immunological role in uterine corpus endometrial carcinoma. BMC Cancer 2022, 22, 1364. [Google Scholar] [CrossRef]
- He, W.; Liu, W.; Liu, X.; Tan, W. The mechanism of L1 cell adhesion molecule interacting with protein tyrosine kinase 2 to regulate the focal adhesion kinase-growth factor receptor-bound protein 2-son of sevenless-rat sarcoma pathway in the identification and treatment of type I high-risk endometrial cancer. CytoJournal 2024, 21, 34. [Google Scholar]
- Visser, N.C.; van der Putten, L.J.; van Egerschot, A.; Van de Vijver, K.K.; Santacana, M.; Bronsert, P.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Addition of IMP3 to L1CAM for discrimination between low- and high-grade endometrial carcinomas: A European Network for Individualised Treatment of Endometrial Cancer collaboration study. Hum. Pathol. 2019, 89, 90–98. [Google Scholar] [CrossRef]
- Visser, N.C.; van der Wurff, A.A.; IntHout, J.; Reijnen, C.; Dabir, P.D.; Soltani, G.G.; Alcala, L.S.; Boll, D.; Bronkhorst, C.M.; Bult, P.; et al. Improving preoperative diagnosis in endometrial cancer using systematic morphological assessment and a small immunohistochemical panel. Hum. Pathol. 2021, 117, 68–78. [Google Scholar] [CrossRef]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef]
- Gast, D.; Riedle, S.; Riedle, S.; Schabath, H.; Schlich, S.; Schneider, A.; Issa, Y.; Stoeck, A.; Fogel, M.; Joumaa, S.; et al. L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int. J. Cancer 2005, 115, 658–665. [Google Scholar] [CrossRef]
- Zeimet, A.G.; Reimer, D.; Huszar, M.; Winterhoff, B.; Puistola, U.; Abdel Azim, S.; Müller-Holzner, E.; Ben-Arie, A.; Van Kempen, L.C.; Petru, E.; et al. L1CAM in early-stage type I endometrial cancer: Results of a large multicenter evaluation. J. Natl. Cancer Inst. 2013, 105, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; Stelloo, E.; Dreef, E.; Nijman, H.W.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Creutzberg, C.L.; Smit, V.T.H.B.M. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: Pooled PORTEC trial results. Eur. J. Cancer 2014, 50, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, T.H.; Smith, D.D.; Ouyang, C.; Warden, C.D.; Williams, J.C.; Han, E.S. L1CAM is an independent predictor of poor survival in endometrial cancer—An analysis of The Cancer Genome Atlas (TCGA). Gynecol. Oncol. 2016, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Smogeli, E.; Davidson, B.; Cvancarova, M.; Holth, A.; Katz, B.; Risberg, B.; Kristensen, G.; Lindemann, K. L1CAM as a prognostic marker in stage I endometrial cancer: A validation study. BMC Cancer 2016, 16, 596. [Google Scholar] [CrossRef]
- van der Putten, L.J.; Visser, N.C.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef]
- Pasanen, A.; Tuomi, T.; Isola, J.; Staff, S.; Butzow, R.; Loukovaara, M. L1 Cell Adhesion Molecule as a Predictor of Disease-Specific Survival and Patterns of Relapse in Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 26, 1465–1471. [Google Scholar] [CrossRef]
- Kommoss, F.; Kommoss, F.; Grevenkamp, F.; Bunz, A.K.; Taran, F.A.; Fend, F.; Brucker, S.Y.; Wallwiener, D.; Schönfisch, B.; Greif, K.; et al. L1CAM: Amending the “low-risk” category in endometrial carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 255–262. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- Klat, J.; Mladenka, A.; Dvorackova, J.; Bajsova, S.; Simetka, O. L1CAM as a Negative Prognostic Factor in Endometrioid Endometrial Adenocarcinoma FIGO Stage IA-IB. Anticancer. Res. 2019, 39, 421–424. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.I.; Kim, N.R.; Kim, H.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Lee, C.; Lee, M. Prognostic significance of L1CAM expression in addition to ProMisE in endometrial cancer. Gynecol. Oncol. 2023, 174, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Joe, S.; Lee, M.; Kang, J.; Kim, J.; Hong, S.H.; Lee, S.J.; Lee, K.H.; Lee, A. Enhanced Risk Stratification in Early-Stage Endometrial Cancer: Integrating POLE through Droplet Digital PCR and L1CAM. Cancers 2023, 15, 4899. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Suh, D.H.; Kim, K.; No, J.H.; Kim, Y.B.; Kim, H. Evaluation of prognostic potential of beta-catenin and L1CAM expression according to endometrial cancer risk group. Gynecol. Oncol. 2024, 184, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zeiter, D.; Vlajnic, T.; Schotzau, A.; Heinzelmann-Schwarz, V.; Montavon, C. L1CAM is not a reliable predictor for lymph node metastases in endometrial cancer, but L1CAM positive patients benefit from radiotherapy. J. Cancer 2021, 12, 6401–6410. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.A.; Kim, H.S.; Chung, H.H.; Park, N.H.; Song, Y.S.; Kang, S.B. L1 cell adhesion molecule expression is associated with pelvic lymph node metastasis and advanced stage in diabetic patients with endometrial cancer: A matched case control study. J. Cancer Prev. 2014, 19, 231–239. [Google Scholar] [CrossRef]
- Tangen, I.L.; Kopperud, R.K.; Visser, N.; Staff, A.C.; Tingulstad, S.; Marcickiewicz, J.; Amant, F.; Bjørge, L.; Pijnenborg, J.; Salvesen, H.B.; et al. Expression of L1CAM in curettage or high L1CAM level in preoperative blood samples predicts lymph node metastases and poor outcome in endometrial cancer patients. Br. J. Cancer 2017, 117, 840–847. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Tuomi, T.; Butzow, R. Preoperative Risk Stratification of Endometrial Carcinoma: L1CAM as a Biomarker. Int. J. Gynecol. Cancer 2017, 27, 1318–1324. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Leung, S.; Magrill, J.; McConechy, M.K.; Yang, W.; Chow, C.; Kobel, M.; Lee, C.H.; Huntsman, D.G.; Talhouk, A.; et al. Evaluation of endometrial carcinoma prognostic immunohistochemistry markers in the context of molecular classification. J. Pathol. Clin. Res. 2017, 3, 279–293. [Google Scholar] [CrossRef]
- Kommoss, F.K.; Karnezis, A.N.; Kommoss, F.; Talhouk, A.; Taran, F.A.; Staebler, A.; Gilks, C.B.; Huntsman, D.G.; Krämer, B.; Brucker, S.Y.; et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br. J. Cancer 2018, 119, 480–486. [Google Scholar] [CrossRef]
- Ravaggi, A.; Capoferri, D.; Ardighieri, L.; Ghini, I.; Ferrari, F.; Romani, C.; Bugatti, M.; Zanotti, L.; Vrede, S.; Tognon, G.; et al. Integrated Biomarker Analysis Reveals L1CAM as a Potential Stratification Marker for No Specific Molecular Profile High-Risk Endometrial Carcinoma. Cancers 2022, 14, 5429. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Butzow, R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod. Pathol. 2020, 33, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Vrede, S.W.; Van Weelden, W.J.; Visser, N.C.M.; Bulten, J.; Van Der Putten, L.J.; Van de Vijver, K.; Santacana, M.; Colas, E.; Gil-Moreno, A.; Moiola, C.P.; et al. Immunohistochemical biomarkers are prognostic relevant in addition to the ESMO-ESGO-ESTRO risk classification in endometrial cancer. Gynecol. Oncol. 2021, 161, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, K.; Kildal, W.; Kleppe, A.; Tobin, K.A.R.; Pradhan, M.; Isaksen, M.X.; Vlatkovic, L.; Danielsen, H.E.; Kristensen, G.B.; Askautrud, H.A. Impact of molecular profile on prognosis and relapse pattern in low and intermediate risk endometrial cancer. Eur. J. Cancer 2024, 200, 113584. [Google Scholar] [CrossRef]
- Aro, K.; Pasanen, A.; Butzow, R.; Loukovaara, M. The impact of estrogen receptor and L1 cell adhesion molecule expression on endometrial cancer outcome correlates with clinicopathological risk group and molecular subgroup. Gynecol. Oncol. 2024, 189, 9–15. [Google Scholar] [CrossRef]
- Kleppe, A.; Lindemann, K.; Kildal, W.; Tobin, K.A.R.; Pradhan, M.; Vlatkovic, L.; Isaksen, M.X.; Danielsen, H.E.; Askautrud, H.A.; Kristensen, G.B. Prognostic and therapeutic implication of molecular classification including L1CAM expression in high-risk endometrial cancer. Gynecol. Oncol. 2025, 192, 80–88. [Google Scholar] [CrossRef]
- Kim, J.C.; Ahn, B.; Lee, Y.J.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T.; Park, E.; Lee, J.Y. Mismatch repair, p53, and L1 cell adhesion molecule status influence the response to chemotherapy in advanced and recurrent endometrial cancer. BMC Cancer 2024, 24, 1586. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Deng, Y.; Wang, P.; Bai, X.; Qin, W. Multifactorial construction of low-grade and high-grade endometrial cancer recurrence prediction models. Int. J. Gynecol. Obstet. 2025, 170, 816–826. [Google Scholar] [CrossRef]
- Abdel Azim, S.; Sprung, S.; Mutz-Dehbalaie, I.; Fessler, S.; Zeimet, A.G.; Marth, C. L1CAM and HER2 Expression in Early Endometrioid Uterine Cancer. Int. J. Gynecol. Pathol. 2017, 36, 356–363. [Google Scholar] [CrossRef]
- van der Putten, L.J.M.; Visser, N.C.M.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Added Value of Estrogen Receptor, Progesterone Receptor, and L1 Cell Adhesion Molecule Expression to Histology-Based Endometrial Carcinoma Recurrence Prediction Models: An ENITEC Collaboration Study. Int. J. Gynecol. Cancer 2018, 28, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Zagidullin, B.; Pasanen, A.; Loukovaara, M.; Butzow, R.; Tang, J. Interpretable prognostic modeling of endometrial cancer. Sci. Rep. 2022, 12, 21543. [Google Scholar] [CrossRef] [PubMed]
- Versluis, M.A.C.; Plat, A.; de Bruyn, M.; Matias-Guiu, X.; Trovic, J.; Krakstad, C.; Nijman, H.W.; Bosse, T.; de Bock, G.H.; Hollema, H. L1CAM expression in uterine carcinosarcoma is limited to the epithelial component and may be involved in epithelial-mesenchymal transition. Virchows Arch. 2018, 473, 591–598. [Google Scholar] [CrossRef]
- Kim, S.R.; Cloutier, B.T.; Leung, S.; Cochrane, D.; Britton, H.; Pina, A.; Storness-Bliss, C.; Farnell, D.; Huang, L.; Shum, K.; et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol. Oncol. 2020, 158, 3–11. [Google Scholar] [CrossRef]
- Asano, H.; Hatanaka, K.C.; Matsuoka, R.; Dong, P.; Mitamura, T.; Konno, Y.; Kato, T.; Kobayashi, N.; Ihira, K.; Nozaki, A.; et al. L1CAM Predicts Adverse Outcomes in Patients with Endometrial Cancer Undergoing Full Lymphadenectomy and Adjuvant Chemotherapy. Ann. Surg. Oncol. 2020, 27, 2159–2168. [Google Scholar] [CrossRef]
- Romani, C.; Capoferri, D.; Reijnen, C.; Lonardi, S.; Ravaggi, A.; Ratti, M.; Bugatti, M.; Zanotti, L.; Tognon, G.; Sartori, E.; et al. L1CAM expression as a predictor of platinum response in high-risk endometrial carcinoma. Int. J. Cancer 2022, 151, 637–648. [Google Scholar] [CrossRef]
- Wojciechowski, M.; Glowacka, E.; Wilczynski, M.; Pekala-Wojciechowska, A.; Malinowski, A. The sL1CAM in sera of patients with endometrial and ovarian cancers. Arch. Gynecol. Obstet. 2017, 295, 225–232. [Google Scholar] [CrossRef]
- Sertel, E.; Demir, M.; Dogan, S.; Corakci, A. Could soluble L1 cell adhesion molecule (sL1CAM) in serum be a new biomarker for endometrial cancer? Ginekol. Pol. 2023, 94, 463–469. [Google Scholar] [CrossRef]
- Torres, A.; Pac-Sosinska, M.; Wiktor, K.; Paszkowski, T.; Maciejewski, R.; Torres, K. CD44, TGM2 and EpCAM as novel plasma markers in endometrial cancer diagnosis. BMC Cancer 2019, 19, 401. [Google Scholar] [CrossRef]
- Bednarikova, M.; Vinklerova, P.; Gottwaldova, J.; Ovesna, P.; Hausnerova, J.; Minar, L.; Felsinger, M.; Valik, D.; Cermakova, Z.; Weinberger, V. The Clinical Significance of DJ1 and L1CAM Serum Level Monitoring in Patients with Endometrial Cancer. J. Clin. Med. 2021, 10, 2640. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirai, S.; Idogawa, M.; Sumi, T.; Uchida, H.; Sakuma, Y. A Newly Developed Anti-L1CAM Monoclonal Antibody Targets Small Cell Lung Carcinoma Cells. Int. J. Mol. Sci. 2024, 25, 8748. [Google Scholar] [CrossRef]
- Gast, D.; Riedle, S.; Issa, Y.; Pfeifer, M.; Beckhove, P.; Sanderson, M.P.; Arlt, M.; Moldenhauer, G.; Fogel, M.; Krüger, A.; et al. The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene 2008, 27, 1281–1289. [Google Scholar] [CrossRef]
- Silveira, C.G.; Finas, D.; Hunold, P.; Köster, F.; Stroschein, K.; Canny, G.O.; Moldenhauer, G.; Altevogt, P.; Rody, A.; Hornung, D. L1 cell adhesion molecule as a potential therapeutic target in murine models of endometriosis using a monoclonal antibody approach. PLoS ONE 2013, 8, e82512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).