Validation of a Manual Method for Measuring Left Atrial Reservoir Strain Against Automated Speckle Tracking Analysis
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Population
Inclusion and Exclusion Criteria
2.2. Study Design
2.3. Echocardiography Evaluation
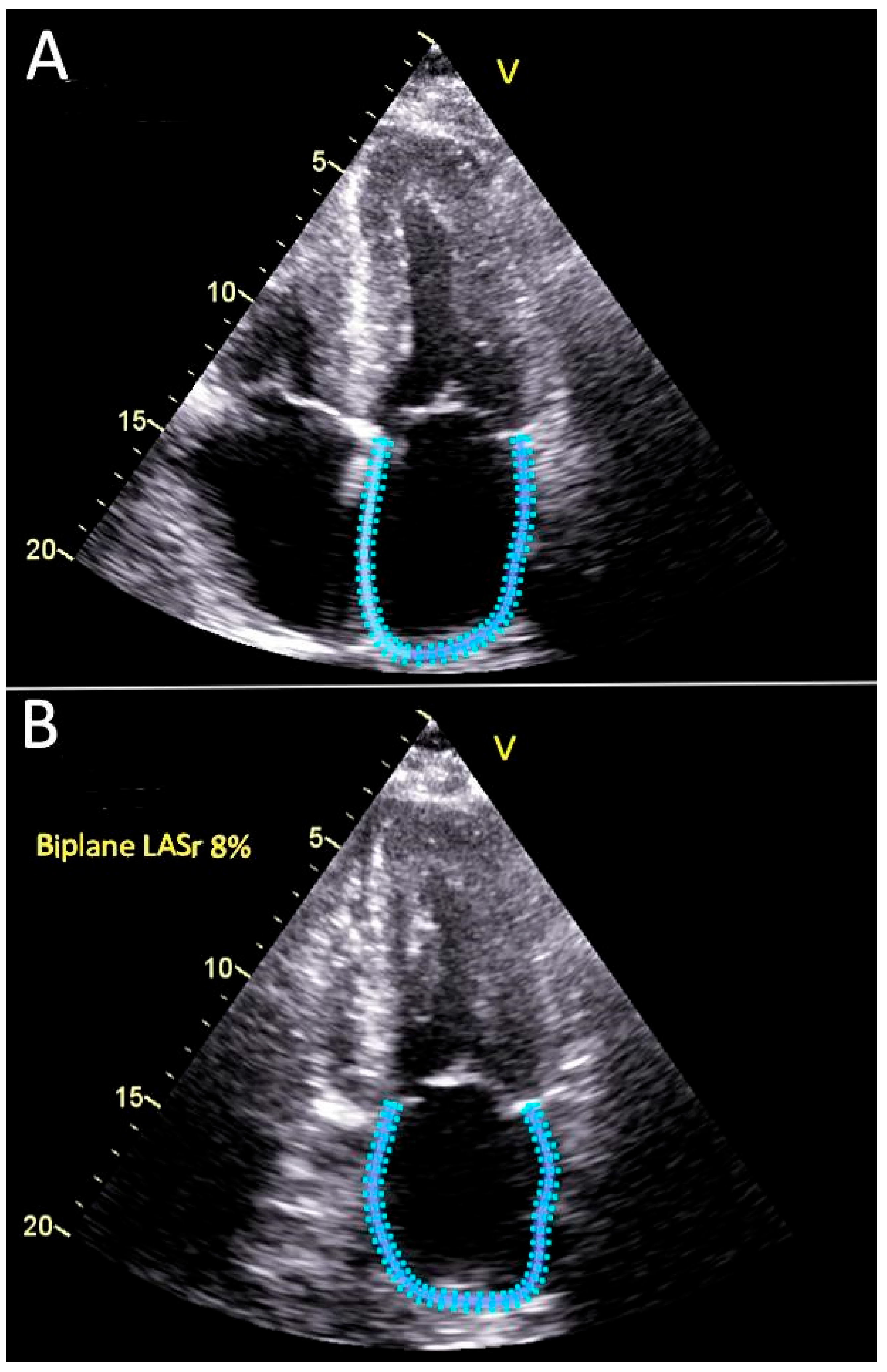
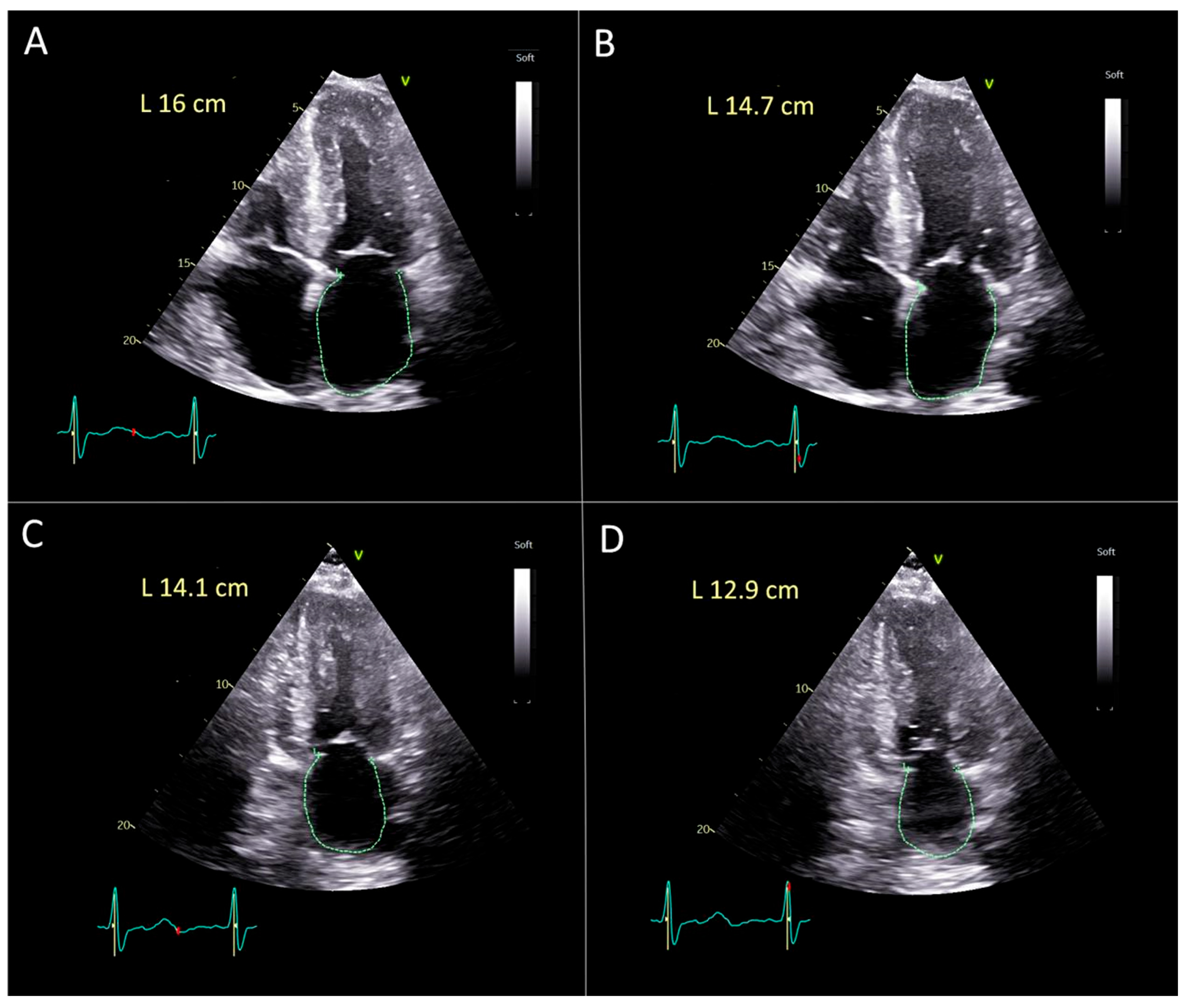
2.4. Statistical Methods
2.5. Ethical Approval
3. Results
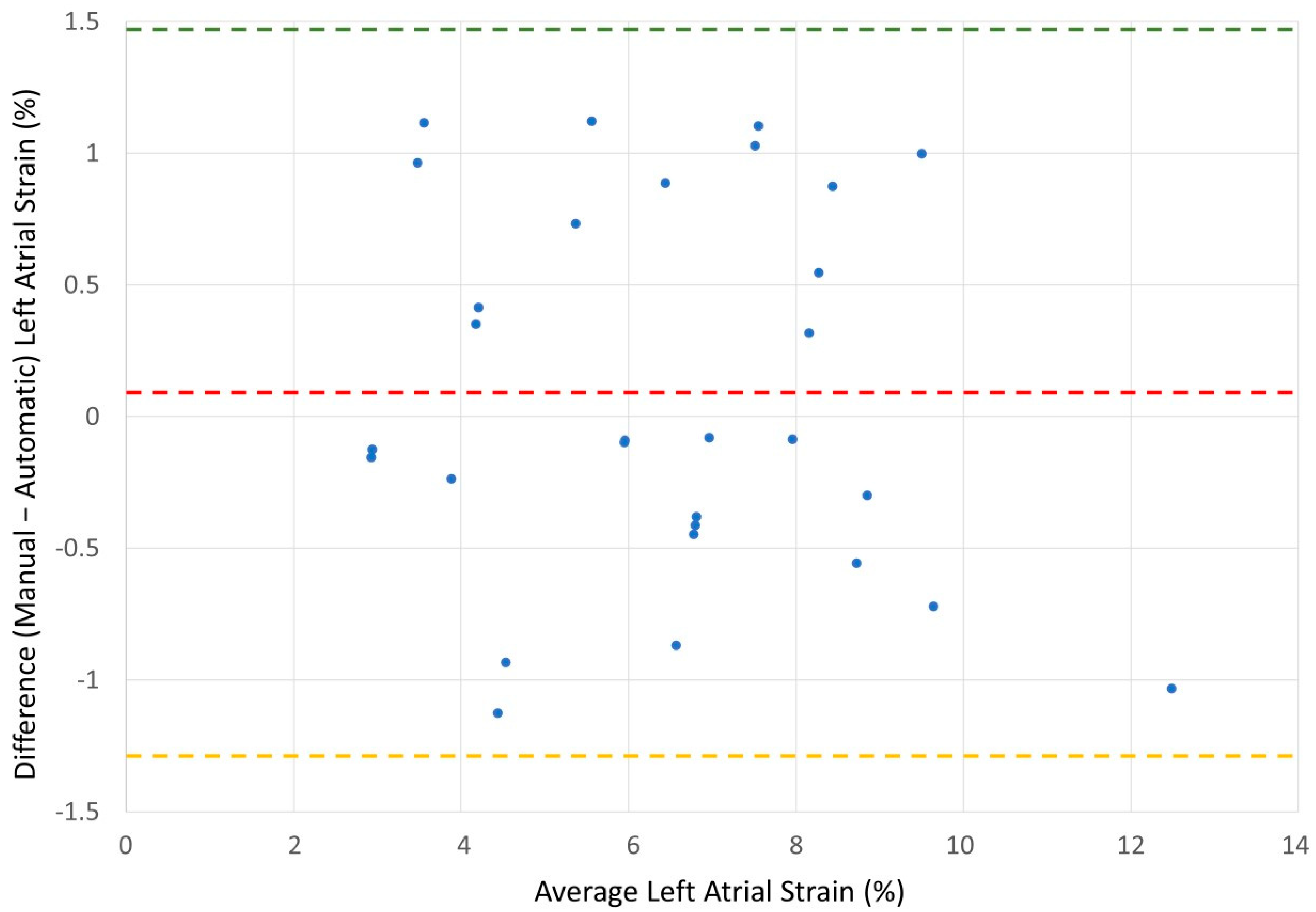
Validation of Manual Method for the Left Atrial Strain Calculation
4. Discussion
4.1. Clinical Relevance of Left Atrial Reservoir Strain
4.2. The Need for Manual Methods
4.3. Diagnostic Accuracy and Measurement Reproducibility
4.4. Broader Implications for Practice and Research
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Köhncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Sanborn, D.Y.; Oh, J.K.; Anderson, B.; Billick, K.; Derumeaux, G.; Klein, A.; Koulogiannis, K.; Mitchell, C.; Shah, A.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography and for Heart Failure With Preserved Ejection Fraction Diagnosis: An Update From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 537–569. [Google Scholar] [CrossRef]
- Ye, Z.; Miranda, W.R.; Yeung, D.F.; Kane, G.C.; Oh, J.K. Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1490–1499. [Google Scholar] [CrossRef]
- Khan, H.R.; Yakupoglu, H.Y.; Kralj-Hans, I.; Haldar, S.; Bahrami, T.; Clague, J.; De Souza, A.; Hussain, W.; Jarman, J.; Jones, D.G.; et al. Left Atrial Function Predicts Atrial Arrhythmia Recurrence Following Ablation of Long-Standing Persistent Atrial Fibrillation. Circ. Cardiovasc. Imaging 2023, 16, e015352. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J.-Cardiovasc. Imaging 2019, 19, 591–600. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Procopio, M.C.; Pica, A.; Vigna, M.; Benfari, G.; Diviggiano, E.E.; Martini, L.; Lunghetti, S.; Focardi, M.; et al. Unveiling the reliability of left atrial strain measurement: A dedicated speckle tracking software perspective in controls and cases. Eur. Heart J. Imaging Methods Pract. 2024, 2, qyae061. [Google Scholar] [CrossRef]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic reference ranges for normal left atrial function parameters: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J.; et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: Importance of left atrial strain. Circ. Cardiovasc. Imaging 2016, 9, e003754. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef]
- Zhang, R.; Li, H.; Wang, Y.; Yu, T.; Li, J.; Wu, Y.; Yu, Z.; Liang, C.; Yu, D.; Xue, L. Left atrial strain predicts paroxysmal atrial fibrillation recurrence after catheter ablation: A 1-year study using three-dimensional speckle-tracking echocardiography. BMC Cardiovasc. Disord. 2025, 25, 78. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Focardi, M.; Reccia, R.; Natali, B.M.; Sparla, S.; Mondillo, S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am. J. Cardiol. 2012, 110, 264–269. [Google Scholar] [CrossRef]
- Choi, J.Y. Expanding Role of Left Atrial Strain in Valvular Heart Disease. Korean Circ. J. 2022, 52, 218–219. [Google Scholar] [CrossRef]
- Di Salvo, G.; Caso, P.; Lo Piccolo, R.; Fusco, A.; Martiniello, A.R.; Russo, M.G.; D’Onofrio, A.; Severino, S.; Calabró, P.; Pacileo, G.; et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation 2005, 112, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, A.; Porcu, L.; Sonaglioni, A.; Genovesi, S. Proposal for a clinical and an echocardiographic score for prediction of left atrial thrombosis in atrial fibrillation patients undergoing early electrical cardioversion. Int. J. Clin. Pract. 2021, 75, e14706. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Arockiam, A.D.; Agrawal, A.; El Dahdah, J.; Haroun, E.; Majid, M.; Grimm, R.A.; Rodriguez, L.L.; Popovic, Z.B.; Griffin, B.P.; et al. Left atrial strain by vendor-neutral echocardiography software in healthy subjects: Vendor comparisons and associated factors. Eur. Heart J. Imaging Methods Pract. 2025, 3, qyaf029. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Fei, H.; Yu, Y.; Ren, S.; Lin, Q.; Li, H.; Tang, Y.; Hou, Y.; Li, M. Left atrial strain reproducibility using vendor-dependent and vendor-independent software. Cardiovasc. Ultrasound 2019, 17, 9. [Google Scholar] [CrossRef]
- Ferkh, A.; Stefani, L.; Trivedi, S.J.; Brown, P.; Byth, K.; Pathan, F.; Thomas, L. Inter-vendor comparison of left atrial strain using layer specific strain analysis. Int. J. Cardiovasc. Imaging 2021, 37, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Mouselimis, D.; Tsarouchas, A.S.; Pagourelias, E.D.; Bakogiannis, C.; Theofilogiannakos, E.K.; Loutradis, C.; Fragakis, N.; Vassilikos, V.P.; Papadopoulos, C.E. Left atrial strain, intervendor variability, and atrial fibrillation recurrence after catheter ablation: A systematic review and meta-analysis. Hell. J. Cardiol. 2020, 61, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Bunting, K.V.; Steeds, R.P.; Slater, K.; Rogers, J.K.; Gkoutos, G.V.; Kotecha, D. A Practical Guide to Assess the Reproducibility of Echocardiographic Measurements. J. Am. Soc. Echocardiogr. 2019, 32, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Peigh, G.; Shah, S.J.; Patel, R.B. Left Atrial Myopathy in Atrial Fibrillation and Heart Failure: Clinical Implications, Mechanisms, and Therapeutic Targets. Curr. Heart Fail. Rep. 2021, 18, 85–98. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Campora, A.; Lisi, M.; Pastore, M.C.; Mandoli, G.E.; Ferrari Chen, Y.F.; Pasquini, A.; Rubboli, A.; Henein, M.Y.; Cameli, M. Atrial Fibrillation, Atrial Myopathy, and Thromboembolism: The Additive Value of Echocardiography and Possible New Horizons for Risk Stratification. J. Clin. Med. 2024, 13, 3921. [Google Scholar] [CrossRef]
- Molnár, A.Á.; Sánta, A.; Pásztor, D.T.; Merkely, B. Atrial Cardiomyopathy in Valvular Heart Disease: From Molecular Biology to Clinical Perspectives. Cells 2023, 12, 1796. [Google Scholar] [CrossRef]
- Pécora, M.; Pastorini, P.; Farolini, R.; Burghi, G.; Hurtado, F.J. Left ventricular systolic longitudinal strain in mechanically ventilated patients in the intensive care unit: Assessment of global and chamber reproducibility. Intensive Care Med. Exp. 2025, 13, 62. [Google Scholar] [CrossRef]
- Hopman, L.H.; Bhagirath, P.; Mulder, M.J.; Demirkiran, A.; El Mathari, S.; van der Laan, A.M.; van Rossum, A.C.; Kemme, M.J.; Allaart, C.P.; Götte, M.J. Left atrial sphericity in relation to atrial strain and strain rate in atrial fibrillation patients. Int. J. Cardiovasc. Imaging 2023, 39, 1753–1763. [Google Scholar] [CrossRef]


| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| Number | 30 | 30 | 1.0 |
| Males | 19 (63.3%) | 19 (63.3%) | 1.0 |
| Females | 11 (36.7%) | 11 (36.7%) | 1.0 |
| Age, years | 79.1 ± 8.7 | 47.4 ± 20.9 | <10−9 |
| Height, cm | 167.2 ± 8.3 | 170 ± 8.6 | 0.21 |
| Weight, kg | 79.2 ± 15.4 | 75.5 ± 13.4 | 0.21 |
| BMI, kg/m2 | 28.3 ± 5.0 | 25.7 ± 3.7 | <0.03 |
| BSA, m2 | 1.88 ± 0.2 | 1.85 ± 0.2 | 0.57 |
| NYHA FC | 2.5 ± 0.8 | 1.1 ± 0.3 | <10−10 |
| Hypertension | 22 (73.3%) | 5 (16.7%) | <10−5 |
| Diabetes | 11 (36.7%) | 3 (10%) | 0.01 |
| IHD | 12 (40%) | 0 | 0.0001 |
| CKD | 12 (40%) | 1 (3.3%) | <0.01 |
| COPD | 12 (40%) | 2 (6.7%) | <0.01 |
| Malignancy | 3 (10%) | 7 (23.3%) | 0.17 |
| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| LVEDD, cm | 4.6 ± 0.6 | 4.6 ± 0.5 | 0.83 |
| LVESD, cm | 3.1 ± 0.8 | 2.7 ± 0.4 | 0.02 |
| EF, % | 47.8 ± 10.5 | 60.5 ± 1.5 | <10−7 |
| IVST, cm | 1.3 ± 0.4 | 1.0 ± 0.2 | <10−4 |
| PWT, cm | 1.1 ± 0.2 | 0.9 ± 0.1 | <10−3 |
| LVMi, g/m2 | 109.3 ± 35.7 | 78.7 ± 21.2 | <10−3 |
| RWT | 0.48 ± 0.14 | 0.40 ± 0.10 | <0.01 |
| LAD, cm | 4.5 ± 0.7 | 3.6 ± 0.4 | <10−7 |
| LAA, cm2 | 30.1 ± 5.6 | 18.1 ± 3.8 | <10−12 |
| LAVi, mL/m2 | 56.7 ± 19.7 | 31.1 ± 10.4 | <10−7 |
| LASr, % | 6.4 ± 2.3 | 30.3 ± 4.68 | <10−31 |
| LASr(m), % | 6.5 ± 2.2 | 29.5 ± 4.4 | <10−32 |
| PAP, mmHg | 44.2 ± 11.0 | 25.2 ± 6.5 | <10−10 |
| MR ≥ 2 degree | 16 (53.3%) | 0 | <10−4 |
| AS ≥ 2 degree | 4 (13.3%) | 0 | <0.04 |
| AI ≥ 2 degree | 1 (3.3%) | 0 | 0.32 |
| TR ≥ 2 degree | 20 (66.7%) | 1 | <10−7 |
| Patients | LASr(m) | LASr | r | p-Value |
|---|---|---|---|---|
| AF | 6.53 ± 2.2 | 6.4 ± 2.3 | r = 0.953 | p < 0.0001 |
| Sinus | 29.5 ± 4.35 | 30.3 ± 4.68 | r = 0.939 | p < 0.0001 |
| Metric | Atrial Fibrillation Group | Sinus Group |
|---|---|---|
| Mean Manual Strain, % | 6.5 ± 2.2 | 29.5 ± 4.4 |
| Mean Automatic Strain, % | 6.4 ± 2.3 | 30.3 ± 4.7 |
| Mean Difference | 0.09 ± 0.71 | −0.80 ± 1.64 |
| Upper Limit of Agreement | 1.49 | 2.41 |
| Lower of Agreement | −1.30 | −4.01 |
| Pearson Correlation (r, p-value) | 0.953, p < 0.0001 | 0.939, p < 0.0001 |
| Interclass Correlation | 0.95 | 0.92 |
| Coefficient of Variation for Manual-Automatic Differences | 11.0% | 5.5% |
| Standard Error of Measurement, % | 0.16 | 0.46 |
| Minimal Detectable Change (95), % | 0.43 | 1.27 |
| Repeatability Coefficient for manual method, % | 0.82 (0.60–1.29) | 10.53 (7.71–16.61) |
| Repeatability Coefficient for automatic method, % | 0.7 (0.51–1.10) | 5.66 (4.15–8.93) |
| Pearson Correlation (r) | 0.995 |
|---|---|
| p-value | <10−60 |
| Intraclass Correlation Coefficient | 0.988 |
| Coefficient of Variation (CV%) | 7.32% |
| Standard Error of Measurement (SEM) | 0.94% |
| Minimal Detectable Change (MDC95) | 2.61% |
| MDC as % of mean | 14.34% |
| Subgroup | N | Mean Absolute Difference, % | Pearson r | ICC |
|---|---|---|---|---|
| Left atrium enlarged (LAVi > 34 mL/m2) | 36 | 0.73 | 0.996 | 0.996 |
| Normal left atrium (LAVi ≤ 34 mL/m2) | 24 | 1.56 | 0.986 | 0.981 |
| Ejection fraction preserved | 48 | 1.16 | 0.994 | 0.992 |
| Ejection fraction reduced | 12 | 0.65 | 0.907 | 0.894 |
| Age ≥ 65 | 36 | 0.78 | 0.997 | 0.993 |
| Age < 65 | 24 | 1.28 | 0.989 | 0.985 |
| Male | 38 | 1.02 | 0.996 | 0.994 |
| Female | 22 | 1.12 | 0.994 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitman, M.; Tyomkin, V. Validation of a Manual Method for Measuring Left Atrial Reservoir Strain Against Automated Speckle Tracking Analysis. Diagnostics 2025, 15, 2073. https://doi.org/10.3390/diagnostics15162073
Leitman M, Tyomkin V. Validation of a Manual Method for Measuring Left Atrial Reservoir Strain Against Automated Speckle Tracking Analysis. Diagnostics. 2025; 15(16):2073. https://doi.org/10.3390/diagnostics15162073
Chicago/Turabian StyleLeitman, Marina, and Vladimir Tyomkin. 2025. "Validation of a Manual Method for Measuring Left Atrial Reservoir Strain Against Automated Speckle Tracking Analysis" Diagnostics 15, no. 16: 2073. https://doi.org/10.3390/diagnostics15162073
APA StyleLeitman, M., & Tyomkin, V. (2025). Validation of a Manual Method for Measuring Left Atrial Reservoir Strain Against Automated Speckle Tracking Analysis. Diagnostics, 15(16), 2073. https://doi.org/10.3390/diagnostics15162073





