The Comparison of Insulin Resistance Between Normal and Early Menopause Women Younger than Fifty Years Old by Machine Learning Methods
Abstract
1. Introduction
- Compares the performance between traditional multiple linear regression (MLR) and ML.
- Uses ML to identify risk factors for IR between women with normal menstrual cycles (NM) and EM.
- Uses Shapley addictive explanation (SHAP) to understand individual-level prediction errors and identify areas of might underperformance.
2. Materials and Methods
2.1. Participants and Study Design
- No history of significant medical diseases such as stroke, myocardial infarction, or heart failure;
- No diagnosis of diabetes;
- No medication for metabolic syndrome.
2.2. Anthropometry and Biochemistry Measurements
Traditional Statistical Analysis
2.3. Study Dataset
2.4. Proposed Machine Learning Scheme
3. Results
4. Discussion
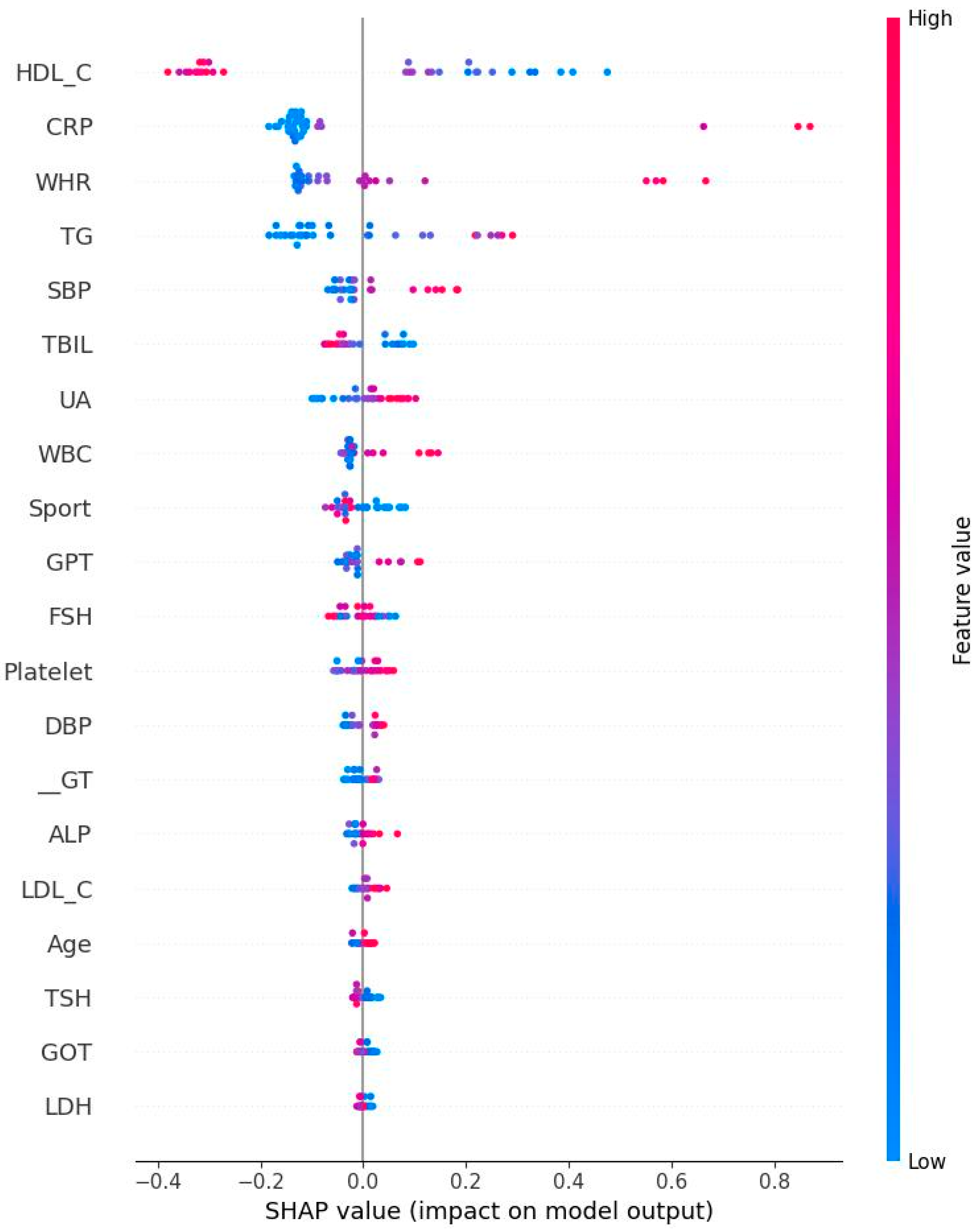
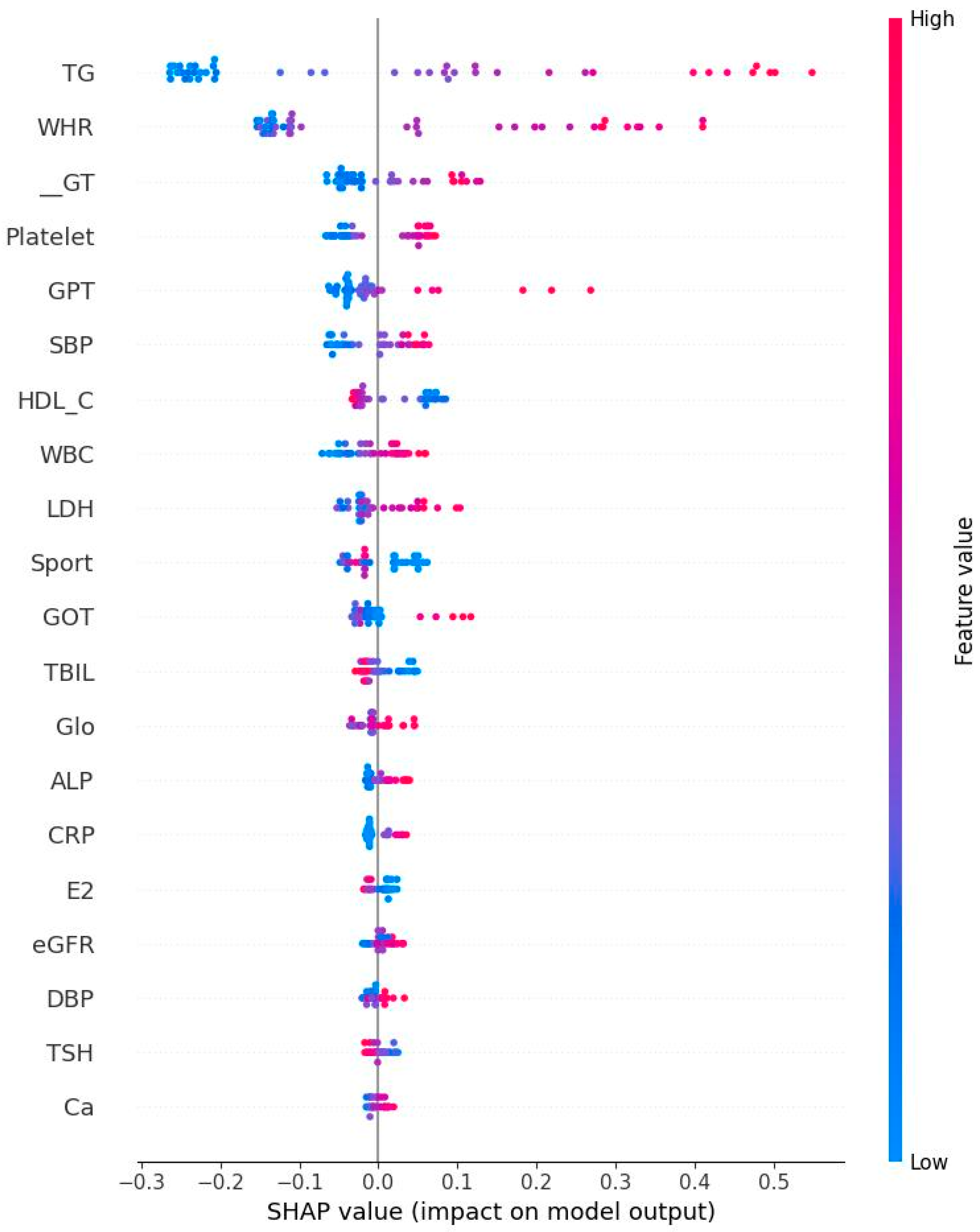
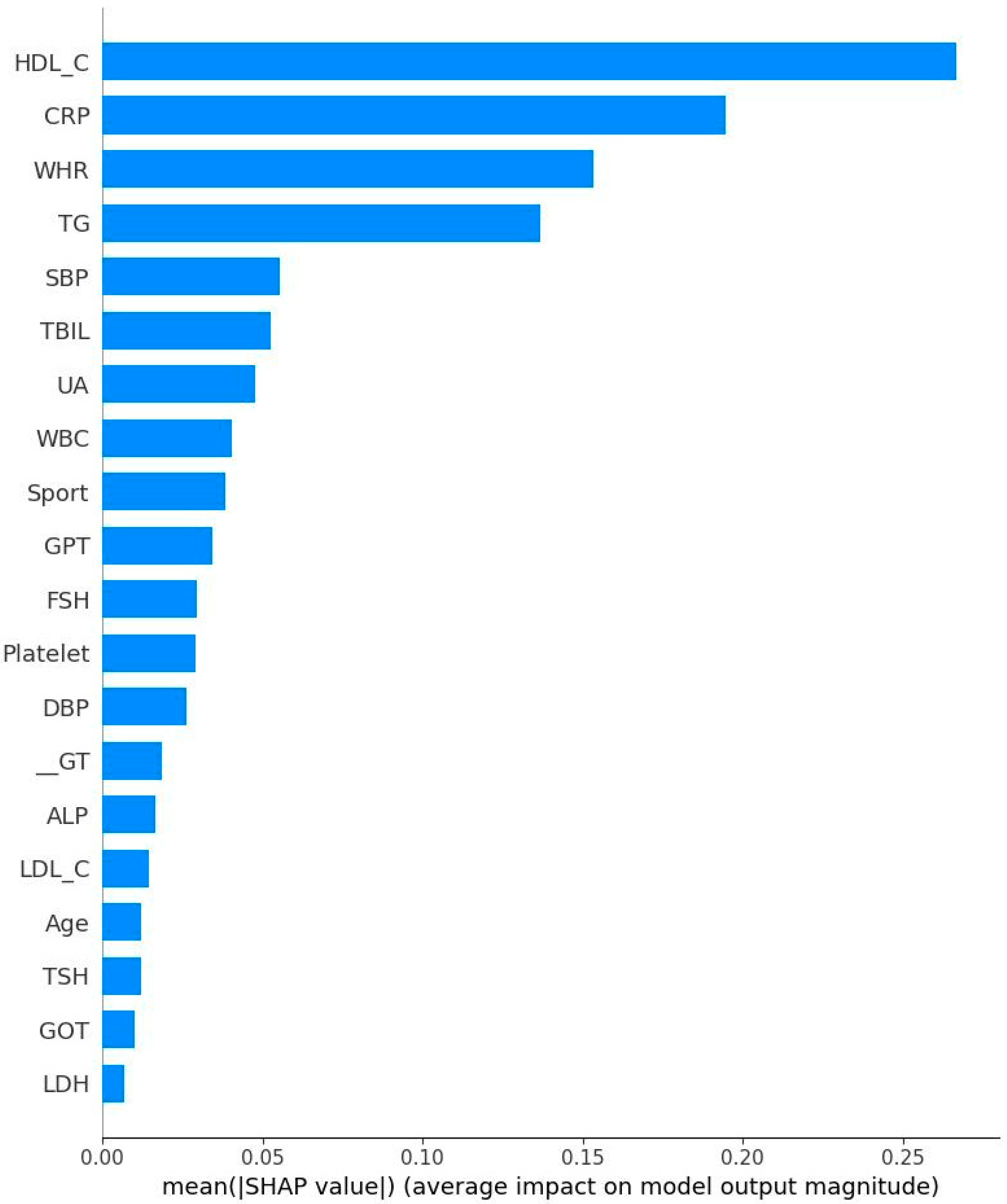
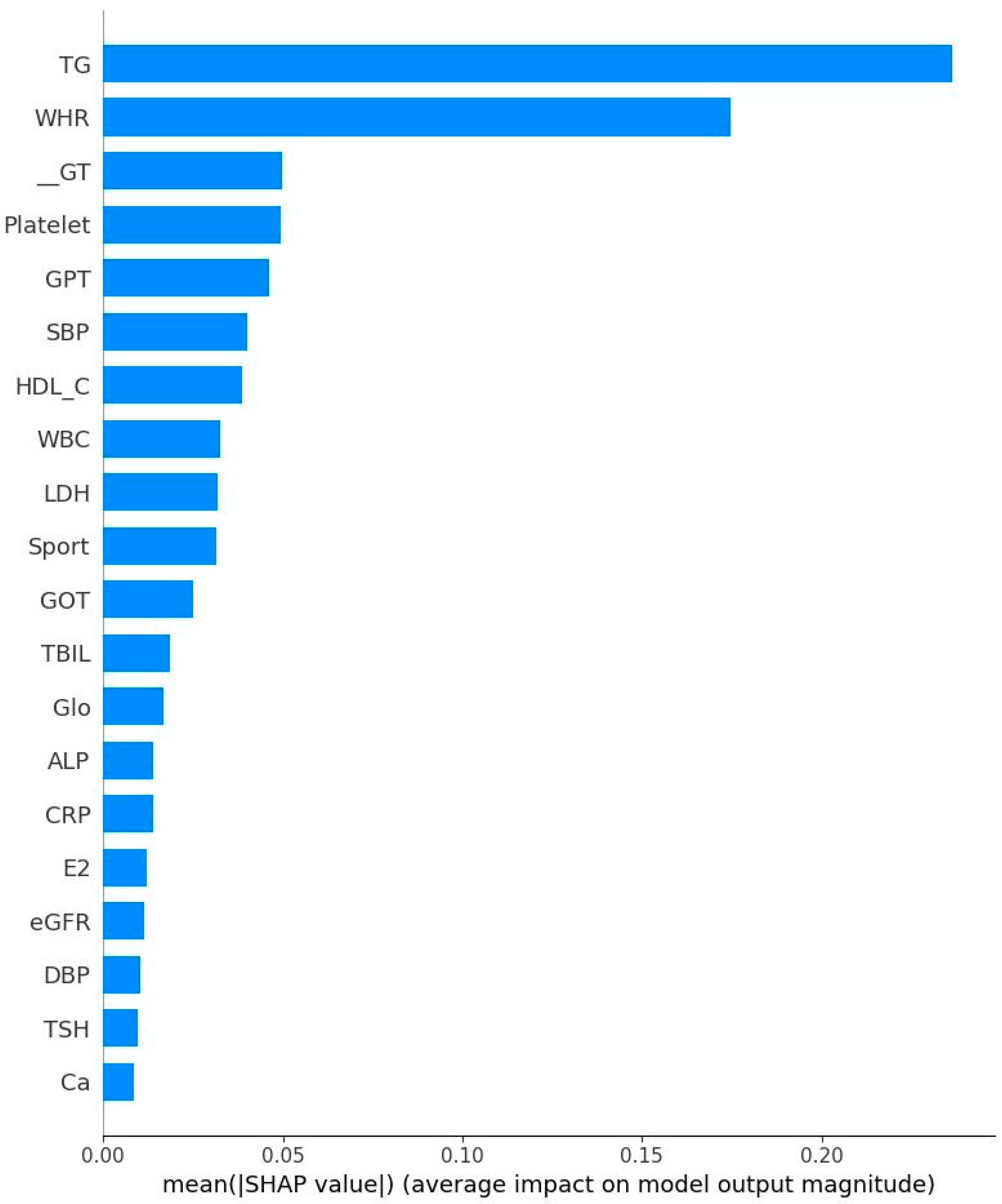
- High TG is a well-known hallmark of dyslipidemia and IR [47]. IR is associated with overproduction of very low-density lipoproteins which contain significant amounts of TG [47]. Due to decreased estrogen levels in menopausal women, the liver produces more TG, which could explain the results of the present study [48].
- GPT could be regarded as a biomarker for IR [49] because that higher GPT levels indicate liver damage which could cause impaired insulin function and glucose metabolism [50]. Menopause is frequently associated with higher GOT and GPT levels due to decreased estrogen levels [51]. Estrogen protects liver cells and helps maintain mitochondrial function. Our finding is consistent with previous results.
- GOT interacts with IR similarly to GPT. Our result showed an independent relationship to IR. This is not surprising since that GPT is mainly found in liver, kidney, heart, and muscle. GOT is distributed more widely in the body than GPT, including in the brain and blood cells [52], potentially explaining this independent relationship.
- Subjects with IR have elevated rates of glycolysis, leading to higher production of pyruvate, a precursor for LDH [53]. Estrogen stimulates production of LDH in some tissues and post-menopausal women typically experience a corresponding decrease in LDH levels [54]. Our finding further confirms previous reports related to this area.
- Both CRP and WBC are markers for inflammation and many studies have confirmed the relationship between inflammation and IR [55,56,57]. For example, CRP is picked out to be one of the important features. This does not mean that their relationship is positive. On the contrary, both CRP and HOMA-IR might be correlated negatively. Therefore, the findings in the present study are not surprising. Inflammatory markers are produced in the adipose tissues and liver, inhibiting insulin signaling pathways and leading to IR [58]. Our findings thus are consistent with previous work.
- Previous studies have established the relationship between blood pressure and IR. Using an euglycemic insulin clamp to quantify IR, Ferrannini et al. reported that DBP is positively correlated with IR (r = 0.18, p < 0.05). As for the underlying mechanism, IR could induce the activity of sympathetic nerve and elevate vasoconstrictors such as angiotensin II, leading to increased blood pressure [59]. Again, our conclusion is consistent with past results.
- TBIL: Pre-menopausal women generally have higher estrogen levels, providing a protective effect for the liver and cardiovascular system. This hormonal environment may help maintain higher or more stable bilirubin levels. After menopause, estrogen levels drop significantly. Previous studies have shown that postmenopausal women often have lower total bilirubin levels compared to premenopausal women. This decrease may be linked to increased risk of hypertension and cardiovascular disease, as bilirubin has antioxidant and anti-inflammatory properties [60].
- Early risk stratifications: WHR and HDL-C could serve as early warning signs for IR.
- Cost-effective screening tools: Variables such was WHR, HDL-C, and CRP could be integrated as a simple composite risk score for integration into electronic health records to flag high-risk individuals for further metabolic assessment.
- Targeted lifestyle interventions: such as exercise, enough sleeping time, and quit smoking.
- As WHR is the strongest predictor, interventions focused on reducing abdominal obesity (via diet, exercise, and behavioral counseling) should be prioritized for women under 50.
- Monitoring inflammatory status in EM Women: EM women, these lose protection of estrogen, so incorporating inflammatory marker monitoring may help guide early preventive strategies during or shortly after the onset of menopause.
- Clinical decision support systems: These findings could inform the development of machine learning-based decision support tools for use in primary care.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Urgent Action Needed as Global Diabetes Cases Increase Four-Fold Over Past Decades. Available online: https://www.who.int/news/item/13-11-2024-urgent-action-needed-as-global-diabetes-cases-increase-four-fold-over-past-decades (accessed on 13 November 2024).
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef]
- Chow, S.N.; Huang, C.C.; Lee, Y.T. Demographic characteristics and medical aspects of menopausal women in Taiwan. J. Formos. Med. Assoc. Taiwan Yi Zhi 1997, 96, 806–811. [Google Scholar]
- Shen, T.-Y.; Strong, C.; Yu, T. Age at menopause and mortality in Taiwan: A cohort analysis. Maturitas 2020, 136, 42–48. [Google Scholar] [CrossRef]
- Al-Maaitah, A.M.; Al-Nasser, M.A.; Al-Khateeb, M.A. Premature menopause: A review. Menopause Int. 2012, 18, 91–100. [Google Scholar]
- Merck Manuals. Premature menopause. In Merck Manuals. Available online: https://www.msdmanuals.com/home/women-s-health-issues/menstrual-disorders-and-abnormal-vaginal-bleeding/premature-menopause (accessed on 2 February 2024).
- Healthline. Causes of Early Menopause. Available online: https://www.healthline.com/health/menopause/causes-early (accessed on 11 January 2022).
- Stachowiak, G.; Pertyński, T.; Pertyńska-Marczewska, M. Metabolic disorders in menopause. Menopause Rev./Przegląd Menopauzalny 2015, 14, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Tsay, S.L.; Tsai, S.T.; Kuo, S.C.; Chou, P. Interrelationship between insulin resistance and menopause on the metabolic syndrome and its individual component among nondiabetic women in the kinmen study. Am. J. Med. Sci. 2007, 333, 208–214. [Google Scholar] [CrossRef]
- Haider, A.W.; Larson, M.G.; Franklin, S.S.; Levy, D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann. Intern. Med. 2003, 138, 10–16. [Google Scholar] [CrossRef]
- Yazdkhasti, M.; Jafarabady, K.; Shafiee, A.; Omran, S.P.; Mahmoodi, Z.; Esmaeilzadeh, S.; Babaheidari, T.B.; Kabir, K.; Peisepar, M.; Bakhtiyari, M. The association between age of menopause and type 2 diabetes: A systematic review and meta-analysis. Nutr. Metab. 2024, 21, 87. [Google Scholar] [CrossRef]
- Nichols, A.R.; Chavarro, J.E.; Oken, E. Reproductive risk factors across the female lifecourse and later metabolic health. Cell Metab. 2024, 36, 240–262. [Google Scholar] [CrossRef]
- Xing, Z.; Chen, H.; Alman, A.C. Discriminating insulin resistance in middle-aged nondiabetic women using machine learning approaches. AIMS Public Health 2024, 11, 667. [Google Scholar] [CrossRef]
- Metwally, A.A.; Heydari, A.A.; McDuff, D.; Solot, A.; Esmaeilpour, Z.; Faranesh, A.Z.; Zhou, M.; Savage, D.B.; Heneghan, C.; Patel, S.; et al. Insulin Resistance Prediction from Wearables and Routine Blood Biomarkers. arXiv 2025, arXiv:2505.03784. [Google Scholar]
- Gao, W.; Deng, Z.; Gong, Z.; Jiang, Z.; Ma, L. AI-driven Prediction of Insulin Resistance in Normal Populations. arXiv 2025, arXiv:2503.05119. [Google Scholar]
- Liu, C.-H.; Chang, C.-F.; Chen, I.-C.; Lin, F.-M.; Tzou, S.-J.; Hsieh, C.-B.; Chu, T.-W.; Pei, D. Machine learning prediction of prediabetes in a young male Chinese cohort with 5.8-year follow-up. Diagnostics 2024, 14, 979. [Google Scholar] [CrossRef] [PubMed]
- MJ Health Resource Center. MJ Health Screening Equipment Use and Replacement Records. MJ Health Resource Center. Available online: http://www.mjhrf.org/upload/user/files/MJHRF-TR-06%20Screening%20Equipment.pdf (accessed on 6 June 2016).
- Tzou, S.-J.; Peng, C.-H.; Huang, L.-Y.; Chen, F.-Y.; Kuo, C.-H.; Wu, C.-Z.; Chu, T.-W. Comparison between linear regression and four different machine learning methods in selecting risk factors for osteoporosis in a Chinese female-aged cohort. J. Chin. Med. Assoc. 2023, 86, 1028–1036. [Google Scholar] [CrossRef]
- Tseng, C.J.; Lu, C.J.; Chang, C.C.; Chen, G.D.; Cheewakriangkrai, C. Integration of data mining classification techniques and ensemble learning to identify risk factors and diagnose ovarian cancer recurrence. Artif. Intell. Med. 2017, 78, 47–54. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, S.H. Developing a novel machine learning-based classification scheme for predicting SPCs in breast cancer survivors. Front. Genet. 2019, 10, 848. [Google Scholar] [CrossRef]
- Shih, C.C.; Lu, C.J.; Chen, G.D.; Chang, C.C. Risk prediction for early chronic kidney disease: Results from an adult health examination program of 19,270 individuals. Int. J. Environ. Res. Public Health 2020, 17, 4875. [Google Scholar] [CrossRef]
- Lee, H.T.; Shin, J.; Min, S.Y.; Lim, Y.H.; Kim, K.S.; Kim, S.G.; Kim, J.H.; Kim, J.K. The relationship between bone mineral density and blood pressure in the Korean elderly population: The Korea National Health and Nutrition Examination Survey, 2008–2011. Clin. Exp. Hypertens. 2015, 37, 212–217. [Google Scholar] [CrossRef]
- Chang, C.C.; Yeh, J.H.; Chen, Y.M.; Jhou, M.J.; Lu, C.J. Clinical predictors of prolonged hospital stay in patients with myasthenia gravis: A study using machine learning algorithms. J. Clin. Med. 2021, 10, 2819. [Google Scholar] [CrossRef]
- Chang, C.C.; Huang, T.H.; Shueng, P.W.; Chen, S.H.; Chen, C.C.; Lu, C.J.; Tseng, Y.J. Developing a stacked ensemble-based classification scheme to predict second primary cancers in head and neck cancer survivors. Int. J. Environ. Res. Public Health 2021, 18, 1223. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.L.; Jhou, M.J.; Lee, T.S.; Lu, C.J.; Chen, M.S. Health data-driven machine learning algorithms applied to risk indicators assessment for chronic kidney disease. Risk Manag. Healthc. Policy 2021, 14, 4401–4411. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.E.; Chen, H.A.; Jhou, M.J.; Chen, Y.N.; Chang, T.J.; Lu, C.J. Evaluating the effect of topical atropine use for myopia control on intraocular pressure by using machine learning. J. Clin. Med. 2020, 10, 118. [Google Scholar] [CrossRef]
- Wu, C.W.; Shen, H.L.; Lu, C.J.; Chen, S.H.; Chen, H.Y. Comparison of different machine learning classifiers for glaucoma diagnosis based on Spectralis OCT. Diagnostics 2021, 11, 1721. [Google Scholar] [CrossRef]
- Wu, C.Z.; Huang, L.Y.; Chen, F.Y.; Kuo, C.H.; Yeih, D.F. Using machine learning to predict abnormal carotid intima-media thickness in type 2 diabetes. Diagnostics 2023, 13, 1342. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Calle, M.; Urrea, V. Letter to the editor: Stability of random forest importance measures. Brief. Bioinform. 2011, 12, 86–89. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Friedman, J.H. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Torlay, L.; Perrone-Bertolotti, M.; Thomas, E.; Baciu, M. Machine learning–XGBoost analysis of language networks to classify patients with epilepsy. Brain Inform. 2017, 4, 159–169. [Google Scholar] [CrossRef]
- Tay, J.K.; Narasimhan, B.; Hastie, T. Elastic net regularization paths for all generalized linear models. J. Stat. Softw. 2023, 106, 1–25. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 31 March 2021).
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC. 2018. Available online: http://www.rstudio.com/ (accessed on 16 May 2018).
- Breiman, L.; Cutler, A.; Liaw, A.; Wiener, M. randomForest: Breiman and Cutler’s Random Forests for Classification and Regression (R Package Version 4.6-14). 2018. Available online: https://CRAN.R-project.org/package=randomForest (accessed on 25 March 2018).
- Greenwell, B.; Boehmke, B.; Cunningham, J. Gbm: Generalized Boosted Regression Models (R Package Version 2.1.8). 2020. Available online: https://CRAN.R-project.org/package=gbm (accessed on 30 July 2020).
- Therneau, T.; Atkinson, B. Rpart: Recursive Partitioning and Regression Trees (R Package Version 4.1.15). 2019. Available online: https://CRAN.R-project.org/package=rpart (accessed on 12 April 2019).
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. Xgboost: Extreme Gradient Boosting (R Package Version 1.5.0.2). 2021. Available online: https://CRAN.R-project.org/package=xgboost (accessed on 21 November 2021).
- Kuhn, M. Caret: Classification and Regression Training (R Package Version 6.0-90). 2021. Available online: https://CRAN.R-project.org/package=caret (accessed on 9 October 2021).
- Sirbu, A.E.; Buburuzan, L.; Kevorkian, S.; Martin, S.; Barbu, C.; Copaescu, C.; Smeu, B.; Fica, S. Adiponectin expression in visceral adiposity is an important determinant of insulin resistance in morbid obesity. Endokrynol. Pol. 2018, 69, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Visceral fat and insulin resistance: Causative or correlative? Br. J. Nutr. 2000, 83 (Suppl. 1), S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Siebel, A.L.; Heywood, S.E.; Kingwell, B.A. HDL and glucose metabolism: Current evidence and therapeutic potential. Front. Pharmacol. 2015, 6, 258. [Google Scholar] [CrossRef]
- Howard, B.V. Insulin resistance and lipid metabolism. Am. J. Cardiol. 1999, 84, 28J–32J. [Google Scholar] [CrossRef]
- Medical News Today. Menopause and Cholesterol: What You Need to Know. Medical News Today. Available online: https://www.medicalnewstoday.com/articles/menopause-and-cholesterol (accessed on 22 May 2023).
- Bonnet, F.; Ducluzeau, P.H.; Gastaldelli, A.; Laville, M.; Anderwald, C.H.; Konrad, T.; Mari, A.; Balkau, B.; RISC Study Group. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011, 60, 1660–1667. [Google Scholar] [CrossRef]
- Kälsch, J.; Bechmann, L.; Heider, D.; Best, J.; Manka, P.; Kälsch, H.; Sowa, J.-P.; Moebus, S.; Slomiany, U.; Jöckel, K.-H.; et al. Normal liver enzymes are correlated with the severity of metabolic syndrome in a large population-based cohort. Sci. Rep. 2015, 5, 13058. [Google Scholar] [CrossRef]
- Aziz, N.M. Effect of postmenopausal on some liver enzymes in Kirkuk women. Al-Qadisiyah J. Pure Sci. 2021, 26, 18–25. [Google Scholar] [CrossRef]
- Hernández Pérez, J.M.; Blanco, I.; Jesús Sánchez Medina, A.; Díaz Hernández, L.; Antonio Pérez Pérez, J. Serum levels of glutamate-pyruvate transaminase, glutamate-oxaloacetate transaminase, and gamma-glutamyl transferase in 1494 patients with various genotypes for the alpha-1 antitrypsin gene. J. Clin. Med. 2020, 9, 3923. [Google Scholar] [CrossRef]
- Maschari, D.; Saxena, G.; Law, T.D.; Walsh, E.; Campbell, M.C.; Consitt, L.A. Lactate-induced lactylation in skeletal muscle is associated with insulin resistance in humans. Front. Physiol. 2022, 13, 951390. [Google Scholar] [CrossRef]
- Nagai, M.A.; Sonohara, S.; Brentani, M.M. Estrogen control of lactate dehydrogenase isoenzyme-5 in human breast cancer. Int. J. Cancer 1988, 41, 10–16. [Google Scholar] [CrossRef]
- Fizelova, M.; Jauhiainen, R.; Kangas, A.J.; Soininen, P.; Ala-Korpela, M.; Kuusisto, J.; Laakso, M.; Stancáková, A. Differential associations of inflammatory markers with insulin sensitivity and secretion: The prospective METSIM study. J. Clin. Endocrinol. Metab. 2017, 102, 3600–3609. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Campbell, L.V. Relationship between inflammation, insulin resistance, and type 2 diabetes: ‘Cause or effect’? Curr. Diabetes Rev. 2006, 2, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Mahdiani, A.; Kheirandish, M.; Bonakdaran, S. Correlation between white blood cell count and insulin resistance in type 2 diabetes. Curr. Diabetes Rev. 2019, 15, 62–66. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Natali, A.; Capaldo, B.; Lehtovirta, M.; Jacob, S.; Yki-Järvinen, H. Insulin resistance, hyperinsulinemia, and blood pressure: Role of age and obesity. European Group for the Study of Insulin Resistance (EGIR). Hypertension 1997, 30, 1144–1149. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S.; Thio, C.; Wang, Y.; Li, M.; Wu, Y.; Lin, R.; Liu, Z.; Snieder, H.; Zhang, Q. Serum total bilirubin and new-onset hypertension in perimenopausal women: A cross-sectional study. Menopause 2022, 29, 944–951. [Google Scholar] [CrossRef]
- Facchini, F.S.; Hollenbeck, C.B.; Jeppesen, J.; Chen, Y.D.I.; Reaven, G.M. Insulin resistance and cigarette smoking. Lancet 1992, 339, 1128–1130. [Google Scholar] [CrossRef]
- Snijder, M.B.; Dekker, J.M.; Visser, M.; Yudkin, J.S.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J.; Nijpels, G.; Seidell, J.C. Larger thigh and hip circumferences are associated with better glucose tolerance: The Hoorn study. Obes. Res. 2003, 11, 104–111. [Google Scholar] [CrossRef]
- Stringhini, S.; Carmeli, C.; Jokela, M.; Avendaño, M.; Muennig, P.; Guida, F.; Ricceri, F.; d’Errico, A.; Barros, H.; Bochud, M.; et al. Socioeconomic status and the 25× 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1· 7 million men and women. Lancet 2017, 389, 1229–1237. [Google Scholar] [CrossRef]


| Variables | Unit and Description |
|---|---|
| Age | Years |
| Marital status, MS | (1) Unmarried (2) Married |
| Income level, IL | NTD/year (1) Below USD 200,000 (2) USD 200,001–USD 400,000 (3) USD 400,001–USD 800,000 (4) USD 800,001–USD 1,200,000 (5) USD 1,200,001–USD 1,600,000 (6) USD 1,600,001–USD 2,000,000 (7) More than USD 2,000,000 |
| Education level, Edu. | (1) Illiterate (2) Elementary school (3) Junior high school (4) High school (vocational) (5) Junior college (6) University (7) Graduate school or above |
| Waist–hip ratio, WHR | Waist circumference/hip circumference |
| Systolic blood pressure, SBP | mmHg |
| Diastolic blood pressure, DBP | mmHg |
| Leukocyte, WBC | ×103/μL |
| Hemoglobin, Hb | ×106/μL |
| Platelets, Plt | ×103/μL |
| Fasting plasma glucose, FPG | mg/dL |
| Fasting plasma insulin, FPI | uU/mL |
| Total bilirubin, TBIL | mg/dL |
| Albumin, Alb | mg/dL |
| Globulin, Glo | g/dL |
| Alkaline Phosphatase, ALP | IU/L |
| Serum glutamic oxaloacetic transaminase (SGOT) | IU/L |
| Serum glutamic pyruvic transaminase, SGPT | IU/L |
| Serum γ-glutamyl transpeptidase, γ-GT | IU/L |
| Lactate dehydrogenase, LDH | mg/dL |
| estimated Glomerular filtration rate, eGFR | mg/dL |
| Uric acid, UA | mg/dL |
| Triglycerides, TG | mg/dL |
| High-density lipoprotein cholesterol, HDL-C | mg/dL |
| Low-density lipoprotein cholesterol, LDL-C | mg/dL |
| Plasma calcium concentration, Ca | mg/dL |
| Plasma phosphate concentration, P | mg/dL |
| Thyroid-stimulating hormone, TSH | μIU/mL |
| C reactive protein, CRP | mg/dL |
| Follicle-stimulating hormone, FSH | mIU/mL |
| Estradiol, E2 | pg/mL |
| Drinking area | - |
| Smoking area | - |
| Sport area | - |
| Sleeping hours, SH | (1) 0~4 h (2) 4~6 h (3) 6~7 h (4) 7~8 h (5) 8~9 h (6) more than 9 h |
| HOMA-IR | FPI (μU/mL) × FPG (mg/dL)/405 |
| Metrics | Description | Calculation Formula |
|---|---|---|
| MAPE | Mean Absolute Percentage Error | |
| SMAPE | Symmetric Mean Absolute Percentage Error | |
| RAE | Relative Absolute Error | |
| RRSE | Root Relative Squared Error | |
| RMSE | Root Mean Squared Error |
| Numeric Variable | Non-Menopause n = 410 Mean ± SD | Early Menopause n = 538 Mean ± SD |
|---|---|---|
| Age | 47.27 ± 0.19 | 46.77 ± 0.19 |
| Waist–hip ratio, WHR | 0.77 ± 0.00 | 0.77 ± 0.00 |
| Systolic blood pressure, SBP | 111.75 ± 0.82 | 112.43 ± 0.66 |
| Diastolic blood pressure, DBP | 72.28 ± 0.54 | 72.08 ± 0.47 |
| Leukocyte, WBC | 5.52 ± 0.08 | 5.83 ± 0.06 ** |
| Hemoglobin, Hb | 13.56 ± 0.04 | 12.85 ± 0.06 *** |
| Platelets, Plt | 237.79 ± 2.52 | 257.33 ± 2.76 *** |
| Fasting plasma glucose, FPG | 101.08 ± 0.85 | 99.99 ± 0.67 |
| Fasting plasma insulin | 7.18 ± 4.33 | 7.15 ± 4.04 |
| Total bilirubin, TBIL | 0.96 ± 0.01 | 0.90 ± 0.01 ** |
| Albumin, Alb | 4.40 ± 0.00 | 4.32 ± 0.00 *** |
| Globulin, Glo | 3.13 ± 0.01 | 3.13 ± 0.01 |
| Alkaline phosphatase, ALP | 62.44 ± 0.91 | 52.90 ± 0.70 *** |
| Serum glutamic oxaloacetic transaminase, SGOT | 23.00 ± 0.44 | 21.67 ± 0.49 |
| Serum glutamic pyruvic transaminase, SGPT | 24.32 ± 0.74 | 22.50 ± 0.77 |
| Serum γ-glutamyl transpeptidase, γ-GT | 26.34 ± 1.37 | 22.79 ± 1.04 * |
| Lactate dehydrogenase, LDH | 165.18 ± 1.61 | 156.21 ± 1.27 *** |
| estimated Glomerular filtration rate, eGFR | 82.03 ± 0.63 | 82.79 ± 0.47 |
| Uric acid, UA | 4.94 ± 0.05 | 4.76 ± 0.04 * |
| Triglycerides, TG | 104.79 ± 3.15 | 92.91 ± 2.28 ** |
| High-density lipoprotein cholesterol, HDL-C | 65.29 ± 0.81 | 63.40 ± 0.64 |
| Low-density lipoprotein cholesterol, LDL-C | 121.45 ± 1.63 | 115.86 ± 1.35 ** |
| Plasma calcium concentration, Ca | 9.58 ± 0.01 | 9.37 ± 0.01 *** |
| Plasma phosphate concentration, P | 4.02 ± 0.02 | 3.72 ± 0.02 *** |
| Thyroid-stimulating hormone, TSH | 1.77 ± 0.08 | 1.76 ± 0.04 |
| C reactive protein, CRP | 0.20 ± 0.01 | 0.21 ± 0.01 |
| Follicle-stimulating hormone, FSH | 48.39 ± 1.72 | 17.42 ± 0.99 *** |
| Estradiol, E2 | 40.92 ± 4.28 | 96.30 ± 4.62 *** |
| HOMA-IR | 1.84 ± 0.06 | 1.81 ± 0.05 |
| Drinking area | 1.36 ± 0.33 | 1.34 ± 0.33 |
| Smoking area | 1.29 ± 0.34 | 0.87 ± 0.26 |
| Sport area | 4.52 ± 0.35 | 4.06 ± 0.24 |
| Range of HOMA-IR | 0.246–8.71 | 0.332–13.68 |
| Ordinal variables | N (%) | N (%) |
| Marital status (MS) | ||
| (1) Unmarried | 90 (24.46) | 140 (28.34) |
| (2) Married | 278 (75.54) | 354 (71.66) |
| Categorical variables | N (%) | N (%) |
| Income level (IL) | ||
| (1) Below USD 200,000 | 24 (11.37) | 31 (10.47) |
| (2) USD 200,001–USD 400,000 | 49 (23.22) | 64 (21.62) |
| (3) USD 400,001–USD 800,000 | 65 (30.81) | 91 (30.74) |
| (4) USD 800,001–USD 1,200,000 | 44 (20.85) | 69 (23.31) |
| (5) USD 1,200,001–USD 1,600,000 | 17 (8.06) | 20 (6.76) |
| (6) USD 1,600,001–USD 2,000,000 | 4 (1.90) | 8 (2.70) |
| (7) More than USD 2,000,000 | 8 (3.79) | 13 (4.39) |
| Education level (Edu.) | ||
| (1) Illiterate | 0 (0.00) | 0 (0.00) |
| (2) Elementary school | 3 (0.82) | 3 (0.60) |
| (3) Junior high school | 12 (3.28) | 12 (2.40) |
| (4) High school (vocational) | 124 (33.88) | 124 (24.85) |
| (5) Junior college | 93 (25.41) | 140 (28.06) |
| (6) University | 99 (27.05) | 165 (33.07) |
| (7) Graduate school or above | 35 (9.56) | 55 (11.02) |
| Sleeping hours (SH) | ||
| (1) 0~4 h | 8 (2.01) | 8 (1.54) |
| (2) 4~6 h | 120 (30.15) | 142 (27.26) |
| (3) 6~7 h | 186 (46.73) | 252 (48.37) |
| (4) 7~8 h | 67 (16.83) | 100 (19.19) |
| (5) 8~9 h | 16 (4.02) | 18 (3.45) |
| (6) more than 9 h | 1 (0.25) | 1 (0.19) |
| Menopause | ||||||
| age | WHR | SBP | DBP | WBC | Hb | |
| HOMA-IR | −0.07 | 0.43 *** | 0.26 *** | 0.24 *** | 0.32 *** | 0.12 ** |
| Plt | FPG | TBIL | Alb | Glo | ALP | |
| HOMA-IR | 0.25 *** | 0.50 *** | −0.24 *** | 0.16 *** | 0.18 *** | 0.25 *** |
| SGOT | SGPT | r-GT | LDH | eGFR | UA | |
| HOMA-IR | 0.30 *** | 0.41 *** | 0.31 *** | 0.14 *** | 0.00 | 0.34 *** |
| TG | HDL-C | LDL-C | Ca | P | TSH | |
| HOMA-IR | 0.45 *** | −0.37 *** | 0.26 *** | 0.11 * | −0.08 | 0.08 |
| CRP | FSH | E2 | Drinking area | Smoking area | Sport area | |
| HOMA-IR | 0.19 *** | −0.09 * | −0.06 | −0.05 | −0.06 | −0.23 *** |
| Early menopause | ||||||
| age | WHR | SBP | DBP | WBC | Hb | |
| HOMA-IR | −0.00 | 0.50 *** | 0.28 *** | 0.28 *** | 0.33 *** | 0.12 ** |
| Plt | FPG | TBIL | Alb | Glo | ALP | |
| HOMA-IR | 0.19 *** | 0.50 *** | −0.25 *** | 0.14 ** | 0.10 * | 0.16 *** |
| SGOT | SGPT | r-GT | LDH | eGFR | UA | |
| HOMA-IR | 0.12 * | 0.36 *** | 0.16 *** | 0.02 | 0.01 | 0.31 *** |
| TG | HDL-C | LDL-C | Ca | P | TSH | |
| HOMA-IR | 0.37 *** | −0.37 *** | 0.20 *** | 0.04 | −0.09 | 0.04 |
| CRP | FSH | E2 | Drinking area | Smoking area | Sport area | |
| HOMA-IR | 0.28 *** | −0.15 ** | −0.04 | −0.04 | −0.01 | −0.03 |
| Menopause | |||||
| Methods | MAPE | SMAPE | RAE | RRSE | RMSE |
| MLR | 0.54 | 0.4293 | 0.974 | 0.9549 | 0.9729 |
| RF | 0.4785 | 0.3567 | 0.8572 | 0.8592 | 0.8753 |
| SGB | 0.5029 | 0.3659 | 0.8852 | 0.9107 | 0.9278 |
| XGBoost | 0.5384 | 0.3636 | 0.9182 | 0.927 | 0.9444 |
| EN | 0.4854 | 0.3671 | 0.8731 | 0.8514 | 0.8673 |
| Early menopause | |||||
| Methods | MAPE | SMAPE | RAE | RRSE | RMSE |
| MLR | 0.5063 | 0.5345 | 1.063 | 1.1386 | 1.1046 |
| RF | 0.4196 | 0.3365 | 0.7619 | 0.8308 | 0.806 |
| SGB | 0.457 | 0.3782 | 0.9129 | 0.9724 | 0.9433 |
| XGBoost | 0.4862 | 0.3981 | 0.9281 | 1.0127 | 0.9824 |
| EN | 0.4765 | 0.3772 | 0.8438 | 0.8815 | 0.8551 |
| RF | SGB | XGBoost | EN | Average | |
|---|---|---|---|---|---|
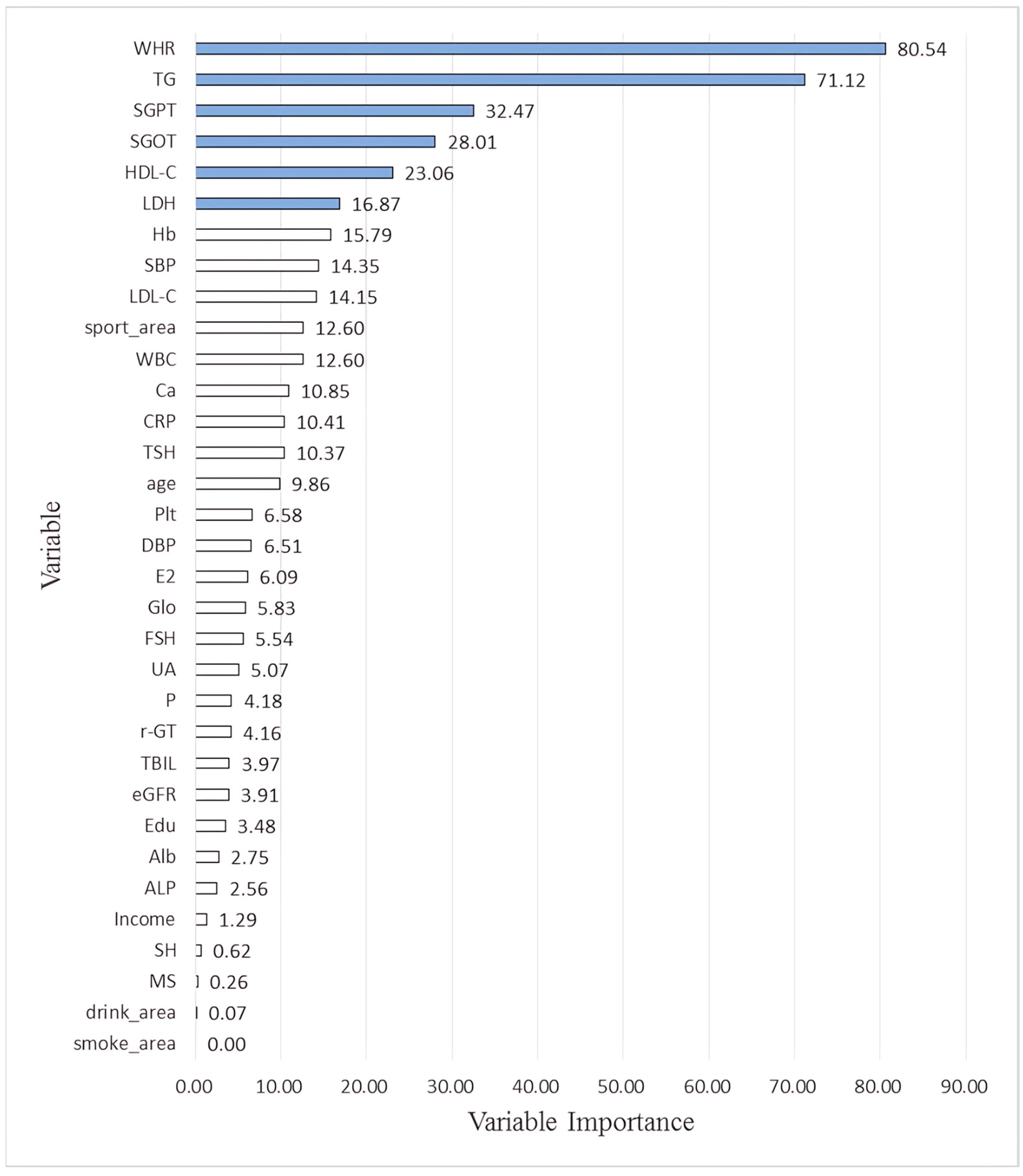
| Age | 7.59 | 22.02 | 9.82 | 0.00 | 9.86 |
| MS | 1.02 | 0.00 | 0.00 | 0.00 | 0.26 |
| Income | 2.57 | 0.64 | 1.96 | 0.00 | 1.29 |
| Edu | 1.99 | 6.06 | 5.87 | 0.00 | 3.48 |
| WHR | 71.50 | 50.64 | 100.00 | 100.00 | 80.54 |
| SBP | 15.53 | 22.39 | 19.47 | 0.00 | 14.35 |
| DBP | 19.52 | 1.65 | 4.63 | 0.23 | 6.51 |
| WBC | 15.27 | 20.13 | 13.81 | 1.17 | 12.60 |
| Hb | 20.85 | 10.17 | 32.12 | 0.02 | 15.79 |
| Plt | 13.06 | 1.77 | 11.44 | 0.05 | 6.58 |
| TBIL | 9.89 | 3.38 | 2.60 | 0.00 | 3.97 |
| Alb | 3.77 | 4.23 | 2.98 | 0.00 | 2.75 |
| Glo | 16.07 | 6.96 | 0.29 | 0.00 | 5.83 |
| ALP | 6.76 | 0.74 | 2.72 | 0.00 | 2.56 |
| SGOT | 25.03 | 40.76 | 46.26 | 0.00 | 28.01 |
| SGPT | 67.80 | 29.96 | 31.45 | 0.67 | 32.47 |
| r-GT | 10.75 | 2.95 | 2.94 | 0.00 | 4.16 |
| LDH | 16.09 | 21.11 | 30.28 | 0.01 | 16.87 |
| eGFR | 9.84 | 2.92 | 2.88 | 0.00 | 3.91 |
| UA | 7.49 | 1.96 | 9.92 | 0.90 | 5.07 |
| TG | 100.00 | 100.00 | 84.22 | 0.26 | 71.12 |
| HDL-C | 18.15 | 33.87 | 40.01 | 0.20 | 23.06 |
| LDL-C | 22.95 | 9.97 | 23.68 | 0.00 | 14.15 |
| Ca | 10.12 | 8.29 | 14.48 | 10.50 | 10.85 |
| P | 8.91 | 3.89 | 3.93 | 0.00 | 4.18 |
| TSH | 13.38 | 16.52 | 11.56 | 0.00 | 10.37 |
| CRP | 7.44 | 19.05 | 7.33 | 7.81 | 10.41 |
| FSH | 8.20 | 5.72 | 8.24 | 0.00 | 5.54 |
| E2 | 7.45 | 11.95 | 4.94 | 0.00 | 6.09 |
| Drinking area | 0.27 | 0.00 | 0.00 | 0.00 | 0.07 |
| Smoking area | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sport area | 6.55 | 8.89 | 34.67 | 0.27 | 12.60 |
| SH | 0.96 | 1.52 | 0.00 | 0.00 | 0.62 |
| RF | SGB | XGBoost | EN | Average | |
|---|---|---|---|---|---|
| Age | 7.43 | 7.15 | 16.04 | 0.00 | 7.66 |
| MS | 0.64 | 0.00 | 0.00 | 0.00 | 0.16 |
| Income | 3.68 | 0.00 | 5.13 | 0.00 | 2.20 |
| Edu | 7.74 | 0.00 | 35.49 | 0.00 | 10.81 |
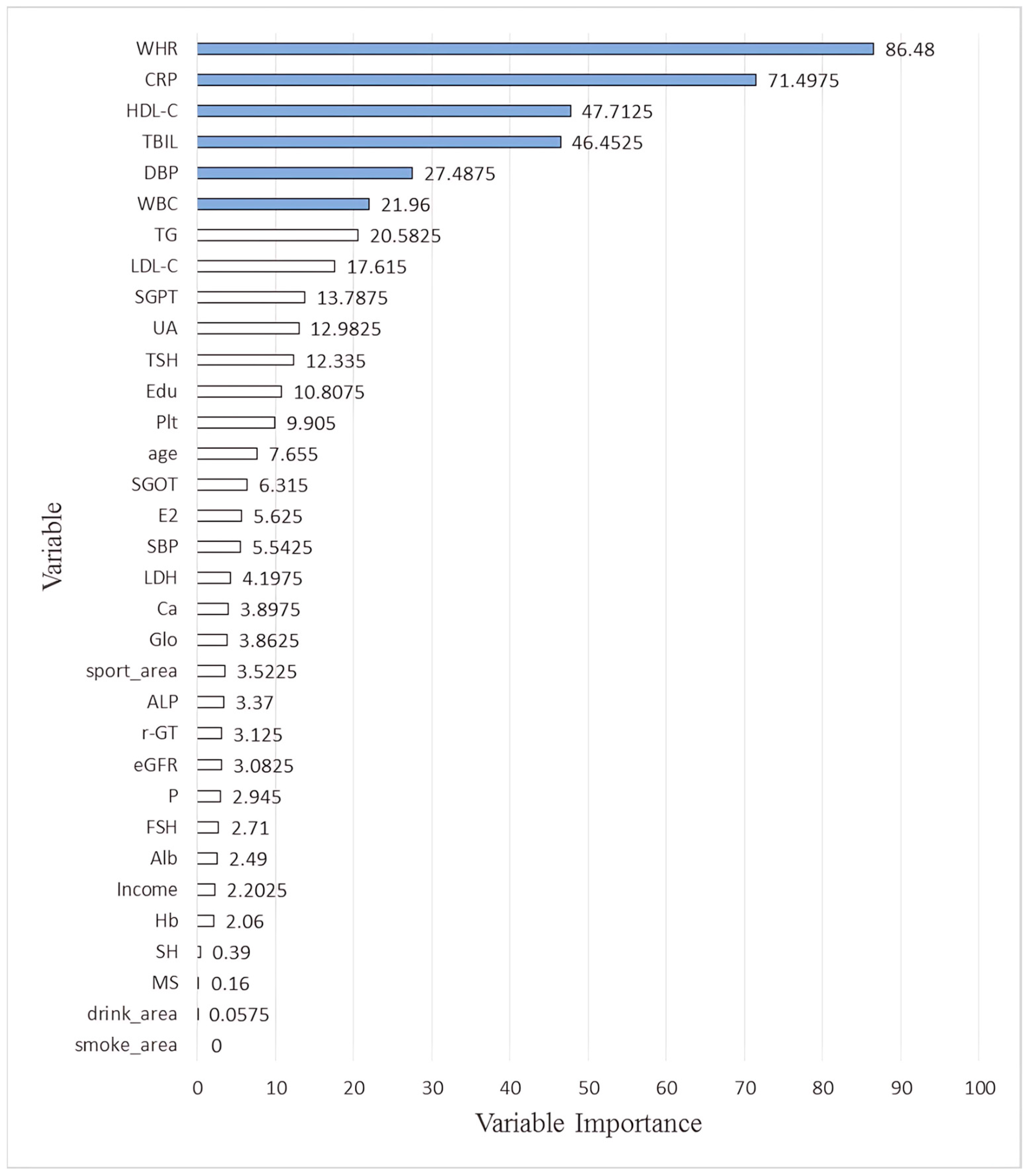
| WHR | 72.08 | 73.84 | 100.00 | 100.00 | 86.48 |
| SBP | 18.36 | 0.00 | 3.80 | 0.01 | 5.54 |
| DBP | 14.35 | 42.15 | 53.20 | 0.25 | 27.49 |
| WBC | 36.24 | 32.52 | 18.28 | 0.80 | 21.96 |
| Hb | 5.46 | 0.00 | 2.78 | 0.00 | 2.06 |
| Plt | 23.41 | 3.50 | 12.71 | 0.00 | 9.91 |
| TBIL | 37.07 | 66.32 | 71.80 | 10.62 | 46.45 |
| Alb | 6.69 | 0.00 | 3.27 | 0.00 | 2.49 |
| Glo | 3.68 | 0.00 | 11.77 | 0.00 | 3.86 |
| ALP | 8.35 | 0.00 | 5.13 | 0.00 | 3.37 |
| SGOT | 5.51 | 8.56 | 11.19 | 0.00 | 6.32 |
| SGPT | 18.94 | 12.56 | 23.40 | 0.25 | 13.79 |
| r-GT | 7.12 | 0.00 | 5.38 | 0.00 | 3.13 |
| LDH | 7.89 | 0.00 | 8.90 | 0.00 | 4.20 |
| eGFR | 7.24 | 2.17 | 2.92 | 0.00 | 3.08 |
| UA | 11.29 | 25.50 | 15.12 | 0.02 | 12.98 |
| TG | 31.26 | 41.57 | 9.50 | 0.00 | 20.58 |
| HDL-C | 48.48 | 50.99 | 91.22 | 0.16 | 47.71 |
| LDL-C | 10.78 | 12.59 | 47.06 | 0.03 | 17.62 |
| Ca | 5.22 | 0.00 | 10.37 | 0.00 | 3.90 |
| P | 5.09 | 2.68 | 4.01 | 0.00 | 2.95 |
| TSH | 9.96 | 13.92 | 25.46 | 0.00 | 12.34 |
| CRP | 100.00 | 100.00 | 83.58 | 2.41 | 71.50 |
| FSH | 6.98 | 0.00 | 3.86 | 0.00 | 2.71 |
| E2 | 3.96 | 6.60 | 11.94 | 0.00 | 5.63 |
| Drinking area | 0.23 | 0.00 | 0.00 | 0.00 | 0.06 |
| Smoking area | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sport area | 7.35 | 0.00 | 6.74 | 0.00 | 3.52 |
| SH | 1.56 | 0.00 | 0.00 | 0.00 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-K.; Pei, D.; Chu, T.-W.; Chiang, K.-J. The Comparison of Insulin Resistance Between Normal and Early Menopause Women Younger than Fifty Years Old by Machine Learning Methods. Diagnostics 2025, 15, 2074. https://doi.org/10.3390/diagnostics15162074
Wang C-K, Pei D, Chu T-W, Chiang K-J. The Comparison of Insulin Resistance Between Normal and Early Menopause Women Younger than Fifty Years Old by Machine Learning Methods. Diagnostics. 2025; 15(16):2074. https://doi.org/10.3390/diagnostics15162074
Chicago/Turabian StyleWang, Chun-Kai, Dee Pei, Ta-Wei Chu, and Kai-Jo Chiang. 2025. "The Comparison of Insulin Resistance Between Normal and Early Menopause Women Younger than Fifty Years Old by Machine Learning Methods" Diagnostics 15, no. 16: 2074. https://doi.org/10.3390/diagnostics15162074
APA StyleWang, C.-K., Pei, D., Chu, T.-W., & Chiang, K.-J. (2025). The Comparison of Insulin Resistance Between Normal and Early Menopause Women Younger than Fifty Years Old by Machine Learning Methods. Diagnostics, 15(16), 2074. https://doi.org/10.3390/diagnostics15162074







