Prognosis of Breast Cancer in Women in Their 20s: Clinical and Radiological Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analysis of Basic Clinical with Histopathological Characteristics
2.3. Radiological Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical and Pathological Characteristics of the Patients and Tumors
3.2. Radiological Characteristics
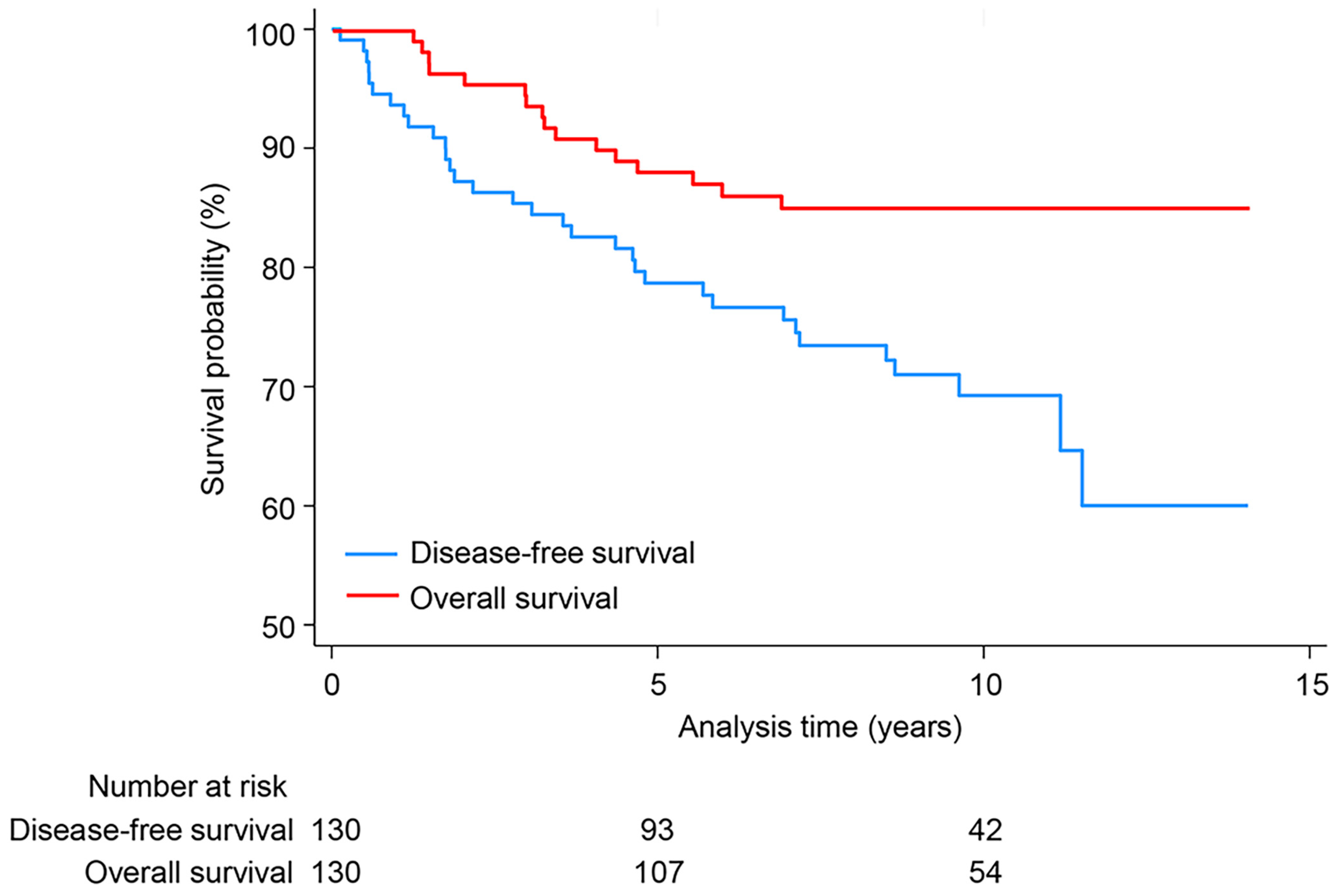
3.3. Follow-Up Outcomes and Prognostic Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| BMI | Body mass index |
| CI | Confidence interval |
| CTX | Chemotherapy |
| DFS | Disease-free survival |
| EIC | Extensive intraductal component |
| ER | Estrogen receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| HRT | Hormone therapy |
| LVI | Lymphovascular invasion |
| MRI | Magnetic resonance imaging |
| NAC | Neoadjuvant chemotherapy |
| OS | Overall survival |
| pCR | Pathologic complete response |
| PR | Progesterone receptor |
| RTx | Radiation therapy |
| TNBC | Triple-negative breast cancer |
| US | Ultrasound |
References
- An, Y.Y.; Kim, S.H.; Kang, B.J.; Park, C.S.; Jung, N.Y.; Kim, J.Y. Breast cancer in very young women (<30 years): Correlation of imaging features with clinicopathological features and immunohistochemical subtypes. Eur. J. Radiol. 2015, 84, 1894–1902. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Gentilini, O.; et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann. Oncol. 2022, 33, 1097–1118. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age < 40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022, 61, 77–83. [Google Scholar] [CrossRef]
- Dibden, A.; Offman, J.; Duffy, S.W.; Gabe, R. Worldwide Review and Meta-Analysis of Cohort Studies Measuring the Effect of Mammography Screening Programmes on Incidence-Based Breast Cancer Mortality. Cancers 2020, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Kim, S.W.; Park, I.A.; Kang, D.; Kim, S.W.; Youn, Y.K.; Oh, S.K.; Choe, K.J.; Noh, D.Y. Young age: An independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer 2004, 4, 82. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Kang, B.J.; Lee, A.; Park, W.C.; Hwang, J. Imaging characteristics of young age breast cancer (YABC) focusing on pathologic correlation and disease recurrence. Sci. Rep. 2021, 11, 20205. [Google Scholar] [CrossRef] [PubMed]
- Eiriz, I.F.; Batista, M.V.; Tomas, T.C.; Neves, M.T.; Guerra-Pereira, N.; Braga, S. Breast cancer in very young women-a multicenter 10-year experience. ESMO Open 2021, 6, 100029. [Google Scholar] [CrossRef] [PubMed]
- Alhaidary, A.A.; Al-Qudimat, A.R.; Arabi, H.; Al-Zoubi, R.M. Imaging Patterns in Breast Cancer for Women Under 40 Years: A Descriptive Cohort Study. J. Epidemiol. Glob. Health 2024, 14, 63–71. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Q.; Cui, C.; Fei, J.; Su, X.; Li, L.; Ma, J.; Zhang, M. Correlation between imaging features and molecular subtypes of breast cancer in young women (≤30 years old). Jpn. J. Radiol. 2020, 38, 1062–1074. [Google Scholar] [CrossRef]
- Langman, E.L.; Kuzmiak, C.M.; Brader, R.; Thomas, S.M.; Alexander, S.L.; Lee, S.S.; Jordan, S.G. Breast cancer in young women: Imaging and clinical course. Breast J. 2021, 27, 657–663. [Google Scholar] [CrossRef]
- Bustreo, S.; Osella-Abate, S.; Cassoni, P.; Donadio, M.; Airoldi, M.; Pedani, F.; Papotti, M.; Sapino, A.; Castellano, I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res. Treat. 2016, 157, 363–371. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Choi, J.S.; Tsunoda, H.; Moon, W.K. Nonmass Lesions on Breast US: An International Perspective on Clinical Use and Outcomes. J. Breast Imaging 2024, 6, 86–98. [Google Scholar] [CrossRef]
- DeMartini, W.B.; Destounis, S.V.; Eby, P.R.; Leung, J.W. BI-RADS update: The edition formerly known as fifth. In Proceedings of the 2023 SBI Breast Imaging Symposium, National Harbor, MD, USA, 4–7 May 2023; Society of Breast Imaging: Richmond, VA, USA, 2023. [Google Scholar]
- Tsunoda, H.; Moon, W.K. Beyond BI-RADS: Nonmass Abnormalities on Breast Ultrasound. Korean J. Radiol. 2024, 25, 134–145. [Google Scholar] [CrossRef]
- Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 27 March 2024).
- Lee, S.K.; Kim, S.W.; Yu, J.H.; Lee, J.E.; Kim, J.Y.; Woo, J.; Lee, S.; Kim, E.K.; Moon, H.G.; Ko, S.S.; et al. Is the high proportion of young age at breast cancer onset a unique feature of Asian breast cancer? Breast Cancer Res. Treat. 2019, 173, 189–199. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, Y.S.; Lee, S.E. Mammographic and Sonographic Features of Breast Cancer in Women Before 30 Years of Age. Curr. Med. Imaging 2020, 16, 1161–1169. [Google Scholar] [CrossRef]
- Hu, X.; Myers, K.S.; Oluyemi, E.T.; Philip, M.; Azizi, A.; Ambinder, E.B. Presentation and characteristics of breast cancer in young women under age 40. Breast Cancer Res. Treat. 2021, 186, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tian, B.; Xu, X.; Zhang, X.; Wang, Y.; Du, L.; Jing, J. Clinical features and prognostic factors of breast cancer in young women: A retrospective single-center study. Arch. Gynecol. Obstet. 2023, 307, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; Schoemaker, M.J.; Wright, L.B.; McGowan, C.; Brook, M.N.; McClain, K.M.; Jones, M.E.; Adami, H.O.; Agnoli, C.; Baglietto, L.; et al. The Premenopausal Breast Cancer Collaboration: A Pooling Project of Studies Participating in the National Cancer Institute Cohort Consortium. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Eric, I.; Petek Eric, A.; Kristek, J.; Koprivcic, I.; Babic, M. Breast Cancer in Young Women: Pathologic and Immunohistochemical Features. Acta Clin. Croat. 2018, 57, 497–502. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.H.; Lee, J.W.; Kim, S.; Choo, K.S. Magnetic resonance imaging characteristics of invasive breast cancer in women aged less than 35 years. Acta Radiol. 2015, 56, 924–932. [Google Scholar] [CrossRef]
- Dufour, O.; Houvenaeghel, G.; Classe, J.M.; Cohen, M.; Faure, C.; Mazouni, C.; Chauvet, M.P.; Jouve, E.; Darai, E.; Azuar, A.S.; et al. Early breast cancer in women aged 35 years or younger: A large national multicenter French population-based case control-matched analysis. Breast 2023, 68, 163–172. [Google Scholar] [CrossRef]
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer (Dove Med. Press) 2021, 13, 241–257. [Google Scholar] [CrossRef]
- Fredholm, H.; Magnusson, K.; Lindstrom, L.S.; Garmo, H.; Falt, S.E.; Lindman, H.; Bergh, J.; Holmberg, L.; Ponten, F.; Frisell, J.; et al. Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Res. Treat. 2016, 160, 131–143. [Google Scholar] [CrossRef]
- Choi, S.B.; Park, J.M.; Ahn, J.H.; Go, J.; Kim, J.; Park, H.S.; Kim, S.I.; Park, B.W.; Park, S. Ki-67 and breast cancer prognosis: Does it matter if Ki-67 level is examined using preoperative biopsy or postoperative specimen? Breast Cancer Res. Treat. 2022, 192, 343–352. [Google Scholar] [CrossRef]
- Bullier, B.; MacGrogan, G.; Bonnefoi, H.; Hurtevent-Labrot, G.; Lhomme, E.; Brouste, V.; Boisserie-Lacroix, M. Imaging features of sporadic breast cancer in women under 40 years old: 97 cases. Eur. Radiol. 2013, 23, 3237–3245. [Google Scholar] [CrossRef]
- Bitencourt, A.G.V.; Eugenio, D.S.G.; Souza, J.A.; Souza, J.O.; Makdissi, F.B.A.; Marques, E.F.; Chojniak, R. Prognostic significance of preoperative MRI findings in young patients with breast cancer. Sci. Rep. 2019, 9, 3106. [Google Scholar] [CrossRef] [PubMed]
- Breast SEER 5-Year Relative Survival Rates, 2014–2020. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 30 March 2024).
- Larson, K.E.; Grobmyer, S.R.; Valente, S.A. Evaluation of recurrence patterns and survival in modern series of young women with breast cancer. Breast J. 2018, 24, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.R.; Choi, W.J.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Chae, E.Y.; Yoon, G.Y. Prognostic factors predicting recurrence in invasive breast cancer: An analysis of radiological and clinicopathological factors. Asian J. Surg. 2019, 42, 613–620. [Google Scholar] [CrossRef]
- Park, A.R.; Chae, E.Y.; Kim, H.J.; Cha, J.H.; Shin, H.J.; Choi, W.J.; Kim, H.H. Effects of Preoperative Breast MRI on Breast Cancer Survival Outcomes in Women Aged 35 Years and Younger. Radiology 2023, 307, e221797. [Google Scholar] [CrossRef] [PubMed]
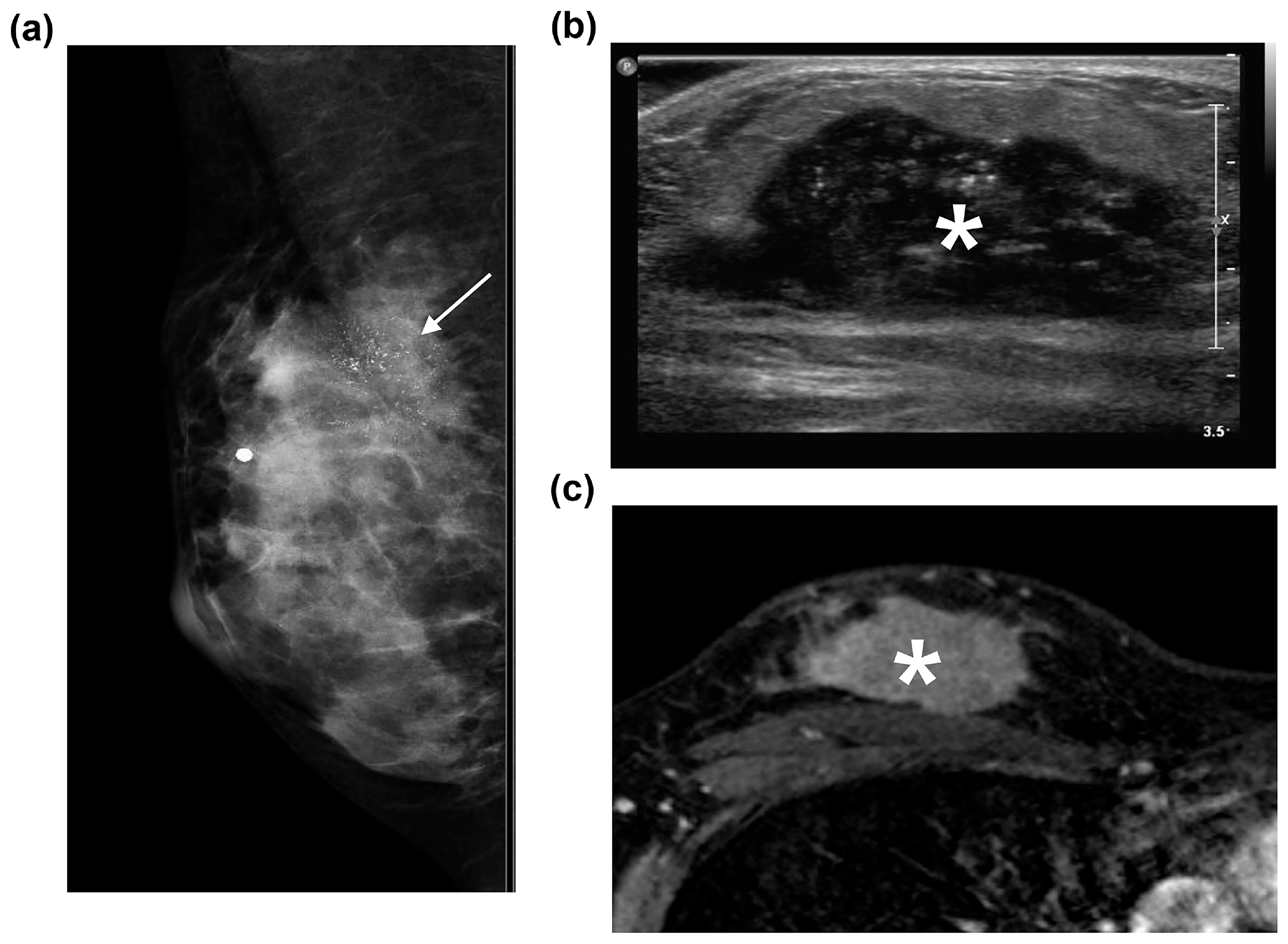

| Characteristics | Value |
|---|---|
| Age (years) | 27.1 ± 1.9 |
| Body mass index (kg/m2) | 21.4 ± 3.1 |
| With family history | 19/132 (14.4%) |
| BRCA mutation test a | |
| Positive | 11/54 (20.4%) |
| BRCA1 | 6/54 (11.1%) |
| BRCA2 | 5/54 (9.3%) |
| Negative | 43/54 (79.6%) |
| Distant metastasis at the time of diagnosis b | 2/132 (1.5%) |
| pCR after neoadjuvant chemotherapy a | 4/27 (14.8%) |
| Method of surgery | |
| Breast-conserving surgery b | 99/132 (75.0%) |
| Without ALND | 60/99 (60.6%) |
| With ALND b | 39/99 (39.4%) |
| Total mastectomy | 33/132 (25.0%) |
| Without ALND | 16/33 (48.5%) |
| With ALND | 17/33 (51.5%) |
| Additional therapy after surgery | |
| Radiation therapy c | 101/132 (76.5%) |
| Chemotherapy | 75/132 (56.8%) |
| Hormone therapy b | 91/132 (68.9%) |
| Recurrence after surgery | |
| Local c | 22/132 (16.7%) |
| Metachronous contralateral breast | 7/132 (5.3%) |
| Distant metastasis b | 21/132 (15.9%) |
| None | 93/132 (70.5%) |
| Survival | |
| Death b | 17/132 (12.9%) |
| Survived | 115/132 (87.1%) |
| Variables | Value |
|---|---|
| Histopathological result a | |
| Invasive ductal carcinoma, no special type b,d | 105/132 (79.5%) |
| Ductal carcinoma in situ | 12/132 (9.1%) |
| Mucinous carcinoma c | 7/132 (5.3%) |
| Metaplastic carcinoma b | 4/132 (3%) |
| Papillary carcinoma c | 2/132 (1.5%) |
| Invasive lobular carcinoma | 2/132 (1.5%) |
| Medullary carcinoma | 1/132 (0.8%) |
| Secretary carcinoma | 1/132 (0.8%) |
| With multiplicity e | 29/132 (22%) |
| With lymphovascular invasion e,f | 53/121 (43.8%) |
| With extensive intraductal component f | 39/111 (35.1%) |
| With nipple–areolar complex involvement | 9/132 (6.8%) |
| Histologic grade of invasive cancer f | |
| Well | 24/109 (22.0%) |
| Moderate e | 46/109 (42.2%) |
| Poor e | 39/109 (35.8%) |
| Surgical staging | |
| Stage 0 | 12/103 (11.7%) |
| Stage 1 | 40/103 (38.8%) |
| Stage 2 | 44/103 (42.7%) |
| Stage 3–4 | 7/103 (6.8%) |
| 29 lesions with chemotherapy before surgery | |
| yp 0 | 7/29 (24.1%) |
| yp 1 | 7/29 (24.1%) |
| yp 2 | 10/29 (34.5%) |
| yp 3–4 d | 5/29 (17.2%) |
| Molecular subtype | |
| HR+/HER2− d | 79/132 (59.8%) |
| HR+/HER2+ | 12/132 (9.1%) |
| HR−/HER2+ | 4/132 (3%) |
| Tripe-negative | 37/132 (28%) |
| Ki-67 (%) | 39.4 ± 29.7 |
| Low, ≤20% e | 50/132 (37.9%) |
| High, >20% e | 82/132 (62.1%) |
| Variables | Value |
|---|---|
| Mammographic breast density | |
| Fatty breast | 3/111 (2.7%) |
| Dense breast a | 108/111 (97.3%) |
| Lesion type | |
| Negative | 11/111 (9.9%) |
| Mass | 34/111 (30.6%) |
| Mass and calcifications a | 44/111 (39.6%) |
| Calcifications only | 12/111 (10.8%) |
| Asymmetry | 8/111 (7.2%) |
| Asymmetry with calcifications | 2/111 (1.8%) |
| Mass (n = 78) a | |
| Shape | |
| Oval/round a | 20/78 (25.6%) |
| Irregular | 58/78 (74.4%) |
| Margin | |
| Circumscribed | 8/78 (10.3%) |
| Not circumscribed a | 70/78 (89.7%) |
| Density | |
| Hyper b | 26/78 (33.3%) |
| Iso b | 52/78 (66.7%) |
| Hypo | |
| Calcifications (n = 58) | |
| Distribution | |
| Segmental b | 22/58 (37.9%) |
| Grouped b | 18/58 (31.0%) |
| Regional | 16/58 (27.6%) |
| Diffuse | 2/58 (3.5%) |
| Shape | |
| Fine linear/pleomorphic b | 35/58 (60.3%) |
| Coarse heterogenous | 5/58 (8.6%) |
| Amorphous b | 18/58 (31.0%) |
| Combined architectural distortion a,c | 25/100 (25.0%) |
| Variables | Value |
|---|---|
| Background echotexture | |
| Homogenous-fatty | - |
| Homogenous-fibroglandular a | 94/112 (83.9%) |
| Heterogenous | 18/112 (16.1%) |
| Lesion type | |
| Negative | 3/112 (2.7%) |
| Mass a | 90/112 (80.4%) |
| Non-mass | 19/112 (17.0%) |
| Mass (n = 90) a | |
| Shape | |
| Oval/round b | 39/90 (44.3%) |
| Irregular b | 51/90 (56.7%) |
| Orientation | |
| Parallel a | 69/90 (76.7%) |
| Nonparallel | 21/90 (23.3%) |
| Margin | |
| Circumscribed | 6/90 (6.7%) |
| Not circumscribed a | 84/90 (93.3%) |
| Echogenicity | |
| Hypoechoic a | 80/90 (88.9%) |
| Isoechoic | 1/90 (1.1%) |
| Hyperechoic | 1/90 (1.1%) |
| Complexed cystic and solid | 5/90 (5.6%) |
| Heterogeneous | 3/90 (3.3%) |
| Echogenic rind b | 19/90 (21.1%) |
| Non-mass (n = 19) | |
| Distribution | |
| Focal | 2/19 (10.5%) |
| Linear/segmental | 13/19 (68.4%) |
| Regional/diffuse | 4/19 (21.1%) |
| Echogenicity | |
| Hypoechoic | 18/19 (94.7%) |
| Isoechoic | 1/19 (5.3%) |
| Intralesional cysts | 3/19 (15.8%) |
| Calcifications in the lesion a,c | 59/109 (54.1%) |
| Architectural distortion b,c | 7/109 (6.4%) |
| Ductal change c | 17/109 (15.6%) |
| Posterior feature c | |
| No | 79/109 (72.5%) |
| Enhancement | 20/109 (18.3%) |
| Shadowing | 10/109 (9.2%) |
| Doppler study (n = 50) d | |
| Avascular | 14/50 (28.0%) |
| Mild b | 20/50 (40.0%) |
| Hypervascular | 16/50 (32.0%) |
| Variables | Value |
|---|---|
| Background parenchymal enhancement | |
| Minimal to mild a | 68/109 (62.4%) |
| Moderate to marked a | 41/109 (37.6%) |
| Lesion type | |
| Mass b | 91/109 (83.5%) |
| Non-mass enhancement | 18/109 (16.5%) |
| Mass (n = 91) b | |
| Shape | |
| Oval/round a | 50/91 (55%) |
| Irregular a | 41/91 (45.1%) |
| Margin | |
| Circumscribed | 9/91 (9.9%) |
| Not circumscribed b | 82/91 (90.1%) |
| Enhancement pattern | |
| Homogenous | 26/91 (28.6%) |
| Heterogenous b | 65/91 (71.4%) |
| Rim enhancement a | 59/91 (64.8%) |
| T2 high signal intensity b | 18/91 (19.8%) |
| Non-mass enhancement (n = 18) | |
| Distribution | |
| Focal | - |
| Segmental | 10/18 (55.6%) |
| Regional | 1/18 (5.6%) |
| Diffuse | 7/18 (38.9%) |
| Enhancement pattern | |
| Homogenous | 7/18 (38.9%) |
| Heterogenous | 11/18 (61.1%) |
| Enhancement kinetics | |
| Persistent | 7/109 (6.4%) |
| Plateau | 23/109 (21.1%) |
| Washout b | 79/109 (72.5%) |
| Variables | Recurrence | Survival | |||||
|---|---|---|---|---|---|---|---|
| Non (n = 93) | Recurrence (n = 37) | p | Survive (n = 115) | Death (n = 15) | p | ||
| Median body mass index, kg/m2 | 20.33 | 20.93 | 0.352 a | 20.33 | 21.57 | 0.040 a | |
| BRCA gene mutation test (n = 54) b | 0.617 c | 0.046 c | |||||
| BRCA1 | 2/28 (7.1) | 4/28 (15.4) | 3/47 (6.4) | 3/7 (42.9) | |||
| BRCA2 | 3/28 (10.7) | 2/28 (7.7) | 5/47 (10.6) | - | |||
| Negative | 23/28 (82.1) | 20/28 (76.9) | 39/47 (83.0) | 4/7 (57.1) | |||
| With preoperative NAC (n = 27) | 18/93 (19.4) | 9/37 (24.3) | 0.513 c | 20/115 (17.4) | 7/15 (46.7) | 0.016 c | |
| Total mastectomy (n = 33) | 0.805 | 0.044 c | |||||
| Without ALND | 11/22 (50.0) | 5/11 (45.5) | 16/28 (57.1) | - | |||
| With ALND | 11/22 (50.0) | 6/11 (54.6) | 12/28 (42.9) | 5/5 (100.0) | |||
| Additional hormone therapy after surgery | 68/93 (73.1) | 21/37 (56.8) | 0.109 | 83/115 (72.2) | 6/15 (40.0) | 0.018 | |
| Recurrence | 24/115 (20.9) | 13/15 (86.7) | <0.001 c | ||||
| Local | 10/115 (8.7) | 9/15 (69.2) | 0.209 c | ||||
| Metachronous contralateral breast | 7/115 (6.1) | - | 0.038 c | ||||
| Distant metastasis | 8/115 (7.0) | 11/15 (73.3) | 0.008 | ||||
| Death (n = 15) | 2/93 (2.2) | 13/37 (35.1) | <0.001 c | ||||
| Mean tumor size (cm) | 3.40 ± 2.25 | 3.25 ± 2.04 | 0.728 d | 3.19 ± 2.04 | 4.63 ± 2.86 | 0.016 d | |
| Histologic grade of invasive b | 0.195 | 0.018 | |||||
| Well differentiated | 20/73 (27.4) | 4/34 (11.8) | 24/93 (25.8) | - | |||
| Moderately differentiated | 29/73 (39.7) | 16/34 (47.1) | 40/93 (43.0) | 5/14 (35.7) | |||
| Poorly differentiated | 24/73 (32.9) | 14/34 (41.2) | 29/93 (31.2) | 9/14 (64.3) | |||
| Positive progesterone receptor | 62/93 (66.7) | 20/37 (54.1) | 0.253 | 79/115 (68.7) | 3/15 (20.0) | 0.001 | |
| Ki-67 | 36.3 ± 29.2 | 63.3 ± 21.4 | 0.001 d | ||||
| ≤20% | 40/93 (43.0) | 9/37 (24.3) | 0.075 c | 49/115 (42.6) | - | 0.001 c | |
| >20% | 53/93 (57.0) | 28/37 (75.7) | 66/115 (57.4) | 15/15 (100.0) | |||
| Ultrasound | |||||||
| Mass—no echogenic rind (n = 88) | 44/61 (40.0) | 26/27 (96.3) | 0.009 c | 60/77 (77.9) | 10/11 (90.9) | 0.448 c | |
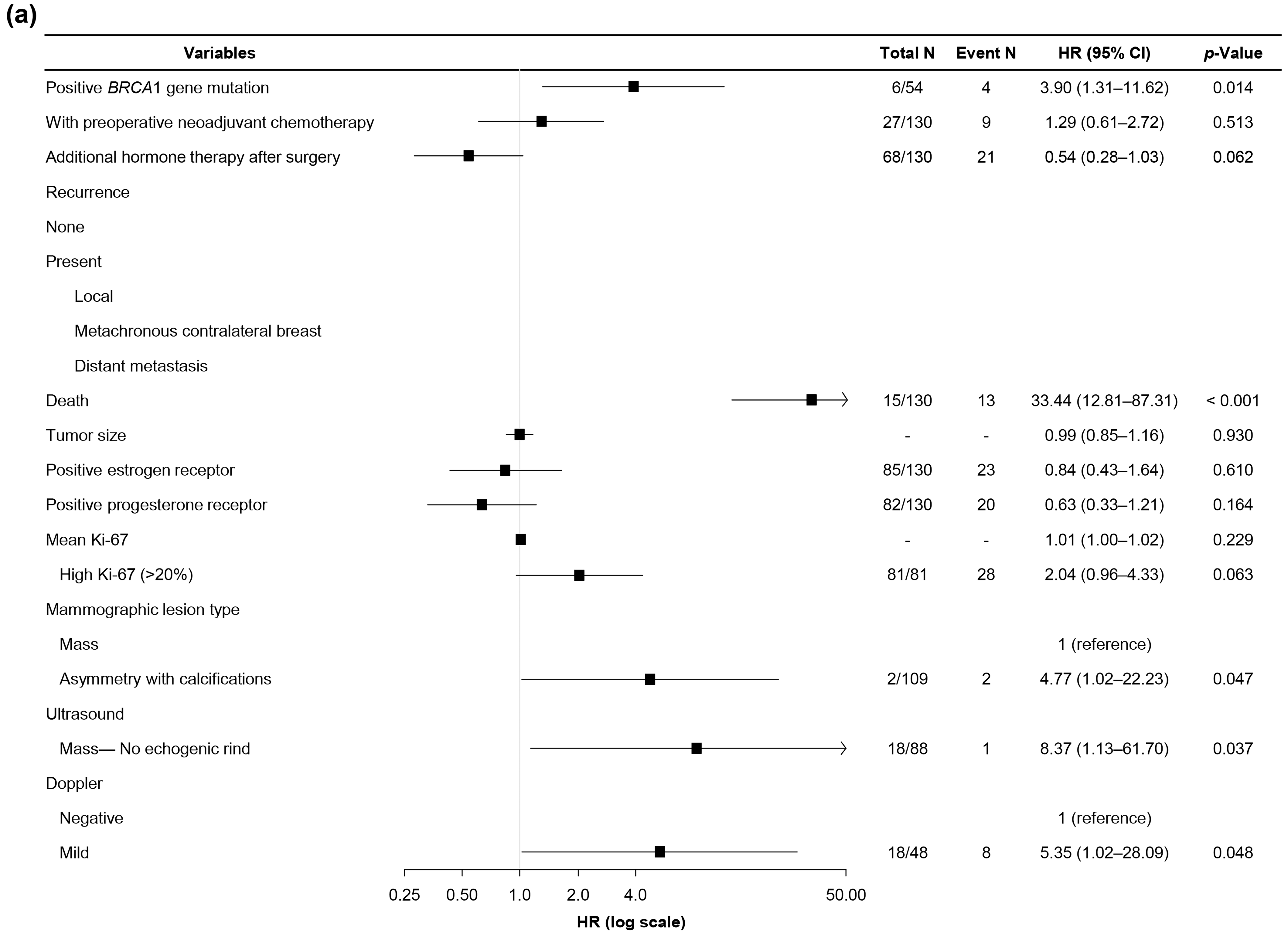
| Variables | DFS | OS | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | ||
| Positive BRCA1 gene mutation | 3.90 | 1.31–11.62 | 0.014 | 10.09 | 2.20–46.21 | 0.003 | |
| With preoperative neoadjuvant chemotherapy | 1.29 | 0.61–2.72 | 0.513 | 3.62 | 1.31– 10.00 | 0.013 | |
| Additional hormone therapy after surgery | 0.54 | 0.28–1.03 | 0.062 | 0.28 | 0.10–0.78 | 0.015 | |
| Recurrence | |||||||
| None | - | 1 (reference) | |||||
| Present | 18.58 | 4.19–82.40 | <0.001 | ||||
| Local | 19.73 | 6.26–62.21 | <0.001 | ||||
| Metachronous contralateral breast | N/A | - | |||||
| Distant metastasis | 40.42 | 8.92–183.12 | <0.001 | ||||
| Death | 33.44 | 12.81–87.31 | <0.001 | - | |||
| Tumor size | 0.99 | 0.85–1.16 | 0.930 | 1.23 | 1.04–1.45 | 0.017 | |
| Positive estrogen receptor | 0.84 | 0.43–1.64 | 0.610 | 0.33 | 0.12–0.94 | 0.038 | |
| Positive progesterone receptor | 0.63 | 0.33–1.21 | 0.164 | 0.13 | 0.04–0.48 | 0.002 | |
| Mean Ki-67 | 1.01 | 1.00–1.02 | 0.229 | 1.03 | 1.01–1.05 | 0.003 | |
| High Ki-67 (>20%) | 2.04 | 0.96–4.33 | 0.063 | N/A | |||
| Mammographic lesion type | |||||||
| Mass | 1 (reference) | 1 (reference) | |||||
| Asymmetry with calcifications | 4.77 | 1.02–22.23 | 0.047 | 4.58 | 0.51–41.12 | 0.174 | |
| Ultrasound | |||||||
| Mass—no echogenic rind | 8.37 | 1.13–61.70 | 0.037 | 2.75 | 0.35–21.48 | 0.335 | |
| Doppler | |||||||
| Negative | 1 (reference) | 1 (reference) | |||||
| Mild | 5.35 | 1.02–28.09 | 0.048 | N/A | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, I.; Ko, E.Y.; Lee, J.E.; Han, B.-K.; Ko, E.S.; Choi, J.S.; Kim, H.; Kim, M.K.; Lee, M.Y.; Moon, S.; et al. Prognosis of Breast Cancer in Women in Their 20s: Clinical and Radiological Insights. Diagnostics 2025, 15, 2072. https://doi.org/10.3390/diagnostics15162072
Youn I, Ko EY, Lee JE, Han B-K, Ko ES, Choi JS, Kim H, Kim MK, Lee MY, Moon S, et al. Prognosis of Breast Cancer in Women in Their 20s: Clinical and Radiological Insights. Diagnostics. 2025; 15(16):2072. https://doi.org/10.3390/diagnostics15162072
Chicago/Turabian StyleYoun, Inyoung, Eun Young Ko, Jeong Eon Lee, Boo-Kyung Han, Eun Sook Ko, Ji Soo Choi, Haejung Kim, Myoung Kyoung Kim, Mi Yeon Lee, Suhyeon Moon, and et al. 2025. "Prognosis of Breast Cancer in Women in Their 20s: Clinical and Radiological Insights" Diagnostics 15, no. 16: 2072. https://doi.org/10.3390/diagnostics15162072
APA StyleYoun, I., Ko, E. Y., Lee, J. E., Han, B.-K., Ko, E. S., Choi, J. S., Kim, H., Kim, M. K., Lee, M. Y., Moon, S., & Kwon, M.-r. (2025). Prognosis of Breast Cancer in Women in Their 20s: Clinical and Radiological Insights. Diagnostics, 15(16), 2072. https://doi.org/10.3390/diagnostics15162072







