Prognostic Value of Automated Bone Scan Index (aBSI) in Patients with mCRPC Undergoing Three vs. Six Cycles of 223Ra Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiopharmaceutical and Imaging Protocol
2.3. Image Analysis
2.4. 223Ra Therapy
2.5. Statistical Analysis
3. Results
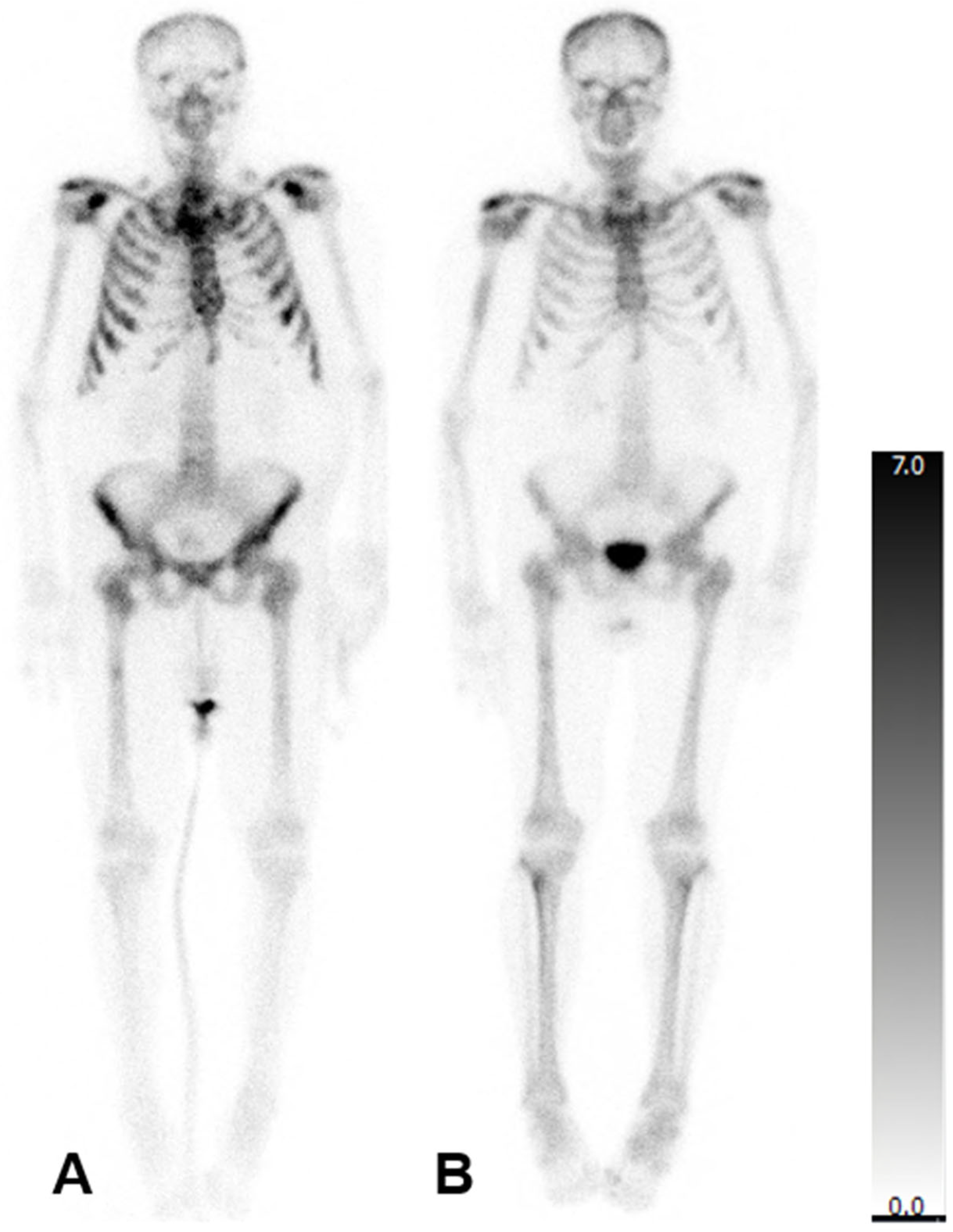
3.1. Bone Scintigraphy, SPECT/CT and Baseline Lab Analysis
3.2. Changes in Clinical and Imaging Parameters Following 223Ra Therapy
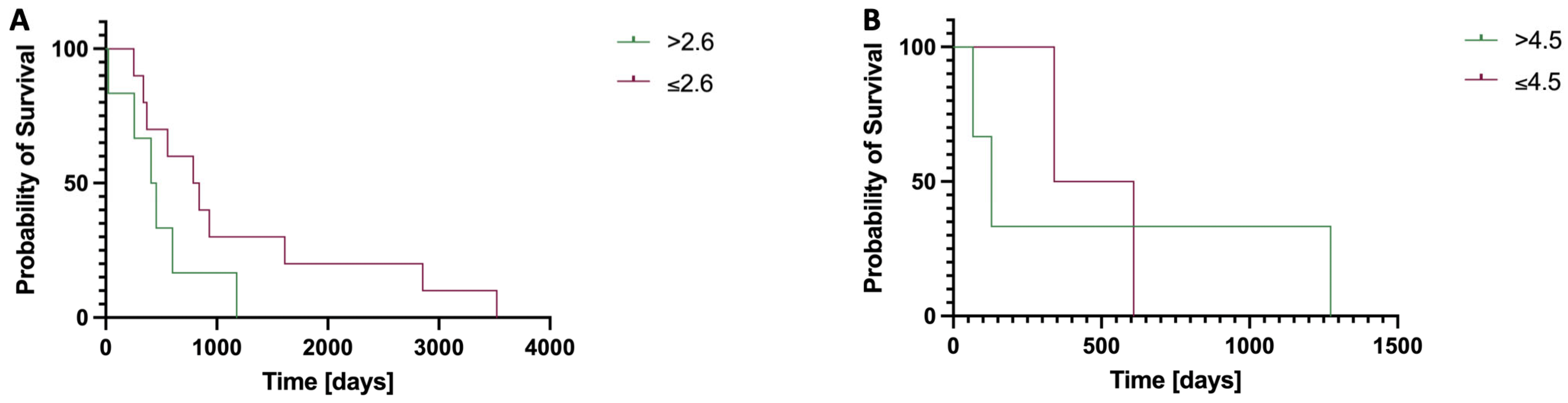
3.3. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aBSI | Automated Bone Scan Index |
| ADT | Androgen deprivation therapy |
| AP | Alkaline phosphatase |
| BS | Bone Scan |
| CR | Complete response |
| mCRPC | Metastatic castration-resistant prostate cancer |
| PD | Progressive disease |
| PR | Partial Response |
| PSA | Prostate-specific antigen |
| Ra | Radium |
| STD | Standard deviation |
| SD | Stable disease |
| TTV | Total tumor volume |
| WBC | White blood cell |
References
- Saad, F.; Hotte, S.J. Guidelines for the management of castrate-resistant prostate cancer. Can. Urol. Assoc. J. 2010, 4, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, S.; Mercinelli, C.; Marandino, L.; Litterio, G.; Marchioni, M.; Schips, L. Metastatic Castration-Resistant Prostate Cancer: Insights on Current Therapy and Promising Experimental Drugs. Res. Rep. Urol. 2023, 15, 243–259. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef]
- Sindhu, K.K.; Nehlsen, A.D.; Stock, R.G. Radium-223 for Metastatic Castrate-Resistant Prostate Cancer. Precis. Radiat. Oncol. 2022, 12, 312–316. [Google Scholar] [CrossRef]
- Brito, A.E.; Etchebehere, E. Radium-223 as an Approved Modality for Treatment of Bone Metastases. Semin. Nucl. Med. 2020, 50, 177–192. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Kitajima, K.; Kuyama, J.; Kawahara, T.; Suga, T.; Otani, T.; Sugawara, S.; Kono, Y.; Tamaki, Y.; Seko-Nitta, A.; Ishiwata, Y.; et al. Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial. Cancers 2023, 15, 2784. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Tsutsumi, S.; Kawahara, T.; Yasui, M.; Uemura, K.; Yoneyama, S.; Yokomizo, Y.; Hayashi, N.; Yao, M.; Uemura, H. Prognostic value of automated bone scan index for predicting overall survival among bone metastatic castration resistant prostate cancer patients treated with radium-223. BJUI Compass 2021, 2, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.H.D.; Osman, S.O.S.; Prise, K.M.; Campfield, C.; Turner, P.G.; Jain, S.F.P.; O’Sullivan, J.M.; Cole, A.J. A novel tool for improving the interpretation of isotope bone scans in metastatic prostate cancer. Br. J. Radiol. 2020, 93, 20200775. [Google Scholar] [CrossRef] [PubMed]
- Bayer. Xofigo® Basiswissen. Available online: https://www.fachinfo.de/fi/pdf/014972 (accessed on 27 July 2024).
- McNamara, M.A.; George, D.J. Pain, PSA flare, and bone scan response in a patient with metastatic castration-resistant prostate cancer treated with radium-223, a case report. BMC Cancer 2015, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Khan, N.; Phillips, C.; Briones, J.; Kapoor, A.; Zalewski, P.; Fleshner, N.E.; Chow, E.; Emmenegger, U. Prevalence and Prognostic Implications of PSA Flares during Radium-223 Treatment among Men with Metastatic Castration Resistant Prostate Cancer. J. Clin. Med. 2023, 12, 5604. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Matsubara, N.; Tabata, K.I.; Satoh, T.; Kamiya, N.; Suzuki, H.; Kawahara, T.; Uemura, H. Prostate-Specific Antigen Flare Phenomenon Induced by Abiraterone Acetate in Chemotherapy-Naive Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 320–325. [Google Scholar] [CrossRef]
- Conteduca, V.; Poti, G.; Caroli, P.; Russi, S.; Brighi, N.; Lolli, C.; Schepisi, G.; Romeo, A.; Matteucci, F.; Paganelli, G.; et al. Flare phenomenon in prostate cancer: Recent evidence on new drugs and next generation imaging. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987654. [Google Scholar] [CrossRef]
- Kessler, E.R. What is behind the flare phenomenon? BJU Int. 2016, 118, 845–846. [Google Scholar] [CrossRef]
- Castello, A.; Macapinlac, H.A.; Lopci, E.; Santos, E.B. Prostate-specific antigen flare induced by (223)RaCl(2) in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2256–2263. [Google Scholar] [CrossRef]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R.; et al. Efficacy and Safety of (177)Lu-labeled Prostate-specific Membrane Antigen Radionuclide Treatment in Patients with Diffuse Bone Marrow Involvement: A Multicenter Retrospective Study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Vogelzang, N.J.; Bruland, Ø.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef]
- Anand, A.; Trägårdh, E.; Edenbrandt, L.; Beckman, L.; Svensson, J.H.; Thellenberg, C.; Widmark, A.; Kindblom, J.; Ullén, A.; Bjartell, A. Assessing Radiographic Response to (223)Ra with an Automated Bone Scan Index in Metastatic Castration-Resistant Prostate Cancer Patients. J. Nucl. Med. 2020, 61, 671–675. [Google Scholar] [CrossRef]
- Naito, M.; Ukai, R.; Hashimoto, K. Bone scan index can be a useful biomarker of survival outcomes in patients with metastatic castration-resistant prostate cancer treated with radium-223. Cancer Rep. 2019, 2, e1203. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Anand, A.; Edenbrandt, L.; Bondesson, E.; Bjartell, A.; Widmark, A.; Sternberg, C.N.; Pili, R.; Tuvesson, H.; Nordle, Ö.; et al. Phase 3 Assessment of the Automated Bone Scan Index as a Prognostic Imaging Biomarker of Overall Survival in Men with Metastatic Castration-Resistant Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 944–951. [Google Scholar] [CrossRef]
- Raimondi, A.; Sepe, P.; Claps, M.; Maccauro, M.; Aliberti, G.; Pagani, F.; Apollonio, G.; Randon, G.; Peverelli, G.; Seregni, E.; et al. Safety and activity of radium-223 in metastatic castration-resistant prostate cancer: The experience of Istituto Nazionale dei Tumori. Tumori 2020, 106, 406–412. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Coleman, R.E.; Michalski, J.M.; Nilsson, S.; O’Sullivan, J.M.; Parker, C.; Widmark, A.; Thuresson, M.; Xu, L.; Germino, J.; et al. Hematologic Safety of Radium-223 Dichloride: Baseline Prognostic Factors Associated with Myelosuppression in the ALSYMPCA Trial. Clin. Genitourin. Cancer 2017, 15, 42–52.e48. [Google Scholar] [CrossRef] [PubMed]
- Leisser, A.; Nejabat, M.; Hartenbach, M.; Duan, H.; Shariat, S.F.; Kramer, G.; Krainer, M.; Hacker, M.; Haug, A.R. Hematopoiesis is prognostic for toxicity and survival of (223)Radium treatment in patients with metastatic castration-resistant prostate cancer. Hell. J. Nucl. Med. 2017, 20, 157. [Google Scholar]
- Higano, C.S.; George, D.J.; Shore, N.D.; Sartor, O.; Miller, K.; Conti, P.S.; Sternberg, C.N.; Saad, F.; Sade, J.P.; Bellmunt, J.; et al. Clinical outcomes and treatment patterns in REASSURE: Planned interim analysis of a real-world observational study of radium-223 in metastatic castration-resistant prostate cancer. eClinicalMedicine 2023, 60, 101993. [Google Scholar] [CrossRef] [PubMed]
- Shagera, Q.A.; Gil, T.; Barraco, E.; Boegner, P.; Kristanto, P.; El Ali, Z.; Sideris, S.; Martinez Chanza, N.; Roumeguère, T.; Flamen, P.; et al. Evaluating response to radium-223 using 68Ga-PSMA PET/CT imaging in patients with metastatic castration-resistant prostate cancer. Ann. Nucl. Med. 2025, 39, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2020, 12, 31. [Google Scholar] [CrossRef]
- Unterrainer, L.M.; Calais, J.; Bander, N.H. Prostate-Specific Membrane Antigen: Gateway to Management of Advanced Prostate Cancer. Annu. Rev. Med. 2024, 75, 49–66. [Google Scholar] [CrossRef]

| Age | Baseline aBSI [%] | Baseline TTV [mL] | Baseline SUVmax | End aBSI [%] | End TTV [mL] | End SUVmax | |
|---|---|---|---|---|---|---|---|
| Mean | 70 | 2.9 | 237.2 | 878.7 | 3.2 | 229.4 | 550.2 |
| STD | 9 | 3.4 | 217.1 | 517.4 | 4.6 | 221.3 | 321.1 |
| Mean6cycles | 2.6 | 217.4 | 891.6 | 2.6 | 208.8 | 548.1 | |
| STD6cycles | 3.3 | 175.8 | 539.6 | 4.3 | 185.2 | 342.2 | |
| Mean3cycles | 4.5 | 356.1 | 801.7 | 6.7 | 353.2 | 562.3 | |
| STD3cycles | 3.8 | 382.9 | 379.3 | 5.2 | 369.2 | 156.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siegmund, S.C.; Ilhan, H.; Gerull, A.; Todica, A.; Unterrainer, M.; Delker, A.; Gildehaus, F.J.; Aydogdu, C.D.; Stief, C.G.; Werner, R.A.; et al. Prognostic Value of Automated Bone Scan Index (aBSI) in Patients with mCRPC Undergoing Three vs. Six Cycles of 223Ra Therapy. Diagnostics 2025, 15, 2007. https://doi.org/10.3390/diagnostics15162007
Siegmund SC, Ilhan H, Gerull A, Todica A, Unterrainer M, Delker A, Gildehaus FJ, Aydogdu CD, Stief CG, Werner RA, et al. Prognostic Value of Automated Bone Scan Index (aBSI) in Patients with mCRPC Undergoing Three vs. Six Cycles of 223Ra Therapy. Diagnostics. 2025; 15(16):2007. https://doi.org/10.3390/diagnostics15162007
Chicago/Turabian StyleSiegmund, Sophie C., Harun Ilhan, Antonia Gerull, Andrei Todica, Marcus Unterrainer, Astrid Delker, Franz Josef Gildehaus, Can D. Aydogdu, Christian G. Stief, Rudolf A. Werner, and et al. 2025. "Prognostic Value of Automated Bone Scan Index (aBSI) in Patients with mCRPC Undergoing Three vs. Six Cycles of 223Ra Therapy" Diagnostics 15, no. 16: 2007. https://doi.org/10.3390/diagnostics15162007
APA StyleSiegmund, S. C., Ilhan, H., Gerull, A., Todica, A., Unterrainer, M., Delker, A., Gildehaus, F. J., Aydogdu, C. D., Stief, C. G., Werner, R. A., Unterrainer, L. M., & Zacherl, M. J. (2025). Prognostic Value of Automated Bone Scan Index (aBSI) in Patients with mCRPC Undergoing Three vs. Six Cycles of 223Ra Therapy. Diagnostics, 15(16), 2007. https://doi.org/10.3390/diagnostics15162007






