Use of Radiomics to Predict Adverse Outcomes in Patients with Pulmonary Embolism: A Scoping Review of an Unresolved Clinical Challenge
Abstract
1. Background
2. Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Article Selection
2.4. Data Extraction and Analysis
2.5. Assessment of Risk of Bias in the Studies
3. Results
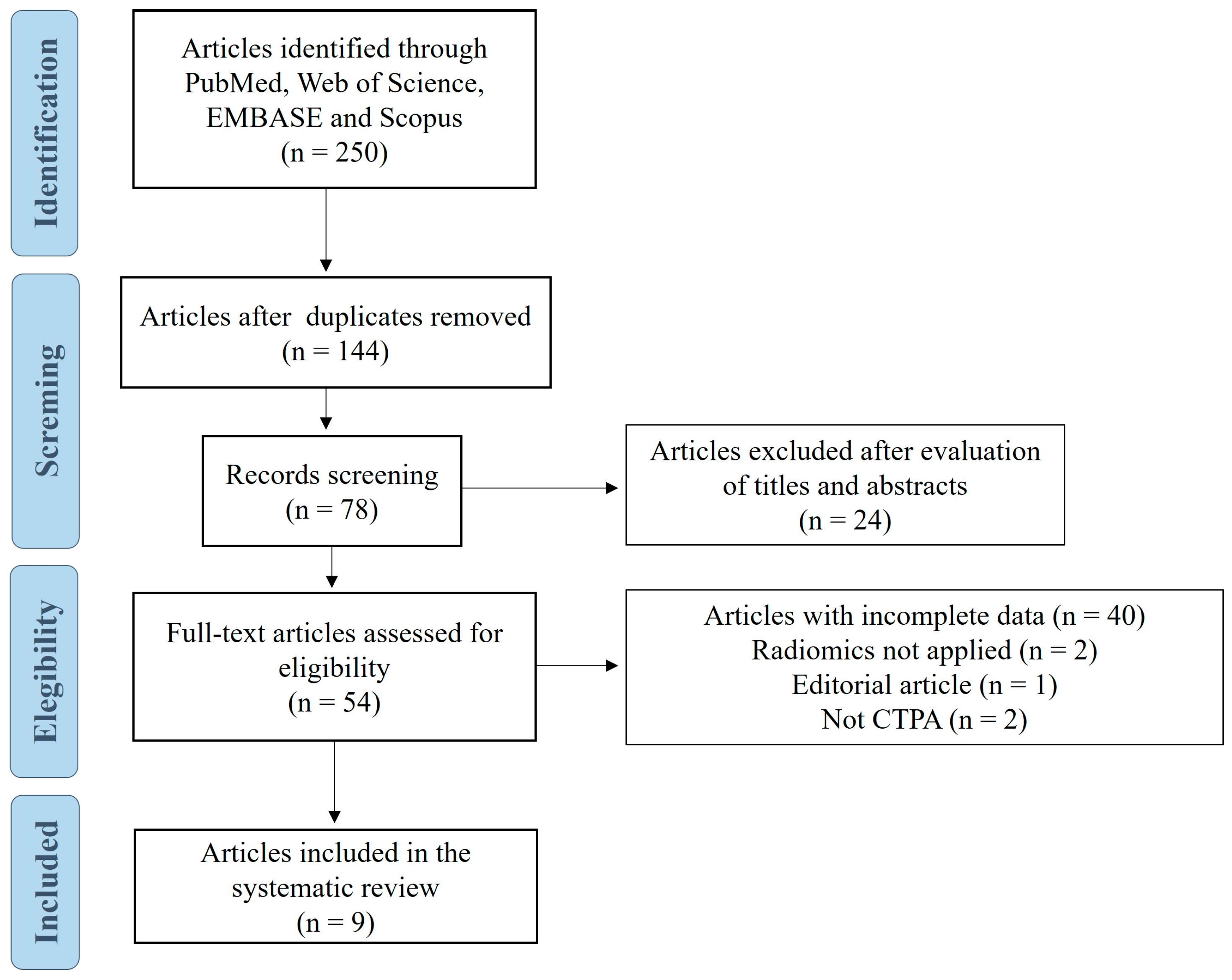
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| APE | Acute pulmonary embolism |
| AUC | Area under a receiver operating characteristic curve |
| CTPA | Computed tomography pulmonary angiography |
| ML | Machine learning |
| NOS | Newcastle–Ottawa Scale |
| NPV | Negative predictive value |
| OR | OR |
| PESI | Pulmonary Embolism Severity Index |
| sPESI | Simplified Pulmonary Embolism Severity Index |
| PPV | Positive predictive value |
| VTE | Venous thromboembolism |
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arter. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous Thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Gal, G.; Fine, M.J.; Roy, P.-M.; Sanchez, O.; Verschuren, F.; Cornuz, J.; Meyer, G.; Perrier, A.; Righini, M.; Aujesky, D.; et al. Prospective validation of the Pulmonary Embolism Severity Index. Thromb. Haemost. 2008, 100, 943–948. [Google Scholar] [CrossRef]
- Kohn, C.G.; Mearns, E.S.; Parker, M.W.; Hernandez, A.V.; Coleman, C.I. Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality. Chest 2015, 147, 1043–1062. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.-N.; Clarke, M. Prognostic models in acute pulmonary embolism: A systematic review and meta-analysis. BMJ Open 2016, 6, e010324. [Google Scholar] [CrossRef] [PubMed]
- Lankeit, M.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuss, G.; Pruszczyk, P.; Konstantinides, S. Predictive value of the high-sensitivity troponin T assay and the simplified Pulmonary Embolism Severity Index in hemodynamically stable patients with acute pulmonary embolism: A prospective validation study. Circulation 2011, 124, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Santagata, D.; Donadini, M.P.; Ageno, W. Use of artificial intelligence and radiomics for risk stratification in patients with pulmonary embolism: New tools for an old problem. Eur. J. Clin. Investig. 2024, 54, e14171. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Vandenbroucke, J.P.; Cevallos, M.; Renehan, A.G.; Altman, D.G.; Egger, M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019, 16, e1002742. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 1 January 2024).
- Zhou, X.; Hou, G. A radiomics nomogram based on CT pulmonary angiographic data for predicting adverse outcomes in non-high-risk acute pulmonary embolism patients. Eur. Respir. J. 2018, 52 (Suppl. S62), OA3596. [Google Scholar]
- Leonhardi, J.; Bailis, N.; Lerche, M.; Denecke, T.; Surov, A.; Meyer, H.-J. Computed Tomography Embolus Texture Analysis as a Prognostic Marker of Acute Pulmonary Embolism. Angiology 2023, 74, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, R.; Yang, Y.; Yang, Z.; Su, Y.; Ji, M.; Pang, Z.; Wang, D. Computed tomography-based radiomics model to predict adverse clinical outcomes in acute pulmonary embolism. J. Thromb. Thrombolysis 2024, 57, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Gotta, J.; Koch, V.; Geyer, T.; Martin, S.S.; Booz, C.; Mahmoudi, S.; Eichler, K.; Reschke, P.; D’ANgelo, T.; Klimek, K.; et al. Imaging-based risk stratification of patients with pulmonary embolism based on dual-energy CT-derived radiomics. Eur. J. Clin. Investig. 2024, 54, e14139. [Google Scholar] [CrossRef]
- Gotta, J.; Gruenewald, L.D.; Martin, S.S.; Booz, C.; Mahmoudi, S.; Eichler, K.; Gruber-Rouh, T.; Biciusca, T.; Reschke, P.; Juergens, L.-J.; et al. From pixels to prognosis: Imaging biomarkers for discrimination and outcome prediction of pulmonary embolism: Original Research Article. Emerg. Radiol. 2024, 31, 303–311. [Google Scholar] [CrossRef]
- Gotta, J.; Gruenewald, L.D.; Geyer, T.; Eichler, K.; Martin, S.S.; Mahmoudi, S.; Booz, C.; Biciusca, T.; Reschke, P.; Juergens, L.-J.; et al. Indicators for Hospitalization in Acute Pulmonary Embolism: Uncover the Association Between D-dimer Levels, Thrombus Volume and Radiomics. Acad. Radiol. 2024, 31, 2610–2619. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zwanenburg, A.; Frohwein, L.J.; Schramm, D.; Meyer, H.J.; Hinnerichs, M.; Moenninghoff, C.; Niehoff, J.H.; Kroeger, J.R.; Borggrefe, J.; et al. Short-term mortality prediction in acute pulmonary embolism: Radiomics values of skeletal muscle and intramuscular adipose tissue. J. Cachexia Sarcopenia Muscle 2024, 15, 1430–1440. [Google Scholar] [CrossRef]
- Surov, A.; Zimmermann, S.; Hinnerichs, M.; Meyer, H.-J.; Aghayev, A.; Borggrefe, J. Radiomics parameters of epicardial adipose tissue predict mortality in acute pulmonary embolism. Respir. Res. 2024, 25, 356. [Google Scholar] [CrossRef]
- Wang, D.; Chen, R.; Wang, W.; Yang, Y.; Yu, Y.; Liu, L.; Yang, F.; Cui, S. Prediction of short-term adverse clinical outcomes of acute pulmonary embolism using conventional machine learning and deep Learning based on CTPA images. J. Thromb. Thrombolysis 2025, 58, 331–339. [Google Scholar] [CrossRef]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Soffer, S.; Klang, E.; Shimon, O.; Barash, Y.; Cahan, N.; Greenspana, H.; Konen, E. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15814. [Google Scholar] [CrossRef]
- Su, H.; Han, Z.; Fu, Y.; Zhao, D.; Yu, F.; Heidari, A.A.; Zhang, Y.; Shou, Y.; Wu, P.; Chen, H.; et al. Detection of pulmonary embolism severity using clinical characteristics, hematological indices, and machine learning techniques. Front. Neurosci. 2022, 16, 1029690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; Ben, S.-Q.; Chen, H.-L.; Ni, S.-S. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: A meta-analysis. Respir. Res. 2012, 13, 111. [Google Scholar] [CrossRef] [PubMed]

| Pulmonary Embolism Severity Index (PESI) | ||
|---|---|---|
| Predictors | Points | |
| Age (years) | Age | |
| Male sex | +10 | |
| Cancer (previous or active) | +30 | |
| Heart failure | +10 | |
| Chronic lung disease | +10 | |
| Pulse ≥ 110 beats per minute | +20 | |
| Systolic blood pressure < 100 mmHg | +30 | |
| Respiratory rate ≥ 30 breaths per minute | +20 | |
| Temperature < 36 °C | +20 | |
| Altered mental status | +60 | |
| Arterial oxygen saturation < 90% | +20 | |
| Risk classes | Risk stratification | Risk of 30-day mortality |
| Class I (≤65 points) | Very low risk | 0.0–1.6% |
| Class II (66–85 points) | Low risk | 1.7–3.5% |
| Class III (86–105 points) | Intermediate risk | 3.2–7.1% |
| Class IV (106–125 points) | High risk | 4.0–11.4% |
| Class V (>125 points) | Very high risk | 10.0–24.5% |
| Simplified Pulmonary Embolism Severity Index (sPESI) | ||
| Predictors | Points | |
| Age > 80 years | +1 | |
| Cancer (previous or active) | +1 | |
| Chronic lung disease | +1 | |
| Pulse ≥ 110 beats per minute | +1 | |
| Systolic blood pressure < 100 mmHg | +1 | |
| Arterial oxygen saturation < 90% | +1 | |
| Risk classes | Risk stratification | Risk of 30-day mortality |
| 0 points | Low risk | 1.1% |
| ≥1 points | High risk | 8.9% |
| Author, Year | Target Population | Country | Study Design | Sample Size | Age, Mean ± SD | Female, n, % | Data Collection Period | Outcome PE Definition | Patients in the Training Set, n | Patients in the Validation Set, n |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al., 2018 [13] | Non-high- risk patients | China | Retrospective cohort | 285 | NR | NR | April 2013 to April 2017 | Adverse outcomes in non-high-risk APE patients | 170 | 115 |
| Leonhardi et al., 2023 [14] | APE patients | Germany | Retrospective cohort | 216 | 65 (17–99) * | 116 (53.7) | 2014 to 2019 | Mortality, ICU admission, and sepsis-related organ failure | NR | NR |
| Yang et al., 2024 [15] | APE patients | China | Retrospective cohort | 74 | 64.64 ± 10.73 | 34 (45.9) | December 2019 to August 2022 | Mortality occurring within 30 days following APE or the requirement for mechanical ventilation, cardiopulmonary resuscitation, thrombolysis, vasopressor therapy, or catheter intervention | 51 | 23 |
| Gotta et al., 2024 [16] | APE patients | Germany | Retrospective cohort | 131 (88 with central APE, 16 with peripheral APE, and 27 in the control group without APE) | 64 ± 15 | 55 (42.0) | January 2015 to March 2022 | Risk stratification and early death in APE | 62 | 42 |
| Gotta et al., 2024 [17] | APE patients | Germany | Retrospective cohort | 131 | 64 ± 15 | 55 (42.0) | January 2015 to March 2022 | Prediction of survival | 79 | 52 |
| Gotta et al., 2024 [18] | APE patients | Germany | Retrospective | 136 | 63 ± 15 | 54 (39.7) | January 2015 to March 2022 | Prediction of complicated courses requiring at least IMCU admission | 81 | 55 |
| Shahzadi et al., 2024 [19] | APE patients | Germany | Retrospective cohort | 829 | 65 * | 385 (46.4) | 2005 to 2021 | 7- and 30-day all-cause mortality | 580 | 249 |
| Surov et al., 2024 [20] | APE patients | Germany | Retrospective cohort | 284 | 64.5 ± 16.6 | 139 (48.9) | 2015 to 2021 | 7-day and 30-day all-cause mortality | 198 | 86 |
| Wang et al., 2025 [21] | APE Patients | China | Retrospective cohort | 321 | 64.2 ± 15.1 | 157 (48.9) | January 2015 to March 2022 | 30-day mortality and prolonged hospital stay (>10 days) | 224 | 97 |
| Author, Year | CT Scanner | Slice Thickness | Tube Voltage (kVp) | Contrast Protocol | CT Scanner Protocol |
|---|---|---|---|---|---|
| Zhou et al., 2018 [13] | NR | NR | NR | NR | NR |
| Leonhardi et al., 2023 [14] | SOMATOM Force (Siemens) | 1 mm | 90/Sn150 | Imeron 400, 80–100 mL at 5 mL/s, bolus tracking | SOMATOM Force (Siemens); DECT; 90/Sn150 kVp; 1 mm; Bv40 kernel |
| Yang et al., 2024 [15] | SOMATOM Force (Siemens) | 1 mm | 90/Sn150 | Iopromide 370, 80 mL at 5 mL/s + 30 mL saline | SOMATOM Force (Siemens); DECT; 90/Sn150 kVp; 1 mm slice; kernel NR |
| Gotta et al., 2024 [16] | SOMATOM Force (Siemens) | 1 mm | 90/Sn150 | Imeron 400, 100 mL at 5 mL/s, bolus tracking | SOMATOM Force (Siemens); DECT; 90/Sn150 kVp; 1 mm; Bv36 kernel |
| Gotta et al., 2024 [17] | SOMATOM Force (Siemens) | 1 mm | 90/Sn150 | Imeron 400, 100 mL at 5 mL/s, bolus tracking | SOMATOM Force (Siemens); DECT; 90/Sn150 kVp; 1 mm; Bv36 kernel |
| Gotta et al., 2024 [18] | SOMATOM Force (Siemens) | 1 mm | 90/Sn150 | Imeron 400, 80–120 mL at 5–6 mL/s | SOMATOM Force (Siemens); DECT; 90/Sn150 kVp; 1 mm slice; kernel NR |
| Shahzadi et al., 2024 [19] | SOMATOM Force (Siemens) | 1 mm | 100 | Iopromide 370, 80 mL at 5 mL/s + 30 mL saline | SOMATOM Force (Siemens); 100 kVp; 1 mm slice; kernel NR |
| Surov et al., 2024 [20] | SOMATOM Definition AS + (Siemens) | 1 mm | 100–140 (modulated) | Accupaque 300 or Imeron 300, 60–150 mL at 3–4 mL/s, bolus tracking | SOMATOM Definition AS + (Siemens); 100–140 kVp (modulated); 1 mm slice thickness |
| Wang et al., 2025 [21] | SOMATOM Definition Flash (Siemens) | 1 mm | 100 | Iopromide 370, 80 mL at 5 mL/s + 30 mL saline | SOMATOM Definition Flash (Siemens); 100–140 kVp (weight-adjusted); 1 mm slice thickness |
| Author, Year | Software Used for Feature Extraction | Anatomical Source of Radiomic Features | Radiomics Features Extracted, n | Models | Radiomics Features Used in Models | AUC (95% CI) of the Model in the Training Set | AUC (95% CI) of the Model in the Validation Set |
|---|---|---|---|---|---|---|---|
| Zhou et al., 2018 [13] | NR | Epicardial fat | NR | Radiomic model | RVD4-CH/LVD4-CH ratio and interventricular septum curvature positive | 0.87 | 0.784 |
| Leonhardi et al., 2023 [14] | MaZda (version 4.7) | Pulmonary embolus | 279 | Radiomic model | -S (5,5) Correlat and S (3,3) SumEntrp correlated with mortality -S (3,−3) AngScMom correlated with sepsis-related organ failure | NR | NR |
| Yang et al., 2024 [15] | PyRadiomics Python package (version 2.2.0) and D-Slicer | Pulmonary embolus | 1037 | Clinical model | Age and sex | 0.778 (0.639–0.882) | 0.833 (0.621–0.954) |
| Radiomic model | RV/LV ≥ 1.0 and radiomics score | 0.907 (0.792–0.970) | 0.817 (0.601–0.945) | ||||
| Combined nomogram | Age, sex, RV/LV ≥ 1.0, and radiomics score | 0.925 (0.816–0.980) | 0.917 (0.724–0.990) | ||||
| Gotta et al., 2024 [16] | PyRadiomics and 3D Slicer software (version 5.1.0–2022-05–20) | Pulmonary embolus | 107 | Radiomic model | Twelve features in the central PE group, seven features in the peripheral PE group, and 15 features in the group with all PE | 0.91 in the central APE cohort | Central APE cohort: 0.86 (0.645–0.956) Peripheral APE cohort: 0.63 (0.38–0.869) |
| Gotta et al., 2024 [17] | PyRadiomics extension package was employed in the 3D Slicer software (version 5.1.0–2022–05-20) | Pulmonary embolus | 107 | Unadjusted radiomic model | Unadjusted radiomic model: voxel number, elongation, flatness, least axis length, major axis length, gray level non-uniformity, surface volume ratio, 10th percentile, root mean squared, energy, skewness, and total energy | NR | 0.991 (0.979–1.000) |
| Radiomic model adjusted by age | 0.991 (0.979–1.000) | ||||||
| Radiomic model adjusted by troponin | 0.989 (0.973–1.000) | ||||||
| Radiomic model adjusted by PESI score | 0.991 (0.979–1.000) | ||||||
| Gotta et al., 2024 [18] | PyRadiomics extension package was employed in the 3D Slicer software (version 5.1.0–2022–05-20) | Pulmonary embolus | 107 | Unadjusted radiomic model | Elongation, flatness, and mesh volume | NR | 0.63 |
| Adjusted radiomic model | Elongation, flatness, mesh volume, voxel volume, 90th percentile, energy, mean, median, and total energy | NR | 0.58 | ||||
| Shahzadi et al., 2024 [19] | ImageJ software (version 1.53) | Skeletal muscle (T12) and intramuscular adipose tissue | 234 | sPESI score 7 days | Age > 80 years, cancer (previous or active), chronic lung disease, pulse ≥ 110 beats per minute, systolic blood pressure < 100 mmHg and arterial oxygen saturation < 90% | 0.74 (0.66–0.82) | 0.73 (0.67–0.79) |
| sPESI score 30 days | 0.72 (0.67–0.77) | 0.74 (0.66–0.82) | |||||
| Radiomic SM 7 days | -stat_rms (root mean square) -morph_pca_elongation (morphological elongation) -szm_sze_2d_fbn_n24 (small zone emphasis in GLSZM) | 0.71 (0.64–0.77) | 0.56 (0.43–0.69) | ||||
| Radiomic SM 30 days | 0.73 (0.67–0.78) | 0.64 (0.53–0.74) | |||||
| Radiomic IMAT 7 days | -morph_comp_1 (morphological compactness) -stat_qcod (quantile coding) -ngl_glnu_d1_a0_2d_fbn_n24 (gray level non-uniformity in NGLDM) | 0.70 (0.63–0.77) | 0.62 (0.50–0.74) | ||||
| Radiomic IMAT 30 days | 0.73 (0.67–0.79) | 0.68 (0.57–0.78) | |||||
| Radiomic SM + IMAT 7 days | -stat_skew (skewness of intensity distribution) -szm_sze_2d_fbn_n24 (small zone emphasis in GLSZM) -morph_pca_elongation (morphological elongation) | 0.74 (0.68–0.80) | 0.57 (0.46–0.67) | ||||
| Radiomic SM + IMAT 30 days | 0.77 (0.72–0.81) | 0.70 (0.60–0.79) | |||||
| Surov et al., 2024 [20] | PyRadiomics | Pulmonary embolus | 107, plus two external validation cohorts of 169 and 186 patients | Logistic regression, random forest | First-order features (energy, kurtosis, and skewness), and texture features (GLRLM, GLSZM, and GLDM) from epicardial adipose tissue | 7-day mortality: 0.724 (0.650–0.798) 30-day mortality: 0.776 (0.709–0.843) | 7-day mortality: 0.750 (0.662–0.839) 30-day mortality: 0.721 (0.633–0.809) |
| Wang et al., 2025 [21] | 3D-Slicer (PyRadiomics) | Pulmonary embolus | 132 | Clinical model | Four key features selected from GLRLM, GLCM, GLDM, and GLSZM matrices. | 0.85 (0.78–0.92) | 0.83 (0.74–0.91) |
| Radiomics model | 0.76 (0.67–0.84) | 0.74 (0.64–0.84) | |||||
| Combined model (clinical plus radiomics) | 0.89 (0.83–0.95) | 0.901 (0.808–0.994) | |||||
| ML models (logistic regression, decision tree, random forest, and SVM) | 0.91 (0.90–0.92) | No external validation | |||||
| DL models (ResNet-50 and VGG-19) | 0.94 (0.93–0.95) | No external validation |
| Author, Year | Selection | Comparability | Outcome | Total Stars | Risk of Bias |
|---|---|---|---|---|---|
| Zhou et al., 2018 [13] | ★★★ | ★★ | ★★ | 7 | Low |
| Leonhardi et al., 2023 [14] | ★★★ | ★★ | ★★★ | 8 | Low |
| Yang et al., 2024 [15] | ★★★ | ★★ | ★★★ | 8 | Low |
| Gotta et al., 2024 [16] | ★★★ | ★★ | ★★★ | 8 | Low |
| Gotta et al., 2024 [17] | ★★★ | ★★ | ★★★ | 8 | Low |
| Gotta et al., 2024 [18] | ★★★ | ★★ | ★★★ | 8 | Low |
| Shahzadi et al., 2024 [19] | ★★★ | ★★ | ★★ | 7 | Low |
| Surov et al., 2024 [20] | ★★★ | ★★ | ★★ | 7 | Low |
| Wang et al., 2025 [21] | ★★★ | ★★ | ★★★ | 8 | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Suela, M.Á.; Torres-Macho, J.; Prada-Alonso, J.; Pastorín-Salis, R.; Martínez de la Casa-Muñoz, A.; Ruiz-Navío, E.; Bustamante-Fermosel, A.; Franco-Moreno, A. Use of Radiomics to Predict Adverse Outcomes in Patients with Pulmonary Embolism: A Scoping Review of an Unresolved Clinical Challenge. Diagnostics 2025, 15, 2022. https://doi.org/10.3390/diagnostics15162022
Casado-Suela MÁ, Torres-Macho J, Prada-Alonso J, Pastorín-Salis R, Martínez de la Casa-Muñoz A, Ruiz-Navío E, Bustamante-Fermosel A, Franco-Moreno A. Use of Radiomics to Predict Adverse Outcomes in Patients with Pulmonary Embolism: A Scoping Review of an Unresolved Clinical Challenge. Diagnostics. 2025; 15(16):2022. https://doi.org/10.3390/diagnostics15162022
Chicago/Turabian StyleCasado-Suela, Miguel Ángel, Juan Torres-Macho, Jesús Prada-Alonso, Rodrigo Pastorín-Salis, Ana Martínez de la Casa-Muñoz, Eva Ruiz-Navío, Ana Bustamante-Fermosel, and Anabel Franco-Moreno. 2025. "Use of Radiomics to Predict Adverse Outcomes in Patients with Pulmonary Embolism: A Scoping Review of an Unresolved Clinical Challenge" Diagnostics 15, no. 16: 2022. https://doi.org/10.3390/diagnostics15162022
APA StyleCasado-Suela, M. Á., Torres-Macho, J., Prada-Alonso, J., Pastorín-Salis, R., Martínez de la Casa-Muñoz, A., Ruiz-Navío, E., Bustamante-Fermosel, A., & Franco-Moreno, A. (2025). Use of Radiomics to Predict Adverse Outcomes in Patients with Pulmonary Embolism: A Scoping Review of an Unresolved Clinical Challenge. Diagnostics, 15(16), 2022. https://doi.org/10.3390/diagnostics15162022






