Investigation of Ocular Blood Flow in Males with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. LSFG Measurements
2.4. Systemic, Laboratory, and Ophthalmic Parameter Measurements
2.5. Diagnosis of MetS
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| LSFG | Laser speckle flowgraphy |
| MBR | Mean blur rate |
| BOS | Blowout score |
| BOT | Blowout time |
| RR | Rising rate |
| ONH | Optic nerve head |
| BMI | Body mass index |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| bpm | Beat per minute |
| D | Diopter |
| IOP | Intraocular pressure |
| FBS | Fasting blood sugar |
| TG | Triglycerides |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| HbA1c | Glycated hemoglobin A1c |
References
- Lakka, H.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Williams, S.M.; Eleftheriadou, A.; Alam, U.; Cuthbertson, D.J.; Wilding, J.P.H. Cardiac autonomic neuropathy in obesity, the metabolic syndrome and prediabetes: A narrative review. Diabetes Ther. 2019, 10, 1995–2021. [Google Scholar] [CrossRef]
- Lim, D.H.; Shin, K.Y.; Han, K.; Kang, S.W.; Ham, D.I.; Kim, S.J.; Park, Y.G.; Chung, T.Y. Differential effect of the metabolic syndrome on the incidence of retinal vein occlusion in the Korean population: A nationwide cohort study. Transl. Vis. Sci. Technol. 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Canani, L.H.; Lisboa, H.R.; Tres, G.S.; Gross, J.L. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet. Med. 2004, 21, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Isono, H.; Kishi, S.; Kimura, Y.; Hagiwara, N.; Konishi, N.; Fujii, H. Observation of choroidal circulation using index of erythrocytic velocity. Arch. Ophthalmol. 2003, 121, 225–231. [Google Scholar] [CrossRef]
- Tamaki, Y.; Araie, M.; Kawamoto, E.; Eguchi, S.; Fujii, H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp. Eye Res. 1995, 60, 373–383. [Google Scholar] [CrossRef]
- Aizawa, N.; Yokoyama, Y.; Chiba, N.; Omodaka, K.; Yasuda, M.; Otomo, T.; Nakamura, M.; Fuse, N.; Nakazawa, T. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin. Ophthalmol. 2011, 5, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Shiba, T.; Nishiwaki, Y.; Kinoshita, A.; Matsumoto, T.; Hori, Y. Influence of age and gender on the pulse waveform in optic nerve head circulation in healthy men and women. Sci. Rep. 2019, 9, 17895. [Google Scholar] [CrossRef]
- Aizawa, N.; Kunikata, H.; Nitta, F.; Shiga, Y.; Omodaka, K.; Tsuda, S.; Nakazawa, T. Age- and sex-dependency of laser speckle flowgraphy measurements of optic nerve vessel microcirculation. PLoS ONE 2016, 11, e0148812. [Google Scholar] [CrossRef]
- Yanagida, K.; Iwase, T.; Yamamoto, K.; Ra, E.; Kaneko, H.; Murotani, K.; Matsui, S.; Terasaki, H. Sex-related differences in ocular blood flow of healthy subjects using laser speckle flowgraphy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4880–4890. [Google Scholar] [CrossRef]
- Muramatsu, R.; Shiba, T.; Takahashi, M.; Hori, Y.; Maeno, T. Pulse waveform analysis of optic nerve head circulation for predicting carotid atherosclerotic changes. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2285–2291. [Google Scholar] [CrossRef]
- Shiba, T.; Takahashi, M.; Matsumoto, T.; Hori, Y. Pulse waveform analysis in ocular microcirculation by laser speckle flowgraphy in patients with left ventricular systolic and diastolic dysfunction. J. Vasc. Res. 2018, 55, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sugiyama, T.; Tokushige, H.; Maeno, T.; Nakazawa, T.; Ikeda, T.; Araie, M. Comparison of CCD-equipped laser speckle flowgraphy with hydrogen gas clearance method in the measurement of optic nerve head microcirculation in rabbits. Exp. Eye Res. 2013, 108, 10–15. [Google Scholar] [CrossRef]
- Luft, N.; Wozniak, P.A.; Aschinger, G.C.; Fondi, K.; Bata, A.M.; Werkmeister, R.M.; Schmidl, D.; Witkowska, K.J.; Bolz, M.; Garhöfer, G.; et al. Ocular blood flow measurements in healthy white subjects using laser speckle flowgraphy. PLoS ONE 2016, 11, e0168190. [Google Scholar] [CrossRef]
- Luft, N.; Wozniak, P.A.; Aschinger, G.C.; Fondi, K.; Bata, A.M.; Werkmeister, R.M.; Schmidl, D.; Witkowska, K.J.; Bolz, M.; Garhöfer, G.; et al. Measurements of retinal perfusion using laser speckle flowgraphy and Doppler optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5417–5425. [Google Scholar] [CrossRef]
- Shiba, T.; Takahashi, M.; Matsumoto, T.; Hori, Y. Relationship between metabolic syndrome and ocular microcirculation shown by laser speckle flowgraphy in a hospital setting devoted to sleep apnea syndrome diagnostics. J. Diabetes Res. 2017, 2017, 3141678. [Google Scholar] [CrossRef]
- Tsuda, S.; Kunikata, H.; Shimura, M.; Aizawa, N.; Omodaka, K.; Shiga, Y.; Yasuda, M.; Yokoyama, Y.; Nakazawa, T. Pulse-waveform analysis of normal population using laser speckle flowgraphy. Curr. Eye Res. 2014, 39, 1207–1215. [Google Scholar] [CrossRef]
- The Examination Committee for Criteria of Metabolic Syndrome. Definition and Criteria of metabolic syndrome. Nihon Naika Gakkai Zasshi. 2005, 94, 794–809. (In Japanese) [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow. Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Shigetoh, Y.; Adachi, H.; Yamagishi, S.; Enomoto, M.; Fukami, A.; Otsuka, M.; Kumagae, S.; Furuki, K.; Nanjo, Y.; Imaizumi, T. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am. J. Hypertens. 2009, 22, 151–155. [Google Scholar] [CrossRef]
- Okamura, T.; Hayakawa, T.; Kadowaki, T.; Kita, Y.; Okayama, A.; Elliott, P.; Ueshima, H.; NIPPONDATA80 Research Group. Resting heart rate and cause-specific death in a 16.5-year cohort study of the Japanese general population. Am. Heart J. 2004, 147, 1024–1032. [Google Scholar] [CrossRef]
- Gillman, M.W.; Kannel, W.B.; Belamger, A.; D’Agostino, R.B. Influence of heart rate mortality among persons with hypertension: The Framingham Study. Am. Heart J. 1993, 125, 1148–1154. [Google Scholar] [CrossRef]
- Oh, S.W.; Lee, S.; Park, C.; Kim, D.J. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab. Res. Rev. 2005, 21, 434–440. [Google Scholar] [CrossRef]
- Wu, S.; Lin, H.; Zhang, C.; Zhang, Q.; Zhang, D.; Zhang, Y.; Meng, W.; Zhu, Z.; Tang, F.; Xue, F.; et al. Association between erythrocyte parameters and metabolic syndrome in urban Han Chinese: A longitudinal cohort study. BMC Public Health 2013, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Fukumoto, Y.; Shiba, N.; Miura, T.; Shimada, K.; Iwama, Y.; Takagi, A.; Matsusaka, H.; Tsutsumi, T.; Yamada, A.; et al. Prevalence and clinical implication of metabolic syndrome in chronic heart failure. Circ. J. 2010, 74, 2612–2621. [Google Scholar] [CrossRef]
- Sharma, A.; Razaghizad, A.; Ferreira, J.P.; Machu, J.L.; Bozec, E.; Girerd, N.; Rossignol, P.; Zannad, F. Metabolic syndrome and the risk of preclinical heart failure: Insights after 17 years of follow-up from the STANISLAS Cohort. Cardiology 2022, 147, 281–287. [Google Scholar] [CrossRef]
- Nagaoka, T.; Sato, E.; Takahashi, A.; Yokota, H.; Sogawa, K.; Yoshida, A. Impaired retinal circulation in patients with type 2 diabetes mellitus: Retinal laser doppler velocimetry study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6729–6734. [Google Scholar] [CrossRef]
- Ueno, Y.; Iwase, T.; Goto, K.; Tomita, R.; Ra, E.; Yamamoto, K.; Terasaki, H. Association of changes of retinal vessels diameter with ocular blood flow in eyes with diabetic retinopathy. Sci. Rep. 2021, 11, 4653. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Ueno, Y.; Tomita, R.; Terasaki, H. Relationship between retinal microcirculation and renal function in patients with diabetes and chronic kidney disease by laser speckle flowgraphy. Life 2023, 13, 424. [Google Scholar] [CrossRef]
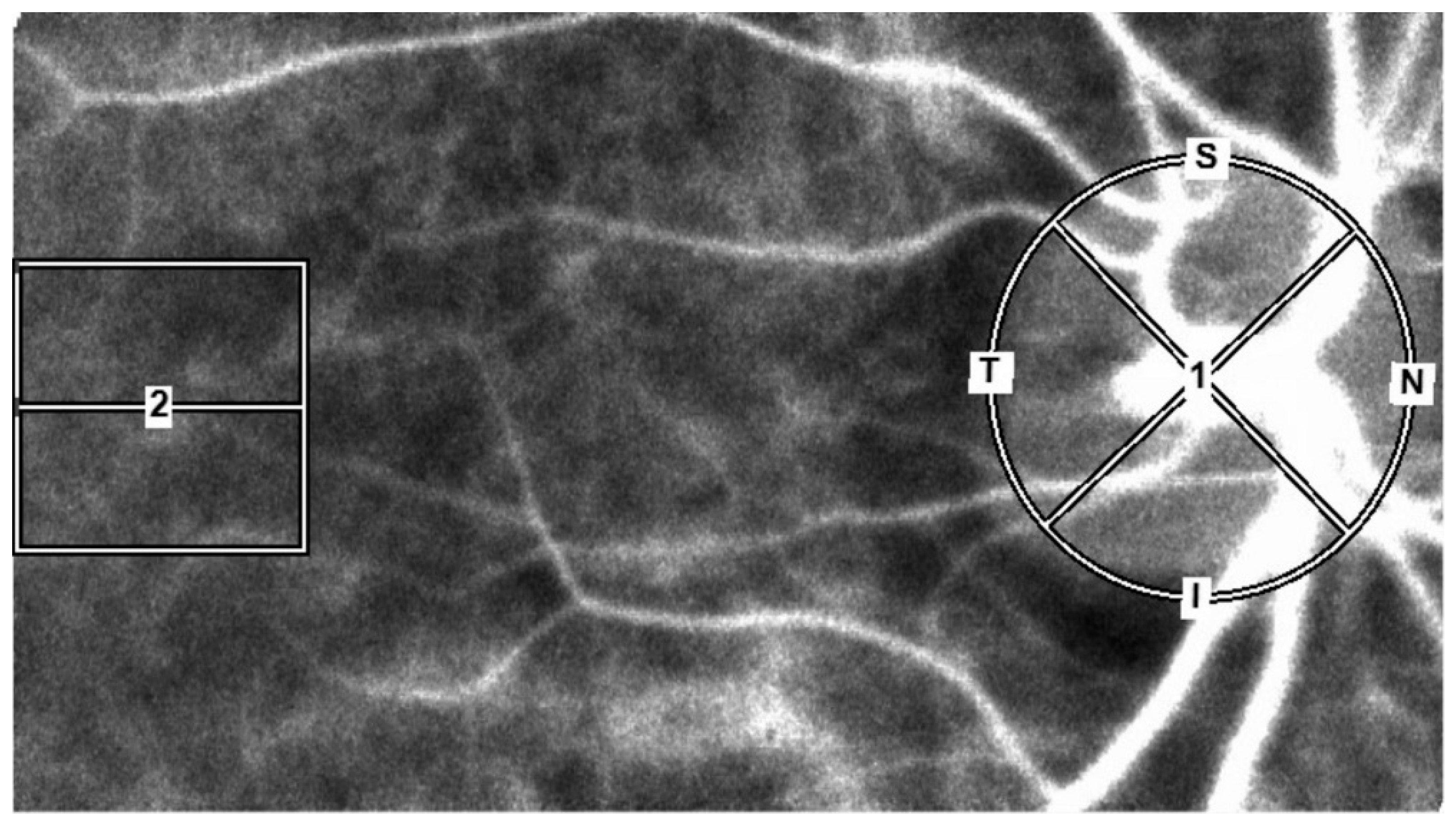
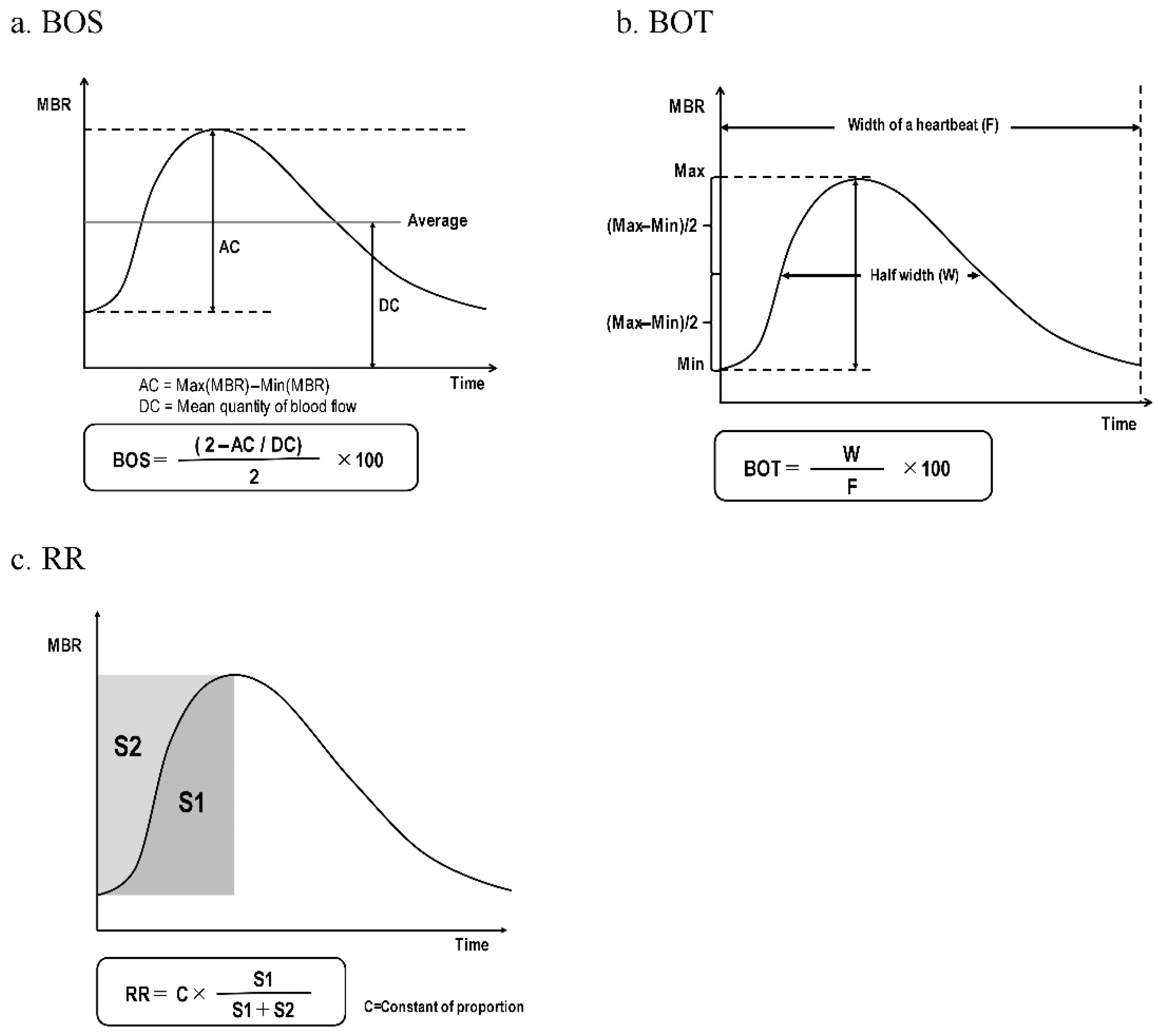
| MetS n = 138 | Control n = 138 | p-Value | |
|---|---|---|---|
| Gender, male | – | – | |
| Age, yrs | 49.95 ± 8.21 | 49.96 ± 8.24 | 0.99 * |
| BMI, kg/m2 | 27.85 ± 3.38 | 22.41 ± 2.53 | <0.001 * |
| Waist circ., cm | 95.24 ± 7.78 | 81.03 ± 7.50 | <0.001 * |
| SBP, mmHg | 137.36 ± 17.14 | 112.75 ± 10.31 | <0.001 * |
| DBP, mmHg | 87.88 ± 12.43 | 70.61 ± 7.22 | <0.001 * |
| Pulse pressure, mmHg | 49.49 ± 11.43 | 42.14 ± 7.03 | <0.001 * |
| Heart rate, bpm | 75.78 ± 10.49 | 67.98 ± 9.26 | <0.001 * |
| FBS, mg/dL | 121.43 ± 31.01 | 95.46 ± 7.80 | <0.001 * |
| TG, mg/dL | 212.91 ± 164.75 | 91.33 ± 28.89 | <0.001 * |
| HDL-C, mg/dL | 52.30 ± 13.38 | 64.98 ± 14.40 | <0.001 * |
| LDL-C, mg/dL | 139.09 ± 35.78 | 129.18 ± 27.14 | 0.010 * |
| Hematocrit, % | 45.92 ± 3.17 | 44.22 ± 3.11 | <0.001 * |
| HbA1c, % | 6.18 ± 0.94 | 5.53 ± 0.26 | <0.001 * |
| Spherical refraction, D | −2.35 ± 2.65 | −2.07 ± 2.67 | 0.38 * |
| IOP, mmHg | 12.61 ± 3.10 | 11.54 ± 2.69 | 0.002 * |
| Glucose tolerance, % | 92 (66.7) | 0 (0) | <0.001 ** |
| Dyslipidemia, % | 115 (83.3) | 0 (0) | <0.001 ** |
| Hypertension, % | 123 (89.1) | 0 (0) | <0.001 ** |
| MBR (AU) | MetS n = 138 | Control n = 138 | p-Value |
|---|---|---|---|
| MBR-All | 24.64 ± 4.09 | 25.20 ± 4.36 | 0.28 |
| MBR-Tissue | 12.67 ± 2.49 | 13.07 ± 2.47 | 0.19 |
| MBR-Vessel | 44.84 ± 6.40 | 44.99 ± 7.06 | 0.85 |
| MBR-Choroid | 8.81 ± 2.82 | 9.59 ± 2.57 | 0.02 |
| Parameter (AU) | MetS n = 138 | Control n = 138 | p-Value |
|---|---|---|---|
| BOS-All | 81.72 ± 4.50 | 80.31 ± 3.80 | 0.005 |
| BOS-Tissue | 78.78 ± 4.95 | 77.64 ± 4.09 | 0.04 |
| BOS-Vessel | 83.02 ± 4.32 | 81.51 ± 3.79 | 0.002 |
| BOS-Choroid | 77.93 ± 5.36 | 76.80 ± 4.99 | 0.07 |
| BOT-All | 52.75 ± 4.66 | 53.18 ± 3.77 | 0.40 |
| BOT-Tissue | 49.92 ± 4.79 | 50.35 ± 3.64 | 0.41 |
| BOT-Vessel | 54.23 ± 4.74 | 54.70 ± 4.04 | 0.38 |
| BOT-Choroid | 48.67 ± 5.03 | 49.32 ± 3.53 | 0.21 |
| RR-All | 12.64 ± 0.93 | 13.37 ± 0.85 | <0.001 |
| RR-Tissue | 12.26 ± 0.85 | 12.91 ± 0.84 | <0.001 |
| RR-Vessel | 12.79 ± 1.01 | 13.62 ± 1.00 | <0.001 |
| RR-Choroid | 12.24 ± 0.91 | 12.78 ± 0.95 | <0.001 |
| Explanatory Variables | r | p-Value |
|---|---|---|
| Age, yrs | −0.054 | 0.37 |
| Heart rate, bpm | 0.049 | 0.42 |
| Hematocrit, % | −0.089 | 0.14 |
| Spherical refraction, D | −0.030 | 0.63 |
| IOP, mmHg | 0.094 | 0.12 |
| MetS component, number | −0.14 | 0.02 |
| Explanatory Variables | Single Regression | Multiple Regression | |||
|---|---|---|---|---|---|
| r | p-Value | β | t-Value | p-Value | |
| SBP, mmHg | −0.12 | 0.05 | |||
| DBP, mmHg | −0.12 | 0.05 | |||
| HbA1c, % | −0.22 | 0.001 | −0.45 | −2.25 | 0.03 |
| FBS, mg/dL | −0.15 | 0.02 | |||
| TG, mg/dL | −0.14 | 0.02 | −0.14 | −0.69 | 0.49 |
| LDL-C, mg/dL | −0.099 | 0.10 | |||
| HDL-C, mg/dL | 0.14 | 0.02 | 0.31 | 1.54 | 0.13 |
| BMI, kg/m2 | −0.11 | 0.01 | |||
| Waist circ., cm | −0.15 | 0.01 | −0.041 | −0.20 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, T.; Shiba, T.; Kobayashi, T.; Takagi, S.; Hori, Y. Investigation of Ocular Blood Flow in Males with Metabolic Syndrome. Diagnostics 2025, 15, 2021. https://doi.org/10.3390/diagnostics15162021
Maruyama T, Shiba T, Kobayashi T, Takagi S, Hori Y. Investigation of Ocular Blood Flow in Males with Metabolic Syndrome. Diagnostics. 2025; 15(16):2021. https://doi.org/10.3390/diagnostics15162021
Chicago/Turabian StyleMaruyama, Takahiro, Tomoaki Shiba, Tatsuhiko Kobayashi, Seiji Takagi, and Yuichi Hori. 2025. "Investigation of Ocular Blood Flow in Males with Metabolic Syndrome" Diagnostics 15, no. 16: 2021. https://doi.org/10.3390/diagnostics15162021
APA StyleMaruyama, T., Shiba, T., Kobayashi, T., Takagi, S., & Hori, Y. (2025). Investigation of Ocular Blood Flow in Males with Metabolic Syndrome. Diagnostics, 15(16), 2021. https://doi.org/10.3390/diagnostics15162021





