Diagnosis and Surgical Treatment of Hydatidiform Mole
Abstract
1. Introduction
2. Diagnosis of Hydatidiform Mole
2.1. Clinical Presentation
2.2. Laboratory Tests
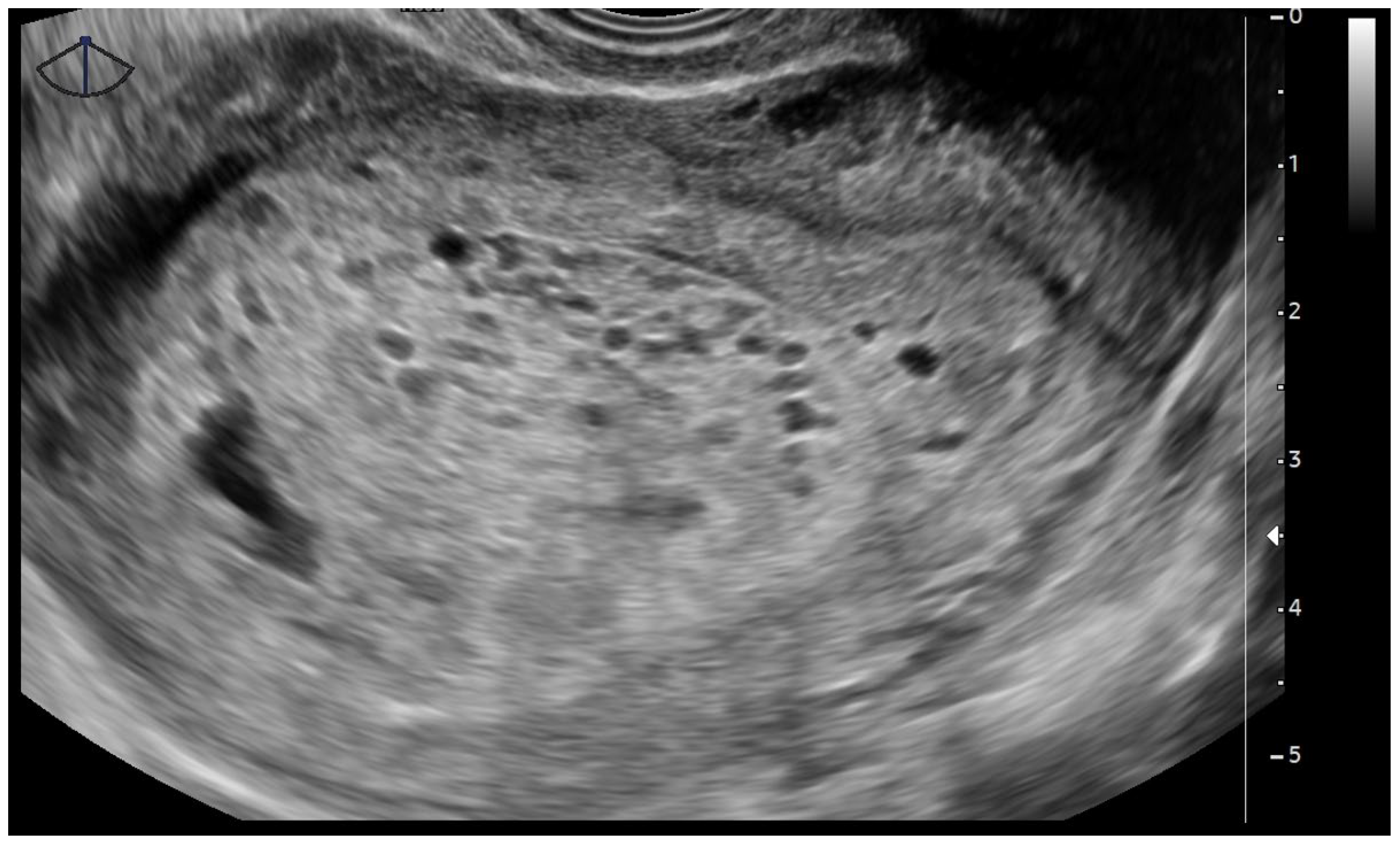
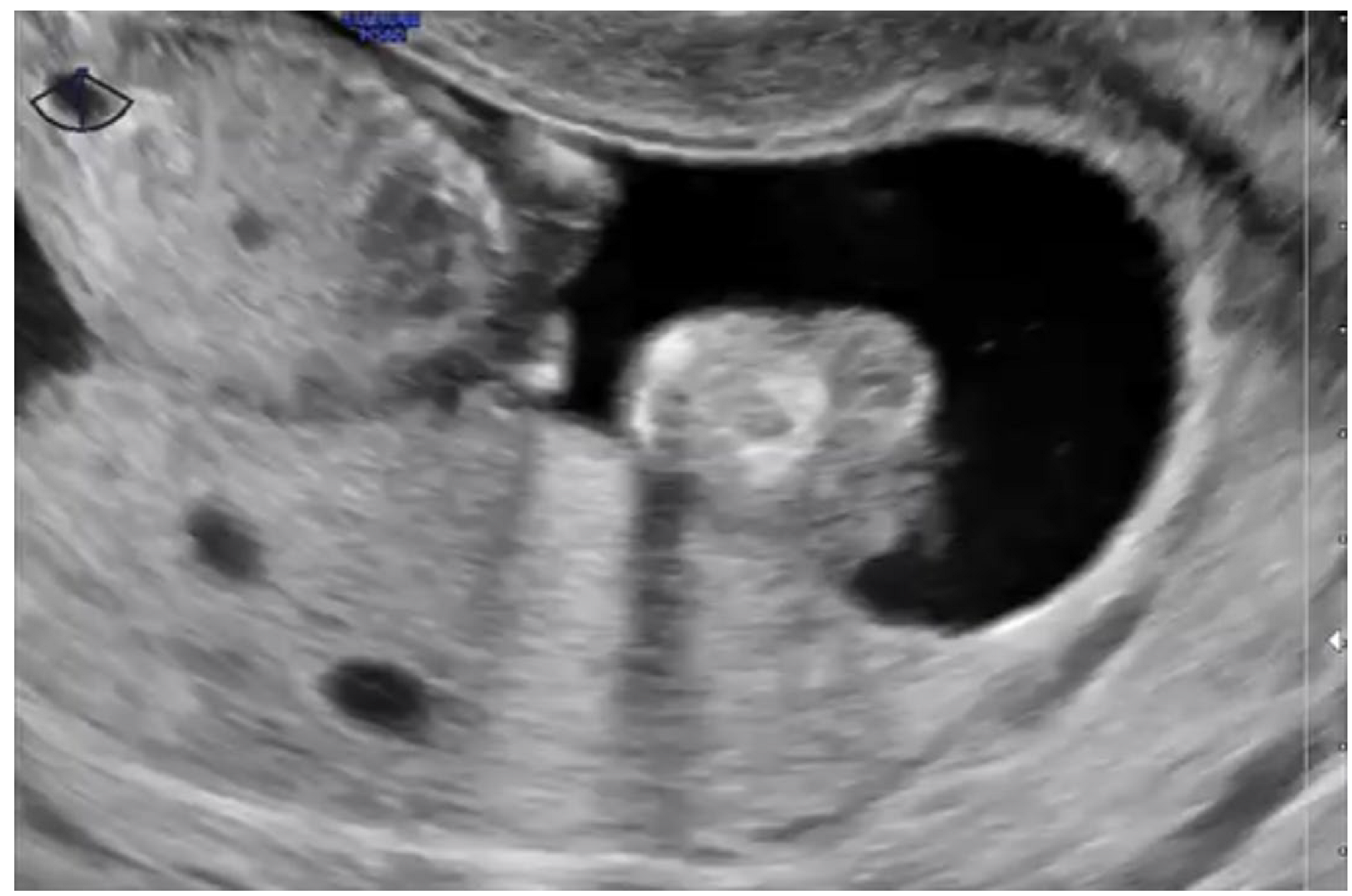
2.3. Pelvic Transvaginal Ultrasound
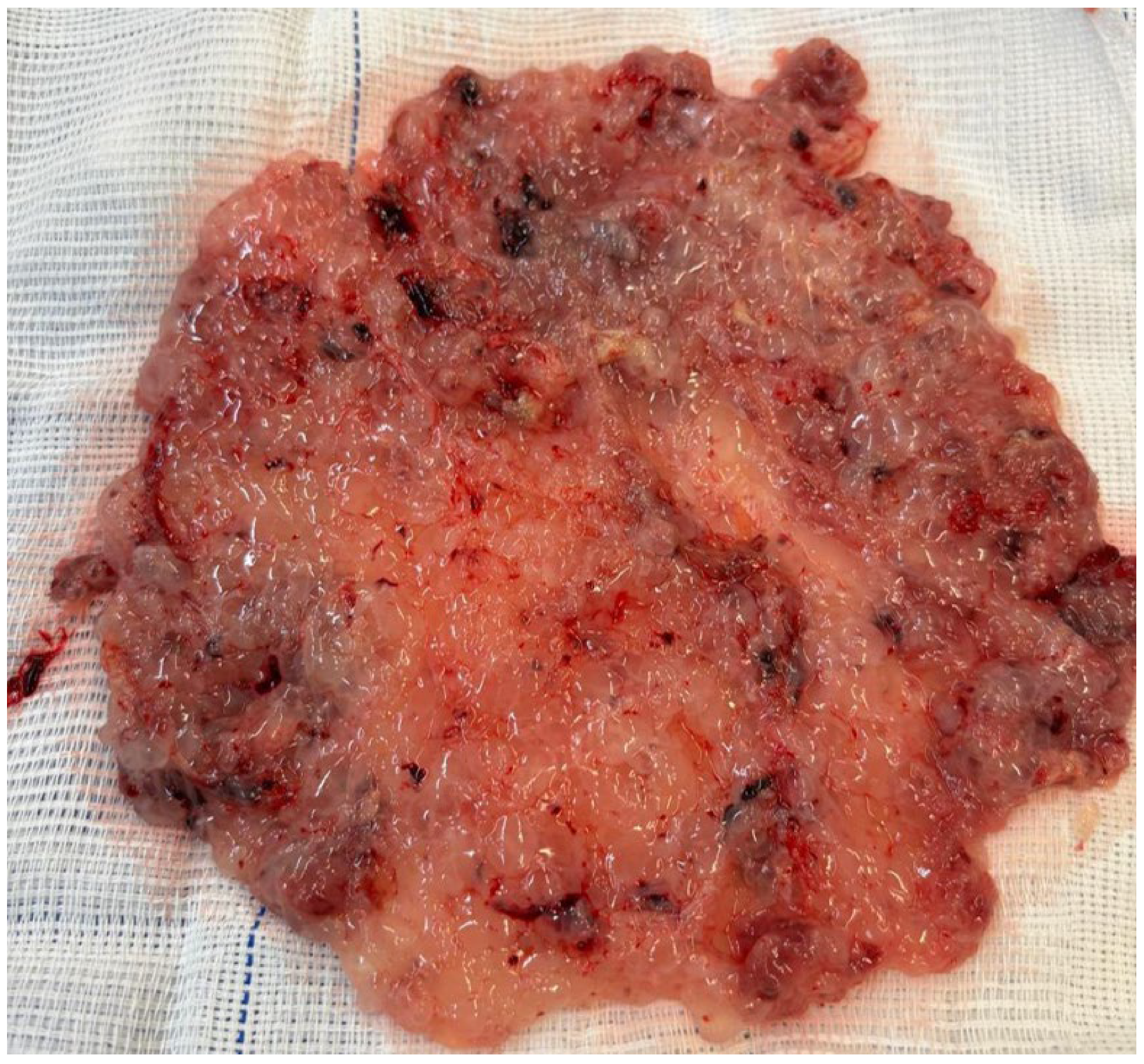
2.4. Histopathology
3. Uterine Evacuation for the Surgical Treatment of Hydatidiform Mole
3.1. Preoperative Evaluation
- ▪
- Coagulation profile (prothrombin time, activated partial thromboplastin time, international normalized ratio, and fibrinogen) to assess bleeding risk and coagulopathy (rare, but possible);
- ▪
- Liver function tests (alanine aminotransferase, aspartate aminotransferase, bilirubin) in cases of early preeclampsia or systemic illness;
- ▪
- Renal function tests (urea and creatinine), especially in patients with hypertension;
- ▪
- Thyroid function tests (thyroid-stimulating hormone and free thyroxine) to exclude hyperthyroidism and prevent a thyroid storm during anesthesia induction;
- ▪
- Fasting glucose for basic pre-surgical metabolic evaluation.
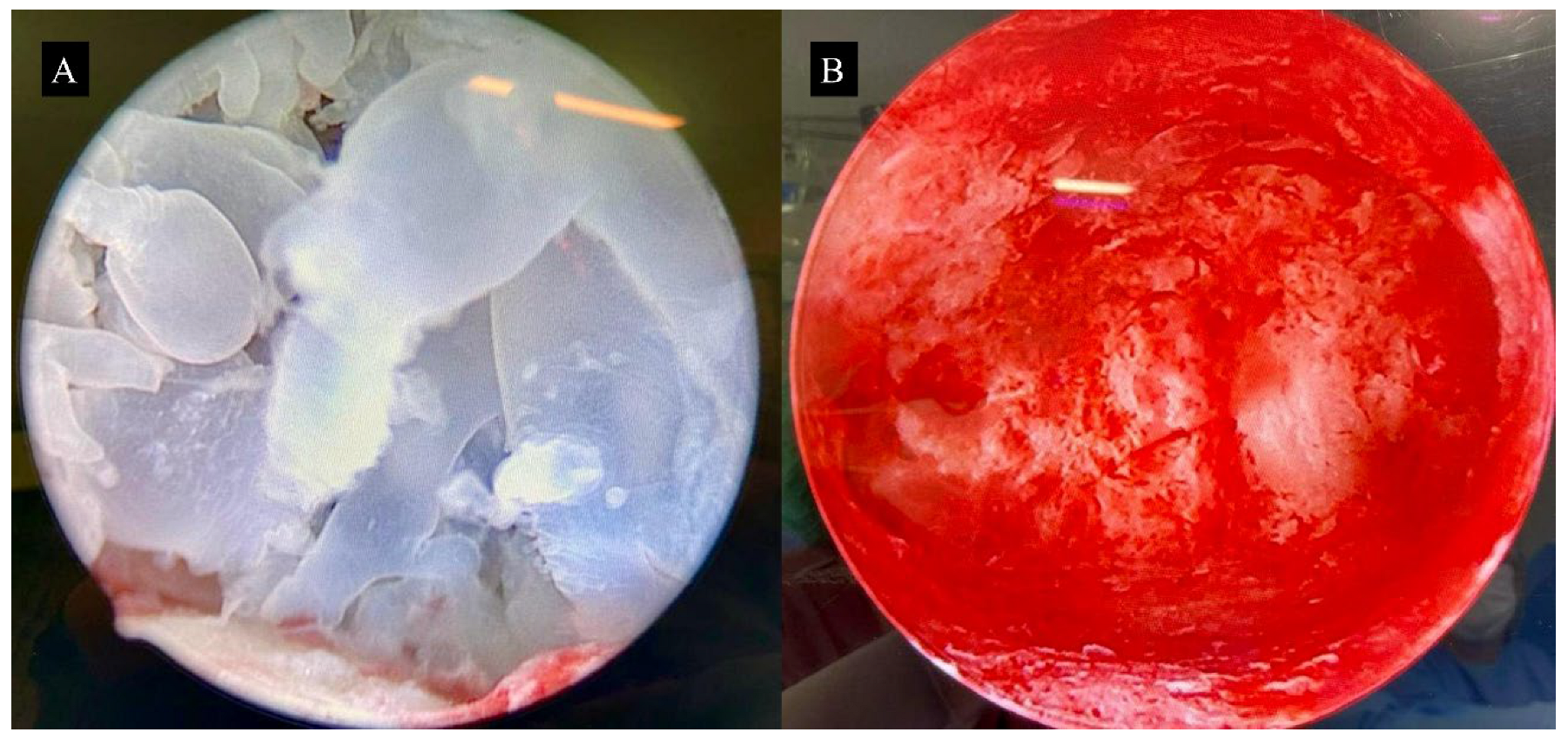
3.2. Surgical Technique for Molar Uterine Evacuation
4. Hysterectomy for the Surgical Treatment of Hydatidiform Mole
Surgical Technique for Hysterectomy
5. Surgical Interventions for Complications of Hydatidiform Mole
5.1. Management of Uterine Perforation
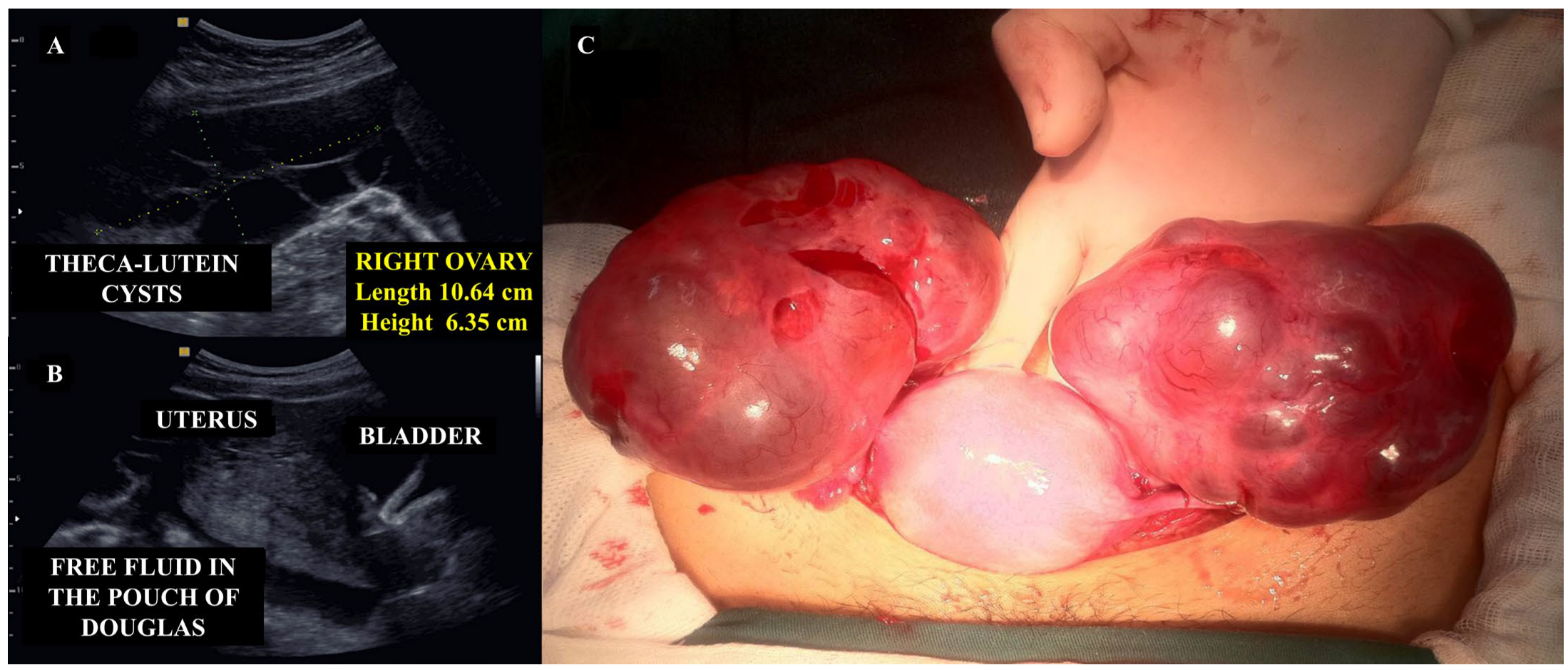
5.2. Management of Ruptured Theca Lutein Ovarian Cysts
5.3. Conservative Surgical Management of Uterine Hemorrhage
5.4. Management of Ectopic Hydatidiform Mole
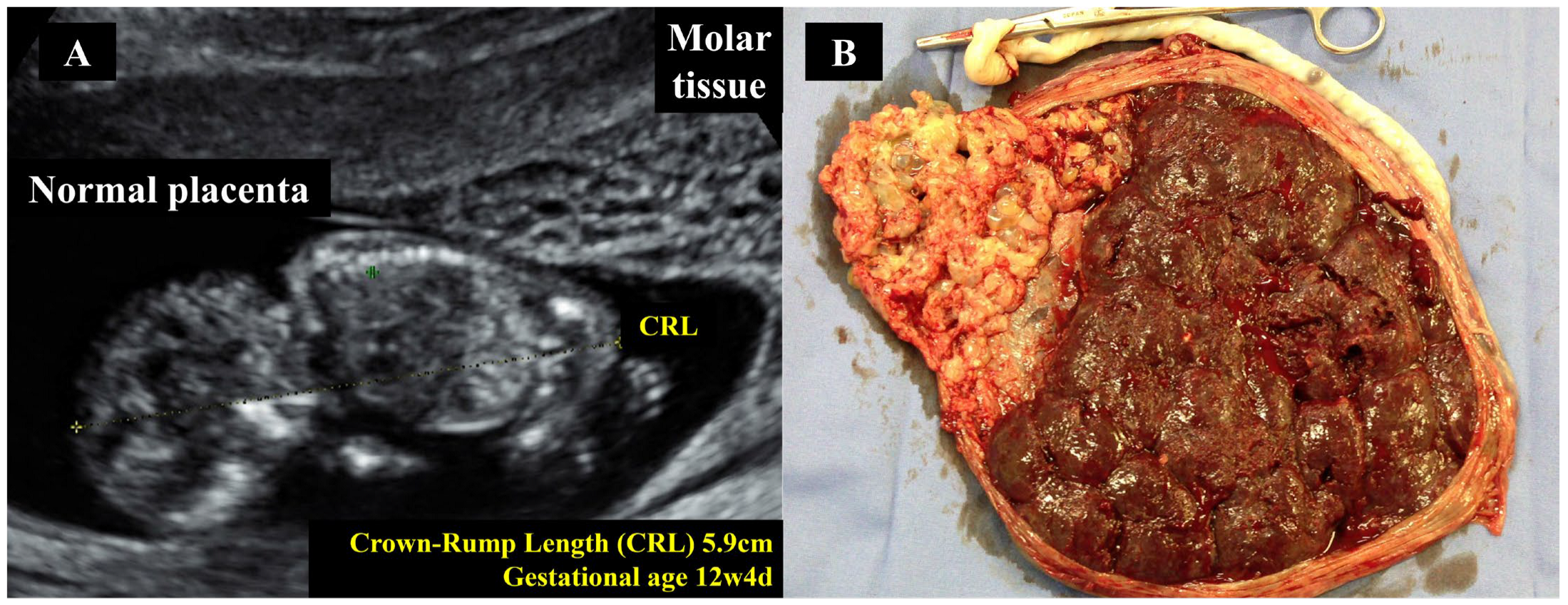
5.5. Cesarean Section in Twin Pregnancies with Coexisting Hydatidiform Mole
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. Gestational Trophoblastic Neoplasia, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 1374–1391. [Google Scholar] [CrossRef]
- Braga, A.; Berkowitz, R.; Horowitz, N. Etiology, Natural History, and Management of Recent Advances in Molar Pregnancy. Obstet. Gynecol. 2025. [Google Scholar] [CrossRef]
- Sun, S.Y.; Goldstein, D.P.; Bernstein, M.R.; Horowitz, N.S.; Mattar, R.; Maestá, I.; Braga, A.; Berkowitz, R.S. Maternal Near Miss According to World Health Organization Classification Among Women with a Hydatidiform Mole: Experience at the New England Trophoblastic Disease Center, 1994–2013. J. Reprod. Med. 2016, 61, 210–214. [Google Scholar]
- Joneborg, U. Epidemiology of Gestational Trophoblastic Disease. Hematol. Oncol. Clin. N. Am. 2024, 38, 1173–1190. [Google Scholar] [CrossRef]
- Hamid, M.; Joyce, C.M.; Carroll, H.K.; Kenneally, C.; Mulcahy, S.; O’Neill, M.K.; Coulter, J.; O’Reilly, S. Challenging gestational trophoblastic disease cases and mimics: An exemplar for the management of rare tumours. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 286, 76–84. [Google Scholar] [CrossRef]
- Lok, C.; van Trommel, N.; Massuger, L.; Golfier, F.; Seckl, M.; Abreu, M.H.; Attia, J.; Balanchandran, K.; Bergamini, A.; Bolze, P.A.; et al. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur. J. Cancer 2020, 130, 228–240. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, T.; Jiang, R.; Lu, X.; Du, Y. The causal relationship between hydatidiform mole and nutrients: A two-sample Mendelian randomization study. Clin. Nutr. ESPEN 2024, 64, 100–106. [Google Scholar] [CrossRef]
- Mangili, G.; Garavaglia, E.; Cavoretto, P.; Gentile, C.; Scarfone, G.; Rabaiotti, E. Clinical presentation of hydatidiform mole in northern Italy: Has it changed in the last 20 years? Am. J. Obstet. Gynecol. 2008, 198, 302.e1–302.e4. [Google Scholar] [CrossRef]
- Brown, J.; Naumann, R.W.; Seckl, M.J.; Schink, J. 15years of progress in gestational trophoblastic disease: Scoring, standardization, and salvage. Gynecol. Oncol. 2017, 144, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; García, Y.; Herrero, A.; Laínez, N.; Fuentes, J.; De Juan, A.; Rodriguez Freixinós, V.; Aparicio, J.; Casado, A.; García-Martinez, E. SEOM clinical guidelines in gestational trophoblastic disease (2017). Clin. Transl. Oncol. 2018, 20, 38–46. [Google Scholar] [CrossRef]
- Fallahian, M.; Sebire, N.J.; Savage, P.M.; Seckl, M.J.; Fisher, R.A. Mutations in NLRP7 and KHDC3L confer a complete hydatidiform mole phenotype on digynic triploid conceptions. Hum. Mutat. 2013, 34, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Descipio, C.; Wagenfuehr, J.; Tandy, S.; Mabray, J.; Beierl, K.; Micetich, K.; Libby, A.L.; Ronnett, B.M. Tetraploid partial hydatidiform mole: A case report and review of the literature. Int. J. Gynecol. Pathol. 2012, 31, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Buza, N. Gestational Trophoblastic Disease: Contemporary Diagnostic Approach. Surg. Pathol. Clin. 2022, 15, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, E.; Seckl, M.J. Future Directions for Gestational Trophoblastic Disease. Hematol. Oncol. Clin. N. Am. 2024, 38, 1265–1276. [Google Scholar] [CrossRef]
- Bolze, P.A.; Riedl, C.; Massardier, J.; Lotz, J.P.; You, B.; Schott, A.M.; Hajri, T.; Golfier, F. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am. J. Obstet. Gynecol. 2016, 214, 390.e1–390.e8. [Google Scholar] [CrossRef]
- Lok, C.; van Trommel, N.; Braicu, E.I.; Planchamp, F.; Berkowitz, R.; Seckl, M.; EOTTD-ESGO-GCIG-ISSTD Guideline Committee. Practical Guidelines for the Treatment of Gestational Trophoblastic Disease: Collaboration of the European Organisation for the Treatment of Trophoblastic Disease (EOTTD)-European Society of Gynaecologic Oncology (ESGO)-Gynecologic Cancer InterGroup (GCIG)-International Society for the Study of Trophoblastic Diseases (ISSTD). J. Clin. Oncol. 2025, 43, JCO2402326. [Google Scholar]
- Braga, A.; Paiva, G.; Barcellos, M.; Elias, K.M.; Horowitz, N.S.; Berkowitz, R.S. Diagnosis and Management of Molar Pregnancies. Hematol. Oncol. Clin. N. Am. 2024, 38, 1149–1159. [Google Scholar] [CrossRef]
- Ngan, H.Y.S.; Seckl, M.J.; Berkowitz, R.S.; Xiang, Y.; Golfier, F.; Sekharan, P.K.; Braga, A.; Garrett, A. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 86–93. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, Y.; Huang, W.; Tong, B.; Lu, W. Total hysterectomy versus uterine evacuation for preventing post-molar gestational trophoblastic neoplasia in patients who are at least 40 years old: A systematic review and meta-analysis. BMC Cancer 2019, 19, 13. [Google Scholar] [CrossRef]
- Usui, H.; Mikiya, A.; Katayama, E.; Nakamura, N.; Sato, A.; Matsui, H.; Shozu, M.; Koga, K. Total human chorionic gonadotropin is a more suitable diagnostic marker of gestational trophoblastic diseases than the free β-subunit of human chorionic gonadotropin. Pract. Lab. Med. 2023, 37, e00343. [Google Scholar] [CrossRef]
- Yeung, C.W.; Cheung, A.N.Y. Negative Pregnancy Test in Patients with Trophoblastic Diseases. Curr. Obstet. Gynecol. Rep. 2013, 3, 102–106. [Google Scholar] [CrossRef]
- Shearer, A.; Saso, S.; Stalder, C.; Jones, B. Rare complications of complete hydatidiform molar pregnancy: The ‘hook effect’ and thyrotoxicosis. BMJ Case Rep. 2024, 17, e259812. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, I.; Spacey, A.; Scragg, B.; Szczepura, K. The diagnostic value and accuracy of ultrasound in diagnosing hydatidiform mole: A systematic review and meta-analysis of the literature. Radiography 2022, 28, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.L.; Maturen, K.E.; Mowers, E.L.; Pasque, K.B.; Wasnik, A.P.; Dalton, V.K.; Bell, J.D. Sonographic diagnosis of partial versus complete molar pregnancy: A reappraisal. J. Clin. Ultrasound 2017, 45, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Seckl, M.J. Gestational trophoblastic disease: Current management of hydatidiform mole. BMJ 2008, 337, a1193. [Google Scholar] [CrossRef]
- Sebire, N.J.; Rees, H.; Paradinas, F.; Seckl, M.; Newlands, E. The diagnostic implications of routine ultrasound examination in histologically confirmed early molar pregnancies. Ultrasound Obstet. Gynecol. 2001, 18, 662–665. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, C.; Zhu, C.; Liang, F.; Lee, A.; Yao, X.; Chen, Q. The impact of pre-evacuation ultrasound examination in histologically confirmed hydatidiform mole in missed abortion. BMC Womens Health 2020, 20, 196. [Google Scholar] [CrossRef]
- Kaur, B. Pathology of Gestational Trophoblastic Disease (GTD). Hematol. Oncol. Clin. N. Am. 2024, 38, 1191–1217. [Google Scholar] [CrossRef]
- Madi, J.M.; Braga, A.; Paganella, M.P.; Litvin, I.E.; Wendland, E.M. Accuracy of p57KIP2 compared with genotyping to diagnose complete hydatidiform mole: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1226–1233. [Google Scholar] [CrossRef]
- Eiriksson, L.; Dean, E.; Sebastianelli, A.; Salvador, S.; Comeau, R.; Jang, J.H.; Bouchard-Fortier, G.; Osborne, R.; Sauthier, P. Guideline No. 408: Management of Gestational Trophoblastic Diseases. J. Obstet. Gynaecol. Can. 2021, 43, 91–105.e1. [Google Scholar] [CrossRef]
- Tidy, J.; Seckl, M.; Hancock, B.W.; on behalf of the Royal College of Obstetricians and Gynaecologists. Management of Gestational Trophoblastic Disease: Green-top Guideline No 38-June 2020. BJOG 2021, 128, e1–e27. [Google Scholar]
- Lurain, J.R. Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am. J. Obstet. Gynecol. 2010, 203, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.L.; Parente, R.C.; Maestá, I.; Amim Junior, J.; de Rezende Filho, J.F.; Montenegro, C.A.B.; Braga, A. Clinical and radiological correlations in patients with gestational trophoblastic disease. Radiol. Bras. 2016, 49, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Asmar, F.T.C.; Braga, A.; de Rezende-Filho, J.; Villas-Boas, J.M.S.; Charry, R.C.; Maesta, I. Uterine artery Doppler flow velocimetry parameters for predicting gestational trophoblastic neoplasia after complete hydatidiform mole, a prospective cohort study. Clinics 2017, 72, 284–288. [Google Scholar] [CrossRef]
- Braga, A.; Padrón, L.; Rezende-Filho, J.; Elias, K.; Horowitz, N.; Berkowitz, R. Treatment of hydatidiform mole using manual vacuum aspiration: Technical and tactical aspects. Int. J. Gynecol. Cancer 2021, 31, 1299–1300. [Google Scholar] [CrossRef]
- López, C.L.; Figueira Gouvêa, A.L.; Rodrigues, F.R.; Braga, A.; Valente Machado, M.D.; Lopes, V.S. Human epidermal growth factor receptor 2 fluorescence in situ hybridization and P57KIP2 immunohistochemistry using tissue microarray: Improving histopathological subtyping of hydatidiform mole. Placenta 2020, 99, 166–172. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Clinical Practice Update: Rh D Immune Globulin Administration After Abortion or Pregnancy Loss at Less Than 12 Weeks of Gestation. Obstet. Gynecol. 2024, 144, e140–e143. [Google Scholar] [CrossRef]
- Fung-Kee-Fung, K.; Wong, K.; Walsh, J.; Hamel, C.; Clarke, G. Guideline No. 448: Prevention of Rh D Alloimmunization. J. Obstet. Gynaecol. Can. 2024, 46, 102449. [Google Scholar] [CrossRef]
- Costa, H.L.; Doyle, P. Influence of oral contraceptives in the development of post-molar trophoblastic neoplasia--a systematic review. Gynecol. Oncol. 2006, 100, 579–585. [Google Scholar] [CrossRef]
- Hagey, J.M.; Drury, K.E.; Kaplan, S.; Davidson, B.A.; Morse, J.E. Contraceptive use following gestational trophoblastic disease: A systematic review. Contraception 2024, 137, 110488. [Google Scholar] [CrossRef]
- Sugrue, R.; Foley, O.; Elias, K.M.; Growdon, W.B.; Sisodia, R.M.C.; Berkowitz, R.S.; Horowitz, N.S. Outcomes of minimally invasive versus open abdominal hysterectomy in patients with gestational trophoblastic disease. Gynecol. Oncol. 2021, 160, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Committee on Gynecologic Practice. ACOG Committee Opinion No. 774 Summary: Opportunistic Salpingectomy as a Strategy for Epithelial Ovarian Cancer Prevention. Obstet. Gynecol. 2019, 133, 842–843. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wan, X.; Xie, X. A metastatic invasive mole arising from iatrogenic uterus perforation. BMC Cancer 2017, 17, 876. [Google Scholar] [CrossRef]
- Maheut, C.; Rollin, I.; Baissas, P.; Panel, P.; Niro, J. Management of uterine rupture during molar pregnancy. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102058. [Google Scholar] [CrossRef]
- Jiang, Q.; Yu, L.; Yang, L.; Wang, H. Laparoscopic management of an invasive mole combined with iatrogenic uterine perforation. J. Rad. Res. Appl. Sci. 2023, 16, 100582. [Google Scholar] [CrossRef]
- Susilo, S.A.; Homenta, C.; Mawardinata, P.; Sinaga, F.I.K.; Suardi, D. Surgical Approach for Uterine Perforation due to Gestational Trophoblastic Neoplasia: A Case Report. Indones. J. Obstet. Gynecol. Sci. 2025, 8, 163–168. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh, S.; Mollabashi, M. Fertility-sparing uterine lesion resection in a woman with hemoperitoneum due to invasive mole: A rare case report. Int. J. Surg. Case Rep. 2021, 84, 106117. [Google Scholar] [CrossRef]
- Lima, L.L.A.; Padron, L.; Câmara, R.; Sun, S.Y.; Rezende, J.; Filho Braga, A. The role of surgery in the management of women with gestational trophoblastic disease. Rev. Colégio Bras. Cir. 2017, 44, 94–101. [Google Scholar] [CrossRef]
- Saloni Potdar, J.; Dahiphale, S.M. A Complete Hydatidiform Mole Complicated by Theca Lutein Cysts in a Teenager: A Rare Case. Cureus 2024, 16, e52240. [Google Scholar] [CrossRef]
- Kurakula, S.; Muralidharan, V.; Appaneravanda, L.C.; Navya, N.; Gaathri, K.B. Rupture of Bilateral Theca Lutein Cysts During Pregnancy: A Case Report. Cureus 2022, 14, e29758. [Google Scholar] [CrossRef]
- Upadhyaya, G.; Goswami, A.; Babu, S. Bilateral theca lutein cysts: A rare cause of acute abdomen in pregnancy. Emerg. Med. Australas. 2004, 16, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Dasari, P.; Prabhu, K.; Chitra, T. Theca lutein cyst rupture—An unusual cause of acute abdomen: A case report. Internet J. Gynecol. Obstet. 2009, 13, 10–15. [Google Scholar]
- Marian Lobo, R.; Taha, M.; Jeremy Herod, J.; Al Ansari, A.; Syed, S.; Al Malik, H.; Farghaly, H. Management of acute haemorrhage following chemotherapy for invasive molar pregnancy by embolization and conservative fertility-sparing surgery. Gynecol. Oncol. Rep. 2020, 32, 100556. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Feng, M.; Cai, J.; Li, H. Case report: Conservative treatment for fertility preservation in a woman with hemoperitoneum due to an invasive mole. Front. Oncol. 2022, 12, 1019082. [Google Scholar] [CrossRef]
- López, C.L.; Lopes, V.G.S.; Resende, F.R.; Steim, J.L.; Padrón, L.; Sun, S.Y.; Júnior, E.A.; Braga, A. Gestational Trophoblastic Neoplasia after Ectopic Molar Pregnancy: Clinical, Diagnostic, and Therapeutic Aspects. Rev. Bras. Ginecol. Obstet. 2018, 40, 294–299. [Google Scholar] [CrossRef]
- Sebire, N.J.; Lindsay, I.; Fisher, R.A.; Savage, P.; Seckl, M.J. Overdiagnosis of complete and partial hydatidiform mole in tubal ectopic pregnancies. Int. J. Gynecol. Pathol. 2005, 24, 260–264. [Google Scholar] [CrossRef]
- Najib, F.S.; Bahrami, S.; Shiravani, Z.; Alavi, S.M.A. Choriocarcinoma in tubal pregnancy: A case report. Clin. Case Rep. 2023, 11, e7977. [Google Scholar] [CrossRef]
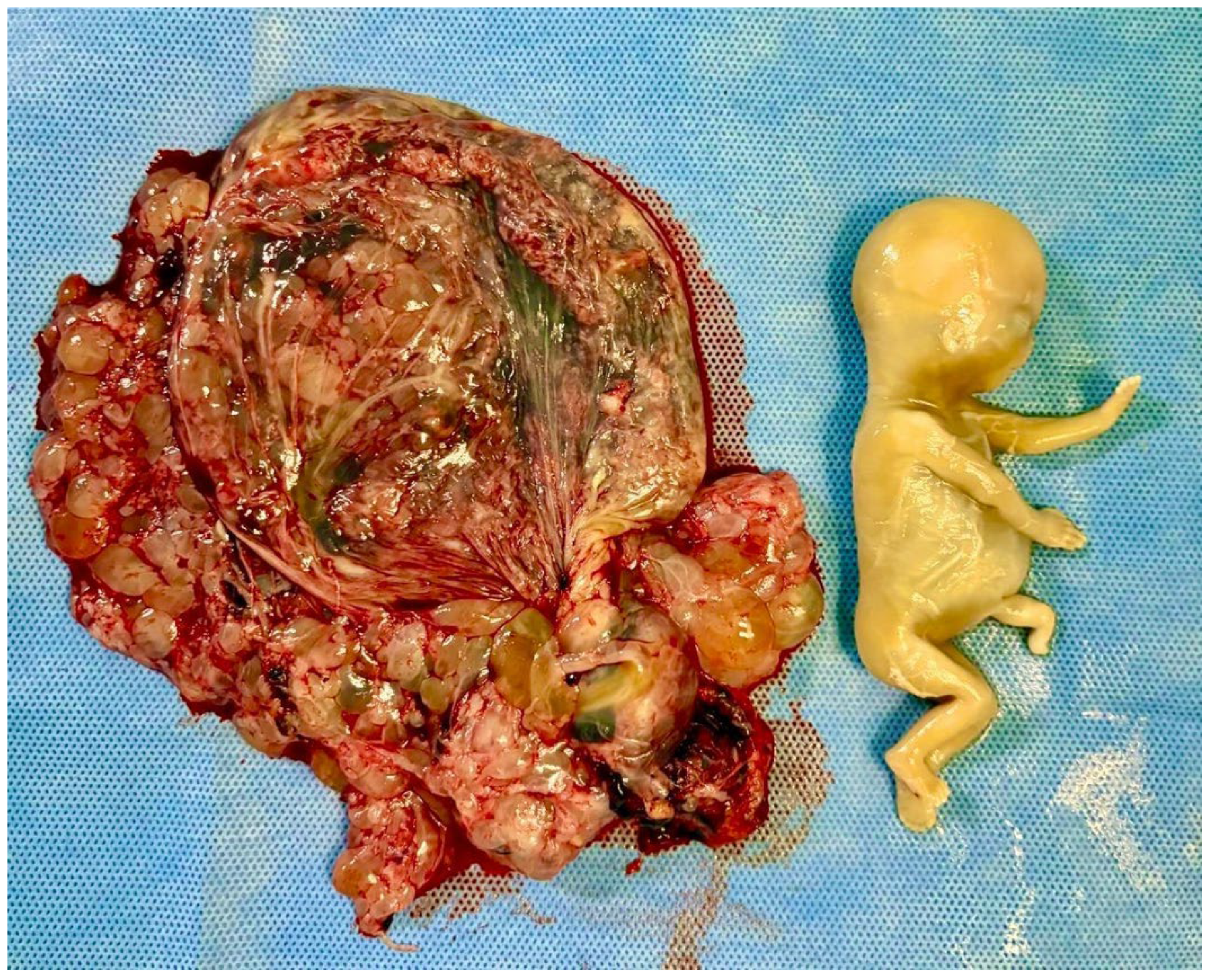
- Braga, A.; Obeica, B.; Werner, H.; Sun, S.Y.; Amim Júnior, J.; Filho, J.R.; Júnior, E.A. A twin pregnancy with a hydatidiform mole and a coexisting live fetus: Prenatal diagnosis, treatment, and follow-up. J. Ultrason. 2017, 17, 299–305. [Google Scholar] [CrossRef]
- Hajri, T.; Massoud, M.; Vergne, M.; Descargues, P.; Allias, F.; You, B.; Lotz, J.P.; Haesebaert, J.; Bolze, P.A.; Golfier, F.; et al. Multiple pregnancy with complete hydatidiform mole and coexisting normal fetus in a retrospective cohort of 141 patients. Am. J. Obstet. Gynecol. 2024, 230, 362.e1–362.e8. [Google Scholar] [CrossRef]
- Sebire, N.J.; Foskett, M.; Paradinas, F.J.; Fisher, R.A.; Francis, R.J.; Short, D.; Newlands, E.S.; Seckl, M.J. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet 2002, 359, 2165–2166. [Google Scholar] [CrossRef]
- Wang, G.; Cao, J.; Xu, X.; Han, X.; Cui, H. Delivery management of a complete hydatidiform mole and co-existing viable fetus: A meta-analysis and systematic review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102269. [Google Scholar] [CrossRef]
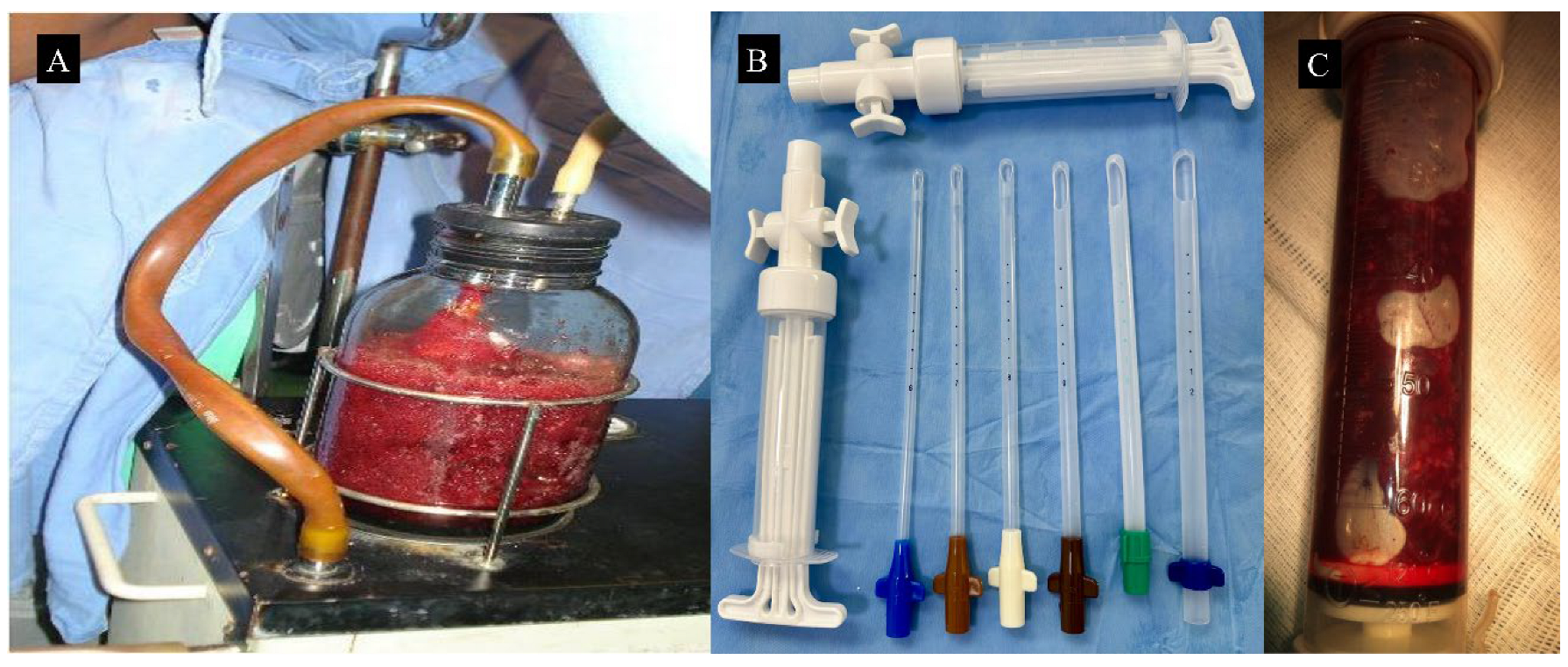

| Characteristic | Electric Vacuum Aspiration | Manual Vacuum Aspiration |
|---|---|---|
| Vacuum source | Electric pump | Manual plastic syringe |
| Negative pressure | Controlled by electric device (±600–700 mmHg) | Manually generated (±400–500 mmHg) |
| Aspiration control | Continuous and adjustable | More operator-dependent |
| Required equipment | Electric pump, cannulas, connection tubing | Sterilizable or disposable syringe, cannula |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, A.; Chagas, M.; Asrani, M.; Soares, J.P.; Sun, S.Y.; Araujo Júnior, E.; Mattar, R.; Amim Junior, J.; Rezende-Filho, J.; Horowitz, N.S.; et al. Diagnosis and Surgical Treatment of Hydatidiform Mole. Diagnostics 2025, 15, 2068. https://doi.org/10.3390/diagnostics15162068
Braga A, Chagas M, Asrani M, Soares JP, Sun SY, Araujo Júnior E, Mattar R, Amim Junior J, Rezende-Filho J, Horowitz NS, et al. Diagnosis and Surgical Treatment of Hydatidiform Mole. Diagnostics. 2025; 15(16):2068. https://doi.org/10.3390/diagnostics15162068
Chicago/Turabian StyleBraga, Antônio, Marcela Chagas, Manisha Asrani, Juliana Pereira Soares, Sue Yazaki Sun, Edward Araujo Júnior, Rosiane Mattar, Joffre Amim Junior, Jorge Rezende-Filho, Neil S. Horowitz, and et al. 2025. "Diagnosis and Surgical Treatment of Hydatidiform Mole" Diagnostics 15, no. 16: 2068. https://doi.org/10.3390/diagnostics15162068
APA StyleBraga, A., Chagas, M., Asrani, M., Soares, J. P., Sun, S. Y., Araujo Júnior, E., Mattar, R., Amim Junior, J., Rezende-Filho, J., Horowitz, N. S., & Berkowitz, R. S. (2025). Diagnosis and Surgical Treatment of Hydatidiform Mole. Diagnostics, 15(16), 2068. https://doi.org/10.3390/diagnostics15162068








