Pelvic Floor Adaptation to a Prenatal Exercise Program: Does It Affect Labor Outcomes or Levator Ani Muscle Injury? A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
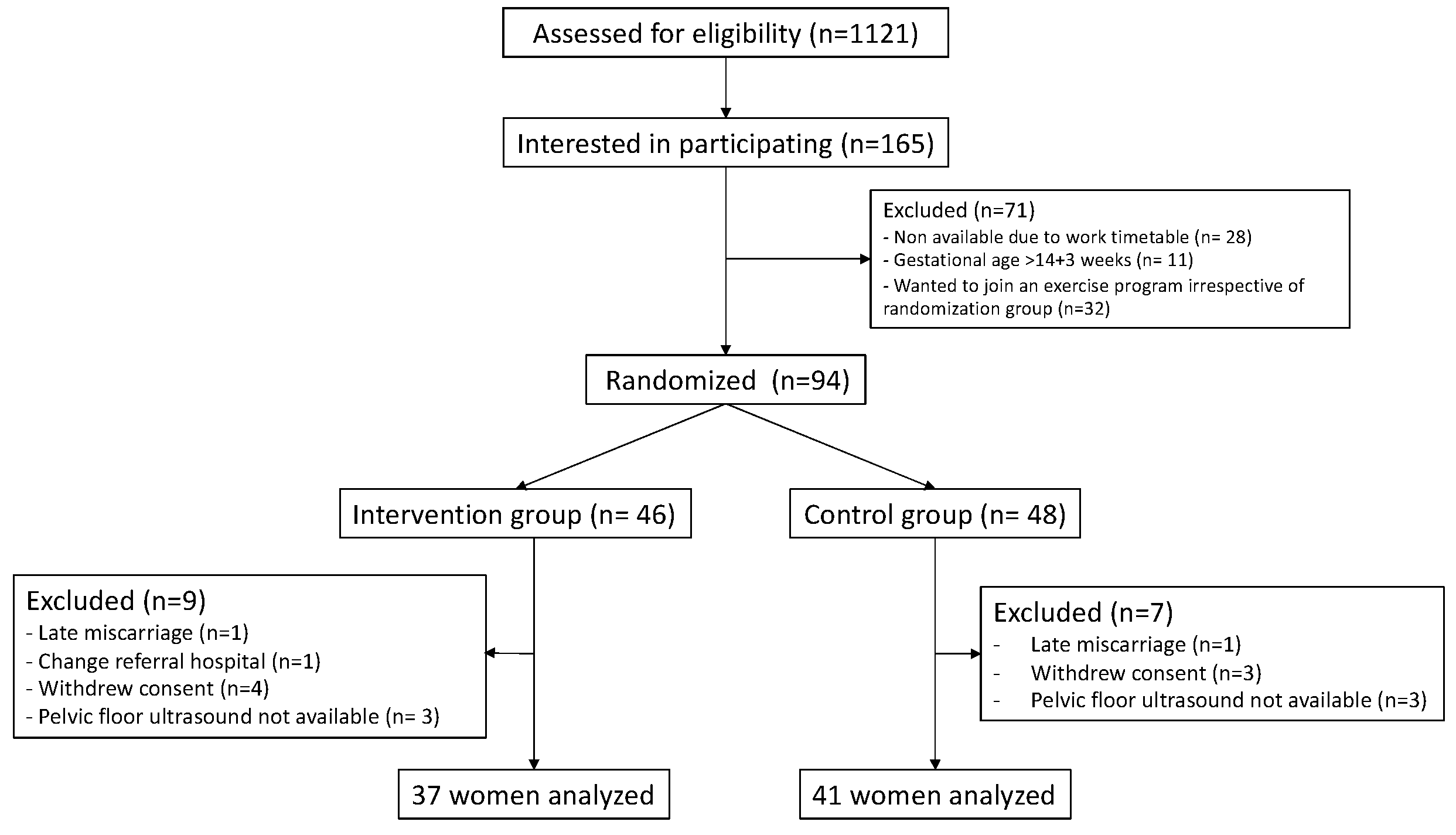
2.1. Trial Design and Participants
2.2. Randomization
2.3. Intervention Program
2.4. Control Group
2.5. Follow-Up
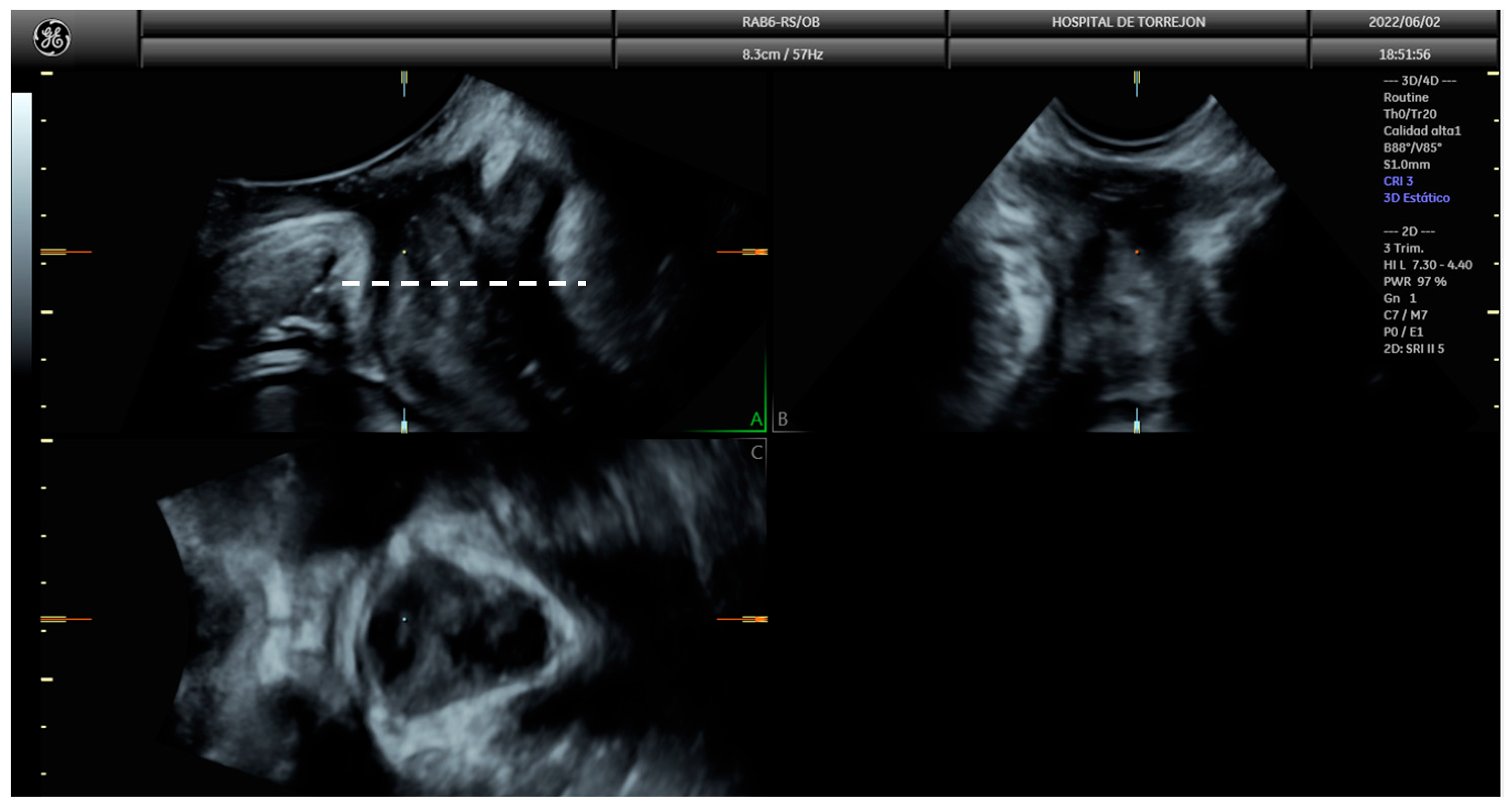
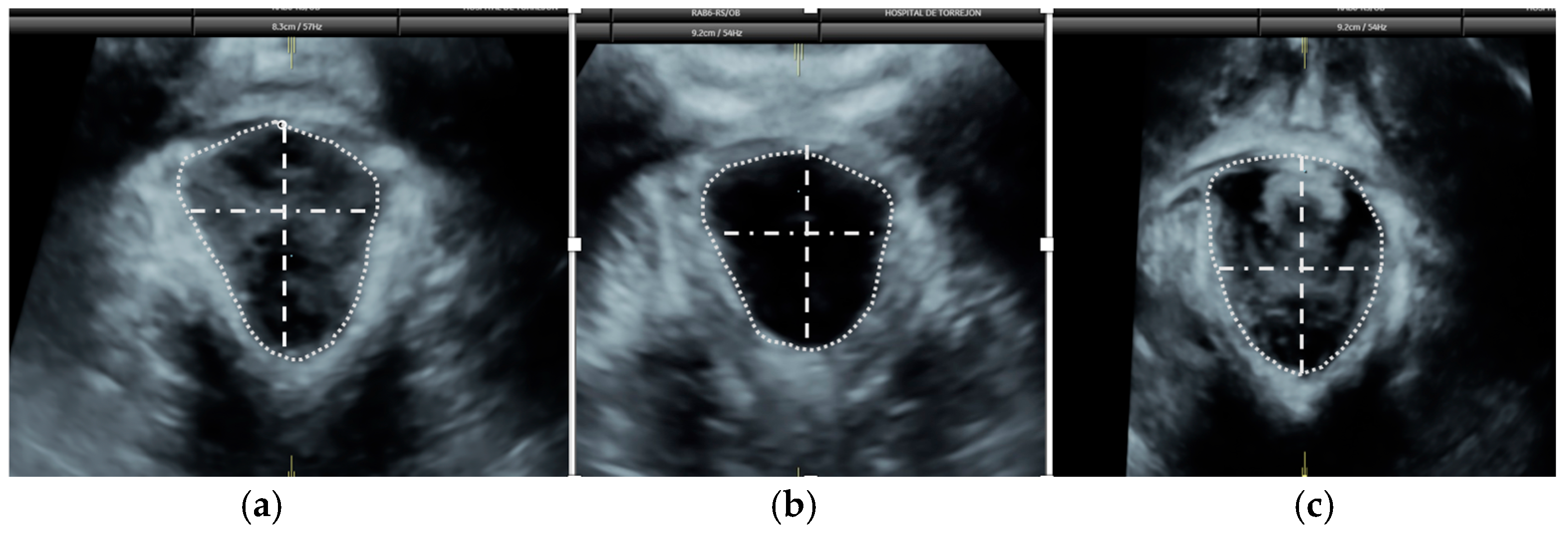
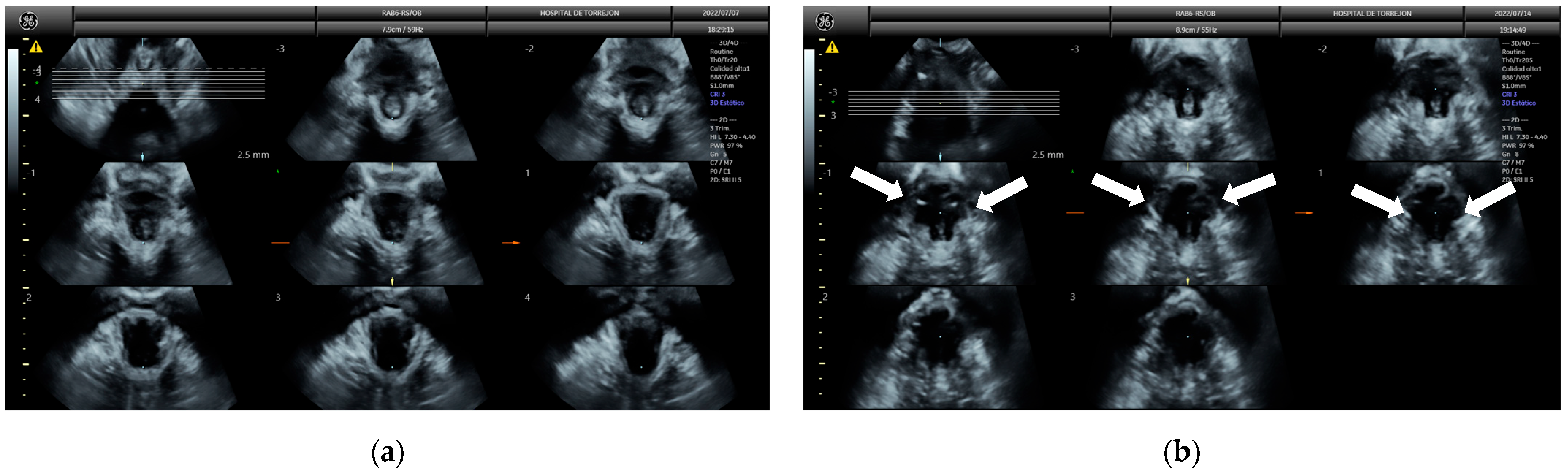
2.6. Pelvic Floor Ultrasound
2.7. Statistical Analysis
2.8. Sample Size Calculation
3. Results
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Study Strengths and Limitations
4.4. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RCT | Randomized controlled clinical trial |
| ACOG | American College of Obstetricians and Gynecologists (ACOG) |
| BMI | Body mass index |
| TUI | Tomographic ultrasound imaging |
| SD | Standard deviation |
| POP | Pelvic organ prolapse |
References
- Artal, R.; O’Toole, M. Exercise During Pregnancy and the Postpartum Period; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2025; Available online: https://www.uptodate.com/contents/exercise-during-pregnancy-and-the-postpartum-period (accessed on 20 June 2025).
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Physical Activity and the Risk of Preeclampsia: A Systematic Review and Meta-Analysis. Epidemiology 2014, 25, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Haakstad, L.A.; Bø, K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: A randomised controlled trial. Eur. J. Contracept. Reprod. Health Care 2011, 16, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Phelan, S. Pregnancy: A “teachable moment” for weight control and obesity prevention. Am. J. Obs. Gynecol. 2010, 202, 135.e1–135.e8. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Prieto, C.; Lardelli-Claret, P.; Olmedo-Requena, R.; Mozas-Moreno, J.; Bueno-Cavanillas, A.; Jiménez-Moleón, J.J. Compliance with leisure-time physical activity recommendations in pregnant women. Acta Obs. Gynecol. Scand. 2011, 90, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice. ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015, 126, e135–e142. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.M.; Strawderman, M.S. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J. Am. Diet. Assoc. 2003, 103, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F. Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J. Pediatr. 1996, 129, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Middleton, P.; Crowther, C.A. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2012, 2012, CD009021. [Google Scholar] [CrossRef] [PubMed]
- Ruchat, S.M.; Mottola, M.F. The important role of physical activity in the prevention and management of gestational diabetes mellitus. Diabetes Metab. Res. Rev. 2013, 29, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.N.; Li, X.L.; Tao, T.J.; Luo, B.R.; Liao, S.J. Physical activity during pregnancy and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2014, 48, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Brik, M.; Fernández-Buhigas, I.; Martin-Arias, A.; Vargas-Terrones, M.; Barakat, R.; Santacruz, B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet. Gynecol. 2019, 53, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Buhigas, I.; Brik, M.; Martin-Arias, A.; Vargas-Terrones, M.; Varillas, D.; Barakat, R.; Santacruz, B. Maternal physiological changes at rest induced by exercise during pregnancy: A randomized controlled trial. Physiol. Behav. 2020, 220, 112863. [Google Scholar] [CrossRef] [PubMed]
- Bø, K. Urinary Incontinence, Pelvic Floor Dysfunction, Exercise and Sport. Sports Med. 2004, 34, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Franco, E.; Perales, M.; López, C.; Mottola, M.F. Exercise during pregnancy is associated with a shorter duration of labor. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 224, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sanda, B.; Vistad, I.; Sagedal, L.R.; Haakstad, L.A.H.; Lohne-Seiler, H.; Torstveit, M.K. What is the effect of physical activity on duration and mode of delivery? Secondary analysis from the Norwegian Fit for Delivery trial. Acta Obstet. Gynecol. Scand. 2018, 97, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Watkins, V.Y.; O’Donnell, C.M.; Perez, M.; Zhao, P.; England, S.; Carter, E.B.; Kelly, J.C.; Frolova, A.; Raghuraman, N. The impact of physical activity during pregnancy on labor and delivery. Am. J. Obstet. Gynecol. 2021, 225, 437.e1–437.e8. [Google Scholar] [CrossRef] [PubMed]
- Bø, K.; Hilde, G.; Stær-Jensen, J.; Siafarikas, F.; Tennfjord, M.K.; Engh, M.E. Does general exercise training before and during pregnancy influence the pelvic floor “opening” and delivery outcome? A 3D/4D ultrasound study following nulliparous pregnant women from mid-pregnancy to childbirth. Br. J. Sports Med. 2015, 49, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Bjerkholt, F.F.; Nyhus, M.Ø.; Mathew, S.; Volløyhaug, I. Are levator hiatal dimensions in mid-pregnancy associated with mode of delivery? Int. Urogynecol. J. 2022, 33, 3529–3534. [Google Scholar] [CrossRef] [PubMed]
- Barca, J.A.; Bravo, C.; Tizón, S.G.; Aracil-Rodriguez, R.; Pina-Moreno, J.M.; Cueto-Hernández, I.; Pintado-Recarte, M.P.; Alvarez-Mon, M.; Ortega, M.A.; De León-Luis, J.A. 3D Ultrasound in Pelvic Floor: Is It Useful as a Prognostic Tool in Type of Labor Development and Subsequent Pelvic Floor Diseases? Int. J. Environ. Res. Public. Health 2022, 19, 11479. [Google Scholar] [CrossRef] [PubMed]
- Guntiñas, A.; Galocha, C.; Madurga, R.; Kirk, J.; Usandizaga, R.; Ángel Rodríguez-Zambrano, M. Application of pelvic floor ultrasound during pregnancy to detect patients at risk of cesarean section due to failure of labor progression in a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 269, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet. Gynecol. 2020, 135, e178–e188. [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R. An exercise program throughout pregnancy: Barakat model. Birth Defects Res. 2021, 113, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Strom, C.J.; McDonald, S.M.; Remchak, M.-M.; Kew, K.A.; Rushing, B.R.; Houmard, J.A.; Tulis, D.A.; Pawlak, R.; Kelley, G.A.; Chasan-Taber, L.; et al. The Influence of Maternal Aerobic Exercise, Blood DHA and EPA Concentrations on Maternal Lipid Profiles. Int. J. Environ. Res. Public Health 2022, 19, 3550. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.P. Ultrasound imaging of the pelvic floor. Part II: Three-dimensional or volume imaging. Ultrasound Obstet. Gynecol. 2004, 23, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Volløyhaug, I.; Mørkved, S.; Salvesen, Ø.; Salvesen, K.Å. Assessment of pelvic floor muscle contraction with palpation, perineometry and transperineal ultrasound: A cross-sectional study. Ultrasound Obstet. Gynecol. 2016, 47, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.P.; Shek, K.L. Tomographic ultrasound imaging of the pelvic floor: Which levels matter most? Ultrasound Obstet. Gynecol. 2009, 33, 698–703. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 22 May 2025).
- Silva-Jose, C.; Sánchez-Polán, M.; Barakat, R.; Díaz-Blanco, Á.; Carrero Martínez, V.; García Benasach, F.; Alzola, I.; Mottola, M.F.; Refoyo, I. Exercise throughout Pregnancy Prevents Excessive Maternal Weight Gain during the COVID-19 Pandemic: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 3392. [Google Scholar] [CrossRef] [PubMed]
- Lanzarone, V.; Dietz, H.P. Three-dimensional ultrasound imaging of the levator hiatus in late pregnancy and associations with delivery outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Montaguti, E.; Dodaro, M.G.; Kamel, R.; Rizzo, N.; Pilu, G. Levator ani muscle coactivation at term is associated with longer second stage of labor in nulliparous women. Ultrasound Obstet. Gynecol. 2019, 53, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; Del Prete, B.; Casadio, P.; Pilu, G.; Youssef, A. The dynamic change of the anteroposterior diameter of the levator hiatus under Valsalva maneuver at term and labor outcome. Neurourol. Urodyn. 2020, 39, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, G.A.; Schweitzer, K.J.; van Hoogenhuijze, N.E.; van der Vaart, C.H. Association between levator hiatal dimensions on ultrasound during first pregnancy and mode of delivery. Ultrasound Obstet. Gynecol. 2015, 45, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Siafarikas, F.; Stær-Jensen, J.; Hilde, G.; Bø, K.; Engh, M.E. Levator Hiatus Dimensions in Late Pregnancy and the Process of Labor: A 3- and 4-Dimensional Transperineal Ultrasound Study. Am. J. Obstet. Gynecol. 2014, 210, 484.e1–484.e7. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, L.; Oom, D.M.J.; Van Bavel, J.; Steegers, E.A.P.; Steensma, A.B. Association of levator injury and urogynecological complaints in women after their first vaginal birth with and without mediolateral episiotomy. Am. J. Obstet. Gynecol. 2019, 220, 93.e1–93.e9. [Google Scholar] [CrossRef] [PubMed]
- Caudwell-Hall, J.; Kamisan Atan, I.; Brown, C.; Guzman Rojas, R.; Langer, S.; Shek, K.L.; Dietz, H.P. Can pelvic floor trauma be predicted antenatally? Acta Obstet. Gynecol. Scand. 2018, 97, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Caudwell-Hall, J.; Kamisan Atan, I.; Martin, A.; Guzman Rojas, R.; Langer, S.; Shek, K.; Dietz, H.P. Intrapartum predictors of maternal levator ani injury. Acta Obstet. Gynecol. Scand. 2017, 96, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Urbankova, I.; Grohregin, K.; Hanacek, J.; Krcmar, M.; Feyereisl, J.; Deprest, J.; Krofta, L. The effect of the first vaginal birth on pelvic floor anatomy and dysfunction. Int. Urogynecol. J. 2019, 30, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Simpson, J. Levator trauma is associated with pelvic organ prolapse. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Bueno, J.A.; Bonomi, M.J.; Suárez-Serrano, C.; Medrano-Sánchez, E.M.; Armijo, A.; Fernández-Palacín, A.; García-Mejido, J.A. Quantification of 3/4D ultrasound pelvic floor changes induced by postpartum muscle training in patients with levator ani muscle avulsion: A parallel randomized controlled trial. Quant. Imaging Med. Surg. 2022, 12, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Hilde, G.; Stær-Jensen, J.; Siafarikas, F.; Engh, M.E.; Bø, K. Postpartum pelvic floor muscle training, levator ani avulsion and levator hiatus area: A randomized trial. Int. Urogynecol. J. 2023, 34, 413–423. [Google Scholar] [CrossRef] [PubMed]
| Control (N = 41) | Exercise (N = 37) | |
|---|---|---|
| Age (years) | 33.9 (4.2) | 32.6 (4.2) |
| Body mass index (kg/m) | 24.5 (4.4) | 24.1 (4.2) |
| Conception method | ||
| Spontaneous | 39 (97.5%) | 32 (88.9%) |
| Assisted | 1 (2.5%) | 4 (11.1%) |
| Ethnicity | ||
| Caucasian | 40 (97.6%) | 35 (94.6%) |
| Latin-American | 1 (2.4%) | 2 (5.4%) |
| Parity | ||
| Nulliparous | 25 (61.0%) | 26 (70.3%) |
| Multiparous | 16 (39.0%) | 11 (29.7%) |
| Smoking before pregnancy | ||
| No | 32 (78.0%) | 30 (81.1%) |
| Yes | 9 (22.0%) | 7 (18.9%) |
| Alcohol before pregnancy | ||
| No | 22 (53.7%) | 20 (54.1%) |
| Yes | 19 (46.3%) | 17 (45.9%) |
| Previous preterm delivery | ||
| No | 38 (97.4%) | 36 (97.3%) |
| Yes | 1 (2.6%) | 1 (2.7%) |
| Previous cesarean delivery | ||
| No | 37 (90.2%) | 35 (97.2%) |
| Yes | 4 (9.8%) | 1 (2.8%) |
| Control (N = 41) | Exercise (N = 37) | p-Value | |
|---|---|---|---|
| Anteroposterior hiatal diameter at rest (mm) | 4.91 (0.76) | 4.60 (0.62) | 0.049 |
| Latero-lateral hiatal diameter at rest (mm) | 3.36 (0.52) | 3.37 (0.67) | 1 |
| Hiatal area at rest (mm) | 12.18 (2.77) | 11.16 (1.92) | 0.180 |
| Pubovisceral muscle thickness (mm) | 1.02 (0.33) | 1.11 (0.92) | 0.387 |
| Avulsion | 0.417 | ||
| No | 36 (90.0%) | 32 (86.5%) | |
| Yes | 3 (7.5%) | 5 (13.5%) | |
| Anteroposterior hiatal diameter at contraction (mm) | 4.13 (0.62) | 3.97 (0.64) | 0.315 |
| Later o-lateral hiatal diameter at contraction (mm) | 3.06 (0.48) | 3.17 (0.48) | 0.270 |
| Hiatal area at contraction (mm) | 9.53 (2.04) | 9.72 (2.06) | 0.771 |
| Pubovisceral muscle thickness at contraction (mm) | 0.94 (0.19) | 0.91 (0.20) | 0.478 |
| Anteroposterior hiatal diameter at Valsalva (mm) | 4.99 (0.77) | 4.93 (0.92) | 0.543 |
| Latero-lateral hiatal diameter at Valsalva (mm) | 3.49 (0.54) | 3.50 (0.63) | 0.921 |
| Hiatal area at Valsalva (mm) | 13.23 (3.22) | 12.96 (3.85) | 0.357 |
| Pubovisceral muscle thickness at Valsalva (mm) | 0.97 (0.18) | 0.98 (0.22) | 0.954 |
| Anteroposterior hiatal diameter contractility (%) | 14.78 (14.16) | 13.50 (11.24) | 0.177 |
| Latero-lateral hiatal diameter contractility (%) | 8.54 (9.48) | 4.04 (11.68) | 0.012 |
| Hiatal area contractility (%) | 20.15 (16.90) | 12.55 (13.18) | 0.020 |
| Anteroposterior hiatal diameter distensibility (%) | 3.21 (13.48) | 8.36 (19.33) | 0.478 |
| Latero-lateral hiatal diameter distensibility (%) | 4.41 (12.41) | 4.89 (14.30) | 0.955 |
| Hiatal area distensibility (%) | 11.49 (23.12) | 17.75 (35.55) | 0.559 |
| Control (N = 41) | Exercise (N = 37) | p-Value | |
|---|---|---|---|
| Gestational age at delivery (weeks) | 39.2 (1.50) | 39.3 (1.53) | 0.766 |
| Labor onset | 0.291 | ||
| Spontaneous labor onset | 22 (53.7%) | 13 (36.1%) | |
| Induction of labor | 16 (39.0%) | 18 (50.0%) | |
| Planned cesarean section | 3 (7.3%) | 5 (13.9%) | |
| Use of oxytocin | 19 (46.3%) | 18 (48.6%) | 1 |
| Use of epidural | 33 (84.6%) | 26 (72.2%) | 0.385 |
| Mode of delivery | 0.692 | ||
| Cesarean section | 5 (12.5%) | 6 (16.7%) | |
| Spontaneous delivery | 29 (72.5%) | 23 (63.9%) | |
| Instrumental delivery | 6 (15.0%) | 7 (19.4%) | |
| Episiotomy | 1 | ||
| No | 34 (97.1%) | 30 (96.8%) | |
| Yes | 1 (2.9%) | 1 (3.2%) | |
| Perineal tear | 0.130 | ||
| No | 12 (30.0%) | 10 (27.8%) | |
| First degree | 14 (35.0%) | 5 (13.9%) | |
| Second degree | 9 (22.5%) | 13 (36.1%) | |
| Third degree | 0 (0%) | 2 (5.6%) | |
| Duration of the first stage (minutes) | 252 (217) | 300 (251) | 0.605 |
| Duration of the second stage (minutes) | 98.1 (86.3) | 102 (97.2) | 0.949 |
| Birthweight (g) | 3150 (448) | 3250 (406) | 0.571 |
| Control (N = 30) | Exercise (N = 24) | p-Value | |
|---|---|---|---|
| Anteroposterior hiatal diameter at rest (mm) | 4.79 (0.75) | 4.72 (0.63) | 0.787 |
| Latero-lateral hiatal diameter at rest (mm) | 3.26 (0.49) | 3.34 (0.44) | 0.632 |
| Hiatal area at rest (mm) | 11.54 (2.33) | 11.51 (2.05) | 0.855 |
| Pubovisceral muscle thickness (mm) | 0.92 (0.17) | 0.93 (0.22) | 0.841 |
| Avulsion | 0.009 | ||
| No | 14 (46.7%) | 19 (79.2%) | |
| Yes | 16 (53.3%) | 5 (20.8%) | |
| Anteroposterior hiatal diameter at contraction (mm) | 4.00 (0.55) | 4.00 (0.64) | 0.470 |
| Latero-lateral hiatal diameter at contraction (mm) | 3.14 (0.58) | 3.04 (0.42) | 0.741 |
| Hiatal area at contraction (mm) | 9.54 (2.43) | 9.42 (1.88) | 0.632 |
| Pubovisceral muscle thickness at contraction (mm) | 1.12 (1.47) | 0.88 (0.16) | 0.663 |
| Anteroposterior hiatal diameter at Valsalva (mm) | 5.32 (0.72) | 5.01 (0.88) | 0.172 |
| Latero-lateral hiatal diameter at Valsalva (mm) | 3.86 (0.72) | 3.58 (0.50) | 0.156 |
| Hiatal area at Valsalva (mm) | 15.61 (4.11) | 13.57 (3.73) | 0.052 |
| Pubovisceral muscle thickness at Valsalva (mm) | 1.02 (0.18) | 0.94 (0.16) | 0.220 |
| Anteroposterior hiatal diameter contractility (%) | 15.78 (9.96) | 14.66 (11.84) | 0.295 |
| Latero-lateral hiatal diameter contractility (%) | 3.56 (13.06) | 8.47 (11.08) | 0.295 |
| Hiatal area contractility (%) | 16.17 (17.52) | 16.92 (15.68) | 0.623 |
| Anteroposterior hiatal diameter distensibility (%) | 12.22 (15.51) | 6.40 (13.42) | 0.215 |
| Latero-lateral hiatal diameter distensibility (%) | 18.89 (16.86) | 7.64 (10.39) | 0.011 |
| Hiatal area distensibility (%) | 36.64 (32.36) | 18.01 (23.46) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Arias, A.; Fernández-Buhigas, I.; Martínez-Campo, D.; Aquise Pino, A.; Rolle, V.; Sánchez-Polan, M.; Silva-Jose, C.; Gil, M.M.; Santacruz, B. Pelvic Floor Adaptation to a Prenatal Exercise Program: Does It Affect Labor Outcomes or Levator Ani Muscle Injury? A Randomized Controlled Trial. Diagnostics 2025, 15, 1853. https://doi.org/10.3390/diagnostics15151853
Martín-Arias A, Fernández-Buhigas I, Martínez-Campo D, Aquise Pino A, Rolle V, Sánchez-Polan M, Silva-Jose C, Gil MM, Santacruz B. Pelvic Floor Adaptation to a Prenatal Exercise Program: Does It Affect Labor Outcomes or Levator Ani Muscle Injury? A Randomized Controlled Trial. Diagnostics. 2025; 15(15):1853. https://doi.org/10.3390/diagnostics15151853
Chicago/Turabian StyleMartín-Arias, Aránzazu, Irene Fernández-Buhigas, Daniel Martínez-Campo, Adriana Aquise Pino, Valeria Rolle, Miguel Sánchez-Polan, Cristina Silva-Jose, Maria M. Gil, and Belén Santacruz. 2025. "Pelvic Floor Adaptation to a Prenatal Exercise Program: Does It Affect Labor Outcomes or Levator Ani Muscle Injury? A Randomized Controlled Trial" Diagnostics 15, no. 15: 1853. https://doi.org/10.3390/diagnostics15151853
APA StyleMartín-Arias, A., Fernández-Buhigas, I., Martínez-Campo, D., Aquise Pino, A., Rolle, V., Sánchez-Polan, M., Silva-Jose, C., Gil, M. M., & Santacruz, B. (2025). Pelvic Floor Adaptation to a Prenatal Exercise Program: Does It Affect Labor Outcomes or Levator Ani Muscle Injury? A Randomized Controlled Trial. Diagnostics, 15(15), 1853. https://doi.org/10.3390/diagnostics15151853








