Molecular Diagnostics in Heart Failure: From Biomarkers to Personalized Medicine
Abstract
1. Introduction
2. Traditional and Emerging Biomarkers in Heart Failure
2.1. Cardiac Troponins and Natriuretic Peptides
2.2. Novel Circulating Biomarkers
3. Genomics and Transcriptomics in HF Diagnostics
3.1. Genetic Risk and Polygenic Scores
3.2. Transcriptomic Profiling
4. Epigenetics and Non-Coding RNAs
5. Proteomics and Metabolomics
5.1. Proteomic Insights
5.2. Metabolomic Profiling
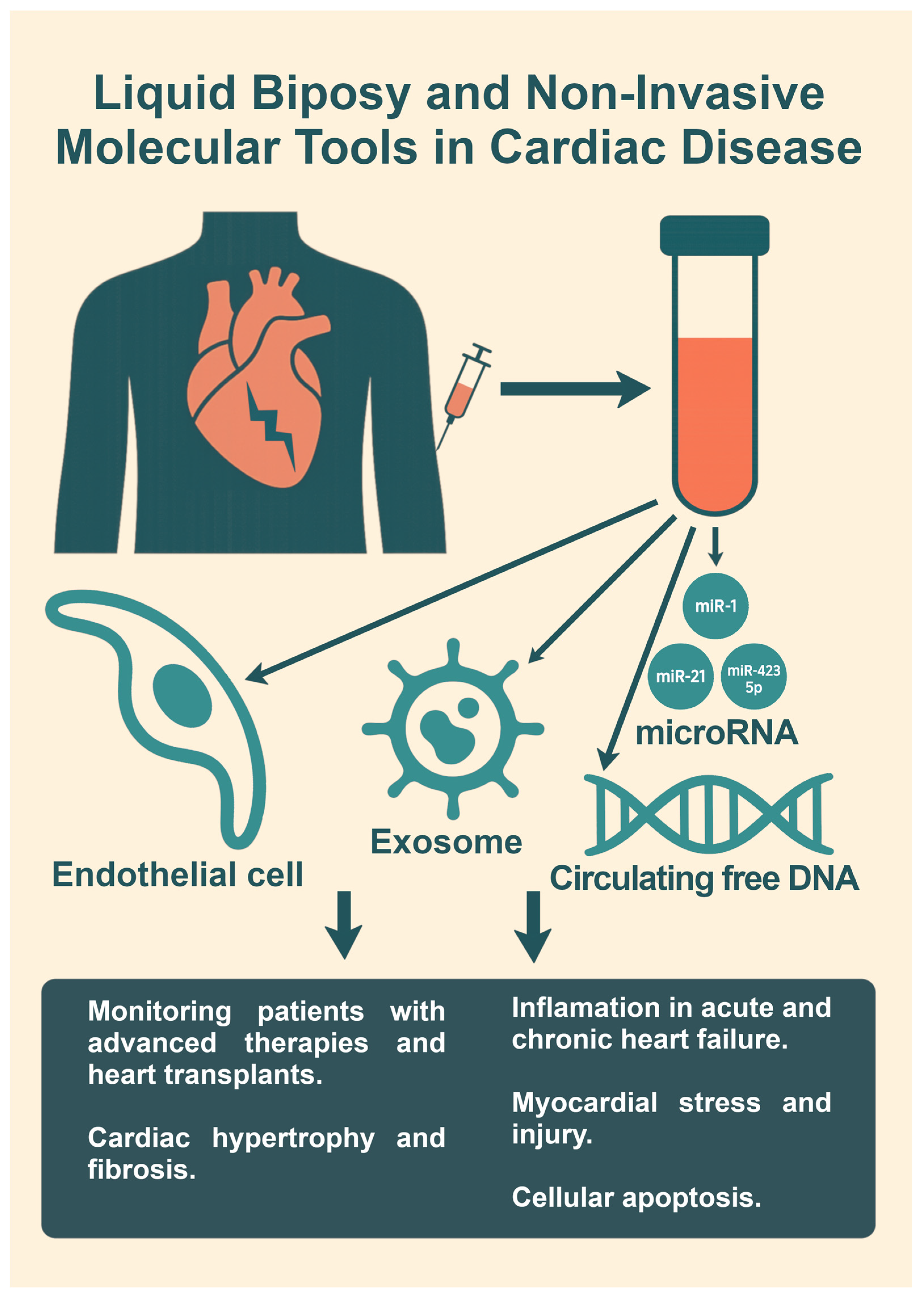
6. Biopsy and Non-Invasive Molecular Tools
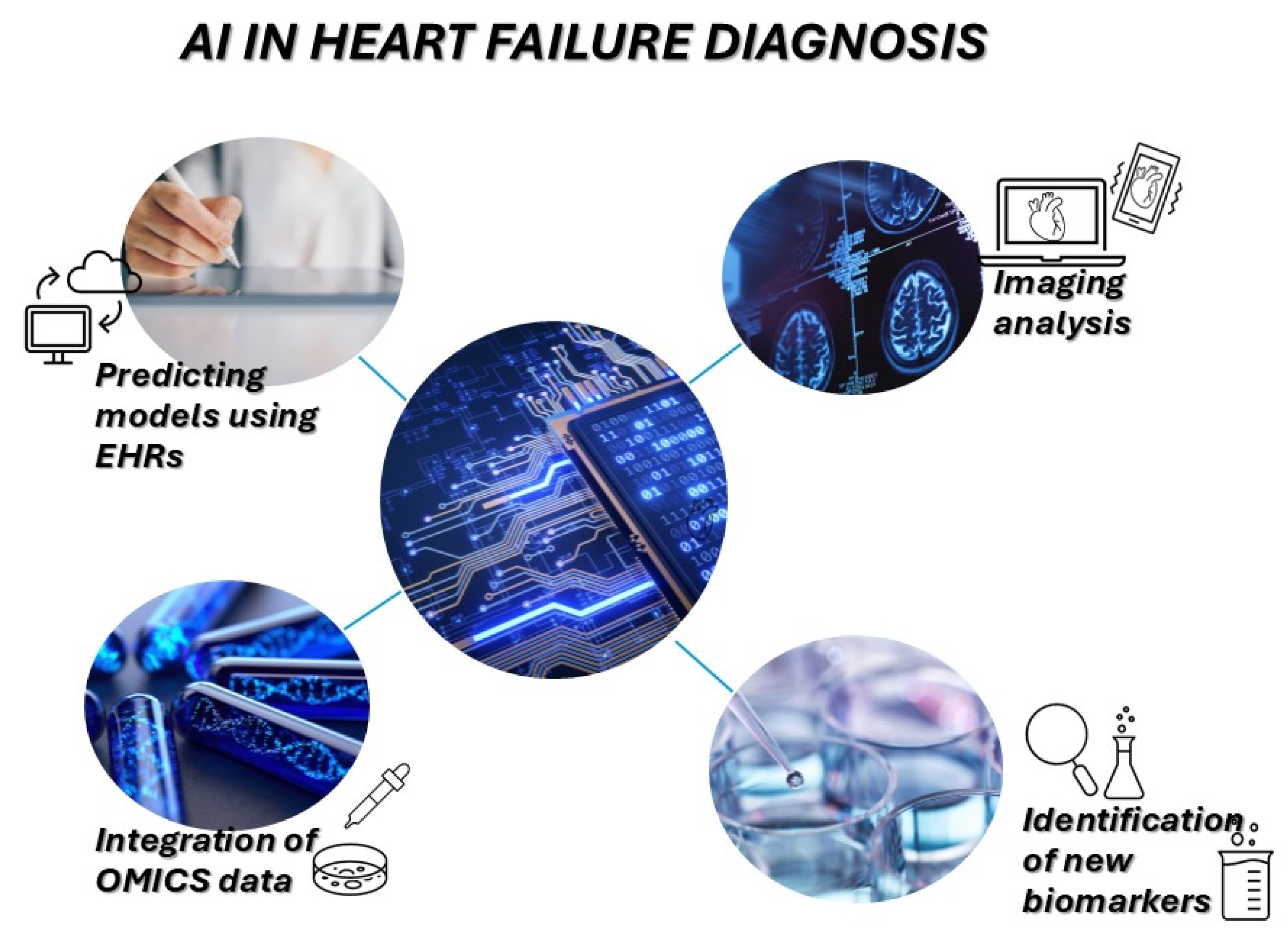
7. Artificial Intelligence and Systems Biology Approaches
8. Clinical Translation: Challenges and Opportunities
9. Limitations
10. Future Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| AI | artificial intelligence |
| ARNIs | angiotensin receptor–neprilysin inhibitors |
| BNP | B-type natriuretic peptide |
| CDSS | clinical decision support systems |
| cfDNA | cell-free DNA |
| CRT | cardiac resynchronization therapy |
| cTnI | cardiac troponins I |
| cTnT | cardiac troponins T |
| DCM | dilated cardiomyopathy |
| DNA | deoxyribonucleic acid |
| EHRs | electronic health records |
| ESC | European Society of Cardiology |
| exRNA | extracellular RNA |
| FGF-23 | fibroblast growth factor-23 |
| GDF-15 | growth differentiation factor-15 |
| GWAS | genome-wide association studies |
| HF | heart failure |
| H-FABP | heart-type fatty acid binding protein |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| HFSA | Heart Failure Society of America |
| IT | information technology |
| lncRNAs | long non-coding RNA |
| LVADs | left ventricular assist devices |
| MI | myocardial infarction |
| miRNAs | micro-RNA |
| ML | machine learning |
| MRAs | mineralocorticoid receptor antagonists |
| MRI | magnetic resonance imaging |
| NGS | next-generation sequencing |
| NLP | natural language processing |
| NT-proBNP | N-terminal proB-type natriuretic peptide |
| PRS | polygenic risk scores |
| PTX-3 | pentraxin-3 |
| RNA | ribonucleic acid |
| scRNA-seq | single-cell RNA sequencing |
| SGLT2 | sodium–glucose cotransporter-2 |
| SNPs | single-nucleotide polymorphisms |
| sST2 | soluble suppression of tumorigenicity-2 |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Țica, O.; Teodorovich, N.; Champsi, A.; Swissa, M. Are the Four Pillars the Ideal Treatment for the Elderly? Cardiology 2023, 148, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Ţica, O.; Khamboo, W.; Kotecha, D. Breaking the Cycle of Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. Card. Fail. Rev. 2022, 8, e32. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.M.; Colaço, B.; Baptista, R.; Gavina, C.; Vitorino, R. Innovations in heart failure management: The role of cutting-edge biomarkers and multi-omics integration. J. Mol. Cell. Cardiol. Plus 2025, 11, 100290. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, D.; Pereira, A.C.; Joseph, J. Drug Discovery and Development for Heart Failure Using Multi-Omics Approaches. Int. J. Mol. Sci. 2025, 26, 2703. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, D.; Giambartolomei, C.; Peloso, G.M.; Dashti, H.; Ferolito, B.R.; Golden, D.; Horimoto, A.; Pietzner, M.; Farber-Eger, E.H.; Wells, Q.S.; et al. Large-scale multi-omics identifies drug targets for heart failure with reduced and preserved ejection fraction. Nat. Cardiovasc. Res. 2025, 4, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, B.; Xie, M.; Li, T. Circulating metabolic signatures of heart failure in precision cardiology. Precis. Clin. Med. 2023, 6, pbad005. [Google Scholar] [CrossRef] [PubMed]
- Karwath, A.; Bunting, K.V.; Gill, S.K.; Tica, O.; Pendleton, S.; Aziz, F.; Barsky, A.D.; Chernbumroong, S.; Duan, J.; Mobley, A.R.; et al. Redefining β-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: A machine learning cluster analysis. Lancet 2021, 398, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, L.; Sun, W.; Zhu, Y.; Zhang, Z.; Chen, L.; Xie, M.; Zhang, L. Artificial Intelligence in Diagnosis of Heart Failure. J. Am. Heart Assoc. 2025, 14, e039511. [Google Scholar] [CrossRef] [PubMed]
- Jelavic, M.M.; Țica, O.; Pintaric, H.; Țica, O. Circulating Neuropeptide Y May Be a Biomarker for Diagnosing Atrial Fibrillation. Cardiology. 2023, 148, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Ahmad, T.; Mulder, H.; Coles, A.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Mark, D.B.; et al. Natriuretic Peptide Response and Outcomes in Chronic Heart Failure with Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Villacorta, H.; Maisel, A.S. Natriuretic Peptide-guided Therapy for Heart Failure. Heart Int. 2022, 16, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Docherty, K.F.; Petrie, M.C.; Januzzi, J.L.; Mueller, C.; Anderson, L.; Bozkurt, B.; Butler, J.; Chioncel, O.; Cleland, J.G.F.; et al. Practical algorithms for early diagnosis of heart failure and heart stress using NT-proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Heck, S.L.; Gulati, G. The Role of Cardioprotection in Cancer Therapy Cardiotoxicity: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Xu, J.; Li, J.; Shaw, W.; Oh, R.; Pfeifer, M.; Butler, J.; Sattar, N.; Mahaffey, K.W.; Neal, B.; et al. Effects of Canagliflozin on Amino-Terminal Pro-B-Type Natriuretic Peptide: Implications for Cardiovascular Risk Reduction. J. Am. Coll. Cardiol. 2020, 76, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic Peptides: Role in the Diagnosis and Management of Heart Failure: A Scientific Statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. J. Card. Fail. 2023, 29, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients with Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Arata, A.; Ricci, F.; Khanji, M.Y.; Mantini, C.; Angeli, F.; Aquilani, R.; Di Baldassarre, A.; Renda, G.; Mattioli, A.V.; Nodari, S.; et al. Sex Differences in Heart Failure: What Do We Know? J. Cardiovasc. Dev. Dis. 2023, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Champsi, A.; Mobley, A.R.; Subramanian, A.; Nirantharakumar, K.; Wang, X.; Shukla, D.; Bunting, K.V.; Molgaard, I.; Dwight, J.; Arroyo, R.C.; et al. Gender and contemporary risk of adverse events in atrial fibrillation. Eur. Heart J. 2024, 45, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.J.; Van Der Meer, P.; de la Porte, P.W.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, M.; Myhre, P.L.; Zelniker, T.A.; Metra, M.; Januzzi, J.L.; Inciardi, R.M. Soluble ST2 in Heart Failure: A Clinical Role beyond B-Type Natriuretic Peptide. J. Cardiovasc. Dev. Dis. 2023, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L., Jr.; Vergaro, G.; Richards, A.M.; Lam, C.S.P.; Latini, R.; Anand, I.S.; Cohn, J.N.; Ueland, T.; Gullestad, L.; et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur. J. Heart Fail. 2020, 22, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Loungani, R.S.; Mentz, R.J.; Agarwal, R.; DeVore, A.D.; Patel, C.B.; Rogers, J.G.; Russell, S.D.; Felker, G.M. Biomarkers in Advanced Heart Failure: Implications for Managing Patients with Mechanical Circulatory Support and Cardiac Transplantation. Circ. Heart Fail. 2020, 13, e006840. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.; Sartini, C.; Suzart-Woischnik, K.; Agrawal, R.; Natani, H.; Gill, S.K.; Wirta, S.B.; Asselbergs, F.W.; Dobson, R.; Denaxas, S.; et al. Identification and Mapping Real-World Data Sources for Heart Failure, Acute Coronary Syndrome, and Atrial Fibrillation. Cardiology 2022, 147, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bai, X.; Lu, J.; Zhang, L.; Yan, X.; Huang, X.; Dai, H.; Wang, Y.; Hou, L.; Wang, S.; et al. Prognostic Value of Multiple Circulating Biomarkers for 2-Year Death in Acute Heart Failure with Preserved Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 779282. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Ripoli, A.; Bayes-Genis, A.; Pascual Figal, D.A.; de Boer, R.A.; Lassus, J.; Mebazaa, A.; Gayat, E.; Breidthardt, T.; et al. Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail. 2017, 5, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.; Wu, A.H.; Fang, J.C.; et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ. Heart Fail. 2011, 4, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Nochioka, K.; Sakata, Y.; Nishimura, K.; Shimokawa, H.; Yasuda, S. Prognostic significance of growth differentiation factor-15 across age in chronic heart failure. ESC Heart Fail. 2024, 11, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Cheng, J.; Qiu, L.; Cheng, X. Copeptin as a Diagnostic and Prognostic Biomarker in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 901990. [Google Scholar] [CrossRef] [PubMed]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Berliner, D.; Bavendiek, U.; Vodovar, N.; Lichtinghagen, R.; David, S.; Patecki, M.; Launay, J.M.; Bauersachs, J.; Haller, H.; et al. Soluble neprilysin, NT-proBNP, and growth differentiation factor-15 as biomarkers for heart failure in dialysis patients (SONGBIRD). Clin. Res. Cardiol. 2020, 109, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Binnenmars, S.H.; Hoogslag, G.E.; Yeung, S.M.H.; Brouwers, F.P.; Bakker, S.J.L.; van Gilst, W.H.; Gansevoort, R.T.; Navis, G.; Voors, A.A.; de Borst, M.H. Fibroblast Growth Factor 23 and Risk of New Onset Heart Failure with Preserved or Reduced Ejection Fraction: The PREVEND Study. J. Am. Heart Assoc. 2022, 11, e024952. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Z.; Lei, W.; Shen, M.; Tang, J.; Xu, X.; Yang, Y.; Zhang, H. Pentraxin 3: A promising therapeutic target for cardiovascular diseases. Ageing Res. Rev. 2024, 93, 102163. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; van Kimmenade, R.R.; et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J. Am. Coll. Cardiol. 2007, 50, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Nijst, P.; Mullens, W. Current Approach to Decongestive Therapy in Acute Heart Failure. Curr. Heart Fail. Rep. 2015, 12, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Jin, J.L.; Cao, Y.X.; Liu, H.H.; Zhang, Y.; Guo, Y.L.; Wu, N.Q.; Zhu, C.G.; Gao, Y.; Xu, R.X.; et al. Heart-type fatty acid binding protein predicts cardiovascular events in patients with stable coronary artery disease: A prospective cohort study. Ann. Transl. Med. 2020, 8, 1349. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Lejeune, S.; Slimani, A.; de Meester, C.; Ahn As, S.A.; Rousseau, M.F.; Mihaela, A.; Ginion, A.; Ferracin, B.; Pasquet, A.; et al. Fibroblast growth factor 23: A biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 2494–2507. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Larsson, A.; Melhus, H.; Lind, L.; Larsson, T.E. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 2009, 207, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Bayés-Genís, A.; Barallat, J.; Galán, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Urrutia, A.; Lupón, J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Hasselblad, V.; Hernandez, A.F.; O’Connor, C.M. Biomarker-guided therapy in chronic heart failure: A meta-analysis of randomized controlled trials. Am. Heart J. 2009, 158, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple Plasma Biomarkers for Risk Stratification in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Henry, A.; Cannie, D.; Lee, M.; Miller, D.; McGurk, K.A.; Bond, I.; Xu, X.; Issa, H.; Francis, C.; et al. Genome-wide association analysis provides insights into the molecular etiology of dilated cardiomyopathy. Nat. Genet. 2024, 56, 2646–2658. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Dixit, P.; Khanom, N.; Sanghera, G.; McGurk, K.A. The Genetic Factors Influencing Cardiomyopathies and Heart Failure across the Allele Frequency Spectrum. J. Cardiovasc. Transl. Res. 2024, 17, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; de Marvao, A.; Dawes, T.J.W.; et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Jolfayi, A.G.; Kohansal, E.; Ghasemi, S.; Naderi, N.; Hesami, M.; MozafaryBazargany, M.; Moghadam, M.H.; Fazelifar, A.F.; Maleki, M.; Kalayinia, S. Exploring TTN variants as genetic insights into cardiomyopathy pathogenesis and potential emerging clues to molecular mechanisms in cardiomyopathies. Sci. Rep. 2024, 14, 5313. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Mo, X.; Finan, C.; Chaffin, M.D.; Speed, D.; Issa, H.; Denaxas, S.; Ware, J.S.; Zheng, S.L.; Malarstig, A.; et al. Genome-wide association study meta-analysis provides insights into the etiology of heart failure and its subtypes. Nat. Genet. 2025, 57, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yang, N.; Luo, Z.; Huang, R.; Li, Q. Key Cell Types and Biomarkers in Heart Failure Identified through Analysis of Single-Cell and Bulk RNA Sequencing Data. Mediat. Inflamm. 2023, 2023, 8384882. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Tsuji, T. One-way flow over uniformly heated U-shaped bodies driven by thermal edge effects. Sci. Rep. 2022, 12, 1929. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Tang, W.H.W. Epigenetics in Cardiac Hypertrophy and Heart Failure. JACC Basic Transl. Sci. 2019, 4, 976–993. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.Y.; Jou, E.; Khong, P.L.; Foo, R.S.Y.; Sia, C.H. Epigenetics in Heart Failure. Int. J. Mol. Sci. 2024, 25, 12010. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Condorelli, G. Epigenetic modifications and noncoding RNAs in cardiac hypertrophy and failure. Nat. Rev. Cardiol. 2015, 12, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Myhre, P.L.; Arthur, V.; Dorbala, P.; Rasheed, H.; Buckley, L.F.; Claggett, B.; Liu, G.; Ma, J.; Nguyen, N.Q.; et al. Large scale plasma proteomics identifies novel proteins and protein networks associated with heart failure development. Nat. Commun. 2024, 15, 528. [Google Scholar] [CrossRef] [PubMed]
- Abou Kamar, S.; Andrzejczyk, K.; Petersen, T.B.; Chin, J.F.; Aga, Y.S.; de Bakker, M.; Akkerhuis, K.M.; Geleijnse, M.; Brugts, J.J.; Sorop, O.; et al. The plasma proteome is linked with left ventricular and left atrial function parameters in patients with chronic heart failure. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Lynch, M.; Yin, X.; Mayr, M. Systems biology-opportunities and challenges: The application of proteomics to study the cardiovascular extracellular matrix. Cardiovasc. Res. 2016, 112, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Short, M.I.; Rasheed, H.; Lin, H.; Jonasson, C.; Yang, Q.; Hveem, K.; Felix, J.F.; Morrison, A.C.; Wild, P.S.; et al. Aptamer-Based Proteomic Platform Identifies Novel Protein Predictors of Incident Heart Failure and Echocardiographic Traits. Circ. Heart Fail. 2020, 13, e006749. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Culler, K.L.; Sinha, A.; Filipp, M.; Giro, P.; Allen, N.B.; Taylor, K.D.; Guo, X.; Thorp, E.; Freed, B.H.; Greenland, P.; et al. Metabolomic profiling identifies novel metabolites associated with cardiac dysfunction. Sci. Rep. 2024, 14, 20694. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Fu, Z.; Jones, P.; Kwee, L.C.; Windsor, S.L.; Ilkayeva, O.; Newgard, C.B.; Margulies, K.B.; Husain, M.; Inzucchi, S.E.; et al. Metabolomic Profiling of the Effects of Dapagliflozin in Heart Failure with Reduced Ejection Fraction: DEFINE-HF. Circulation 2022, 146, 808–818. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Artner, T.; Sharma, S.; Lang, I.M. Nucleic acid liquid biopsies in cardiovascular disease: Cell-free DNA liquid biopsies in cardiovascular disease. Atherosclerosis 2024, 398, 118583. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J. Current and Future Perspectives of Cell-Free DNA in Liquid Biopsy. Curr. Issues Mol. Biol. 2022, 44, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Kalligosfyri, P.M.; Lamprou, E.; Kalogianni, D.P. Emerging Sensing Technologies for Liquid Biopsy Applications: Steps Closer to Personalized Medicine. Sensors 2024, 24, 7902. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Prosperi, S.; Severino, P.; Myftari, V.; Correale, M.; Perrone Filardi, P.; Badagliacca, R.; Fedele, F.; Vizza, C.D.; Palazzuoli, A. MicroRNA and Heart Failure: A Novel Promising Diagnostic and Therapeutic Tool. J. Clin. Med. 2024, 13, 7560. [Google Scholar] [CrossRef] [PubMed]
- Melman, Y.F.; Shah, R.; Das, S. MicroRNAs in heart failure: Is the picture becoming less miRky? Circ. Heart Fail. 2014, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Tomaniak, M.; Błaszczyk, O.; Kołtowski, Ł.; Filipiak, K.J.; Sitkiewicz, D. Circulating microribonucleic acids miR-1, miR-21 and miR-208a in patients with symptomatic heart failure: Preliminary results. Arch. Cardiovasc. Dis. 2015, 108, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Ziegler, O.; Yeri, A.; Liu, X.; Murthy, V.; Rabideau, D.; Xiao, C.Y.; Hanspers, K.; Belcher, A.; Tackett, M.; et al. MicroRNAs Associated with Reverse Left Ventricular Remodeling in Humans Identify Pathways of Heart Failure Progression. Circ. Heart Fail. 2018, 11, e004278. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; Benincasa, G.; Della Mura, N.; Nicoletti, G.F.; Napoli, C. Epigenetic-sensitive liquid biomarkers and personalised therapy in advanced heart failure: A focus on cell-free DNA and microRNAs. J. Clin. Pathol. 2020, 73, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Pereira, P.; Tuma, P. Quality improvement at times of crisis. BMJ 2021, 373, n928. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, A.; Belmonte, M.; Masetti, M.; Bergamaschi, L.; Paolisso, P.; Borgese, L.; Angeli, F.; Armillotta, M.; Dierckx, R.; Verstreken, S.; et al. CTA-Derived Pericoronary Fat Attenuation Index Predicts Allograft Rejection and Cardiovascular Events in Heart Transplant Recipients. JACC Cardiovasc. Imaging 2025, 18, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Guo, J.; Gu, Z.; Tang, W.; Tao, H.; You, S.; Jia, D.; Sun, Y.; Jia, P. Machine learning and multi-omics integration: Advancing cardiovascular translational research and clinical practice. J. Transl. Med. 2025, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Karwath, A.; Uh, H.W.; Cardoso, V.R.; Gu, Z.; Barsky, A.; Slater, L.; Acharjee, A.; Duan, J.; Dall’Olio, L.; et al. Artificial intelligence to enhance clinical value across the spectrum of cardiovascular healthcare. Eur. Heart J. 2023, 44, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Bunting, K.V.; Brind, D.; Thorley, A.; Karwath, A.; Lu, W.; Zhou, D.; Wang, X.; Mobley, A.R.; et al. Development of automated neural network prediction for echocardiographic left ventricular ejection fraction. Front. Med. 2024, 11, 1354070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mobley, A.R.; Tica, O.; Okoth, K.; Ghosh, R.E.; Myles, P.; Williams, T.; Haynes, S.; Nirantharakumar, K.; Shukla, D.; et al. Systematic approach to outcome assessment from coded electronic healthcare records in the DaRe2THINK NHS-embedded randomized trial. Eur. Heart J. Digit. Health 2022, 3, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.W.; Abraham, W.T.; Bhatt, A.S.; Dunn, J.; Felker, G.M.; Jain, S.S.; Lindsell, C.J.; Mace, M.; Martyn, T.; Shah, R.U.; et al. Artificial Intelligence in Cardiovascular Clinical Trials. J. Am. Coll. Cardiol. 2024, 84, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Windecker, D.; Baj, G.; Shiri, I.; Kazaj, P.M.; Kaesmacher, J.; Gräni, C.; Siontis, G.C.M. Generalizability of FDA-Approved AI-Enabled Medical Devices for Clinical Use. JAMA Netw. Open 2025, 8, e258052. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Schutte, N.M.; Buttigieg, S.; Novillo-Ortiz, D.; Sutherland, E.; Anderson, M.; de Witte, B.; Peolsson, M.; Unim, B.; Pavlova, M.; et al. Mapping the regulatory landscape for artificial intelligence in health within the European Union. NPJ Digit. Med. 2024, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Manríquez Roa, T.; Biller-Andorno, N. Black box algorithms in mental health apps: An ethical reflection. Bioethics 2023, 37, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Adewale, B.A.; Huang, C.J.; Nta, M.T.; Ademiju, P.O.; Pathmarajah, P.; Hang, M.K.; Adesanya, O.; Abdullateef, R.O.; Babatunde, A.O.; et al. A scoping review of reporting gaps in FDA-approved AI medical devices. NPJ Digit. Med. 2024, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Chulde-Fernández, B.; Enríquez-Ortega, D.; Guevara, C.; Navas, P.; Tirado-Espín, A.; Vizcaíno-Imacaña, P.; Villalba-Meneses, F.; Cadena-Morejon, C.; Almeida-Galarraga, D.; Acosta-Vargas, P. Classification of Heart Failure Using Machine Learning: A Comparative Study. Life 2025, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Liastuti, L.D.; Budi Siswanto, B.; Sukmawan, R.; Jatmiko, W.; Nursakina, Y.; Putri, R.Y.I.; Jati, G.; Nur, A.A. Detecting Left Heart Failure in Echocardiography through Machine Learning: A Systematic Review. Rev. Cardiovasc. Med. 2022, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.W.; Singh, P.; Reeder, C.; Claggett, B.; Marti-Castellote, P.M.; Lau, E.S.; Khurshid, S.; Batra, P.; Lubitz, S.A.; Maddah, M.; et al. Natural Language Processing for Adjudication of Heart Failure Hospitalizations in a Multi-Center Clinical Trial. medRxiv 2023. [Google Scholar] [CrossRef]
- Ebad, S.A.; Alhashmi, A.; Amara, M.; Miled, A.B.; Saqib, M. Artificial Intelligence-Based Software as a Medical Device (AI-SaMD): A Systematic Review. Healthcare 2025, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Ntinginya, N.E.; Kuchaka, D.; Orina, F.; Mwebaza, I.; Liyoyo, A.; Miheso, B.; Aturinde, A.; Njeleka, F.; Kiula, K.; Msoka, E.F.; et al. Unlocking the health system barriers to maximise the uptake and utilisation of molecular diagnostics in low-income and middle-income country setting. BMJ Glob. Health 2021, 6, e005357. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.A.; Hua, N.; Buchanan, J.; Christensen, K.D.; Frederix, G.W.J.; Goranitis, I.; Ijzerman, M.; Jansen, J.P.; Lavelle, T.A.; Regier, D.A.; et al. Paving the path for implementation of clinical genomic sequencing globally: Are we ready? Health Aff. Sch. 2024, 2, qxae053. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Ahmad, T.; Anstrom, K.J.; Adams, K.F.; Cooper, L.S.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L.; Leifer, E.S.; et al. Rationale and design of the GUIDE-IT study: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure. JACC Heart Fail. 2014, 2, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Ferreira, J.P.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Lang, C.C.; Metra, M.; et al. Multimarker profiling identifies protective and harmful immune processes in heart failure: Findings from BIOSTAT-CHF. Cardiovasc. Res. 2022, 118, 1964–1977. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhães, P.; Schmidt, S.; Vale, N. Advancing Precision Medicine: A Review of Innovative In Silico Approaches for Drug Development, Clinical Pharmacology and Personalized Healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Liu, P.P.; Lanfear, D.E.; de Boer, R.A.; González, A.; Thum, T.; Emdin, M.; Januzzi, J.L. Omics phenotyping in heart failure: The next frontier. Eur. Heart J. 2020, 41, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.E.; Cocciolo, A.; Slavov, D.; Jones, K.L.; Sweet, J.R.; Graw, S.L.; Reece, T.B.; Ambardekar, A.V.; Bristow, M.R.; Mestroni, L.; et al. Transcriptome analysis of human heart failure reveals dysregulated cell adhesion in dilated cardiomyopathy and activated immune pathways in ischemic heart failure. BMC Genom. 2018, 19, 812. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gray, A.; Xue, L.; Farb, M.G.; Ayalon, N.; Andersson, C.; Ko, D.; Benjamin, E.J.; Levy, D.; Vasan, R.S.; et al. Metabolomic Profiles, Ideal Cardiovascular Health, and Risk of Heart Failure and Atrial Fibrillation: Insights from the Framingham Heart Study. J. Am. Heart Assoc. 2023, 12, e028022. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, A.; Počivavšek, K.; Petrovič, D.; Peterlin, B. The Role of microRNAs in Heart Failure: A Systematic Review. Front. Cardiovasc. Med. 2020, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Acar, E.; Izci, S.; Donmez, I.; Yilmaz, M.F.; Ozgul, N.; Kayabası, O.; Gokce, M.; Güneş, Y.; Izgi, I.A.; Kirma, C. The Left Distal transradial access site could give a safe alternate site for transradial coronary intervention (The Litaunent Study). Angiology 2024, 75, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Pfeifer, J.D. Pitfalls in molecular diagnostics. Semin. Diagn. Pathol. 2019, 36, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Czepluch, F.S.; Wollnik, B.; Hasenfuß, G. Genetic determinants of heart failure: Facts and numbers. ESC Heart Fail. 2018, 5, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mudd-Martin, G.; Cirino, A.L.; Barcelona, V.; Fox, K.; Hudson, M.; Sun, Y.V.; Taylor, J.Y.; Cameron, V.A. Considerations for Cardiovascular Genetic and Genomic Research with Marginalized Racial and Ethnic Groups and Indigenous Peoples: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2021, 14, e000084. [Google Scholar] [CrossRef] [PubMed]
- Crea, F. Challenges in heart failure: From actionability of genetic variants in cardiopmyopathies to new therapeutic targets. Eur. Heart J. 2022, 43, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.; Baker, W.L.; Bosak, K.; Breathett, K.; Carter, S.; Drazner, M.H.; Dunlay, S.M.; Fonarow, G.C.; et al. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics An Updated 2024 Report from the Heart Failure Society of America. J. Card. Fail. 2025, 31, 66–116. [Google Scholar] [CrossRef] [PubMed]
- Zakiyah, N.; Marulin, D.; Alfaqeeh, M.; Puspitasari, I.M.; Lestari, K.; Lim, K.K.; Fox-Rushby, J. Economic Evaluations of Digital Health Interventions for Patients with Heart Failure: Systematic Review. J. Med. Internet Res. 2024, 26, e53500. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.T.; Zheng, J.; Kalwani, N.M.; Gupta, A.; Calma, J.; Skye, M.; Lan, R.; Yu, B.; Spertus, J.A.; Heidenreich, P.A. Impact of Patient-Reported Outcome Measurement in Heart Failure Clinic on Clinician Health Status Assessment and Patient Experience: A Substudy of the PRO-HF Trial. Circ. Heart Fail. 2023, 16, e010280. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Giamouzis, G.; Kitai, T.; Skoularigis, J.; Starling, R.C.; Xanthopoulos, A. A Holistic View of Advanced Heart Failure. Life 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Hajishah, H.; Kazemi, D.; Safaee, E.; Amini, M.J.; Peisepar, M.; Tanhapour, M.M.; Tavasol, A. Evaluation of machine learning methods for prediction of heart failure mortality and readmission: Meta-analysis. BMC Cardiovasc. Disord. 2025, 25, 264. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Cyr, D.D.; Ward, J.H.; Hernandez, A.F.; Lepage, S.; Morrow, D.A.; Sharma, K.; Claggett, B.L.; Starling, R.C.; Velazquez, E.J.; et al. Association of Sacubitril/Valsartan vs. Valsartan with Blood Pressure Changes and Symptomatic Hypotension: The PARAGLIDE-HF Trial. J. Card. Fail. 2024, 30, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.J.; Zareba, K.M.; Ni, H.; Bello-Pardo, E.; Huggins, G.S.; Hershberger, R.E. Validating an Idiopathic Dilated Cardiomyopathy Diagnosis Using Cardiovascular Magnetic Resonance: The Dilated Cardiomyopathy Precision Medicine Study. Circ. Heart Fail. 2022, 15, e008877. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Luciano, J.S.; Andersson, B.; Batchelor, C.; Bodenreider, O.; Clark, T.; Denney, C.K.; Domarew, C.; Gambet, T.; Harland, L.; Jentzsch, A.; et al. The Translational Medicine Ontology and Knowledge Base: Driving personalized medicine by bridging the gap between bench and bedside. J. Biomed. Semant. 2011, 2 (Suppl. S2), S1. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, F.; Randhawa, V.K. Singling out the Heart: Revolutionizing Heart Failure by Harnessing Translational Technologies. J. Card. Fail. 2023, 29, 939–942. [Google Scholar] [CrossRef] [PubMed]
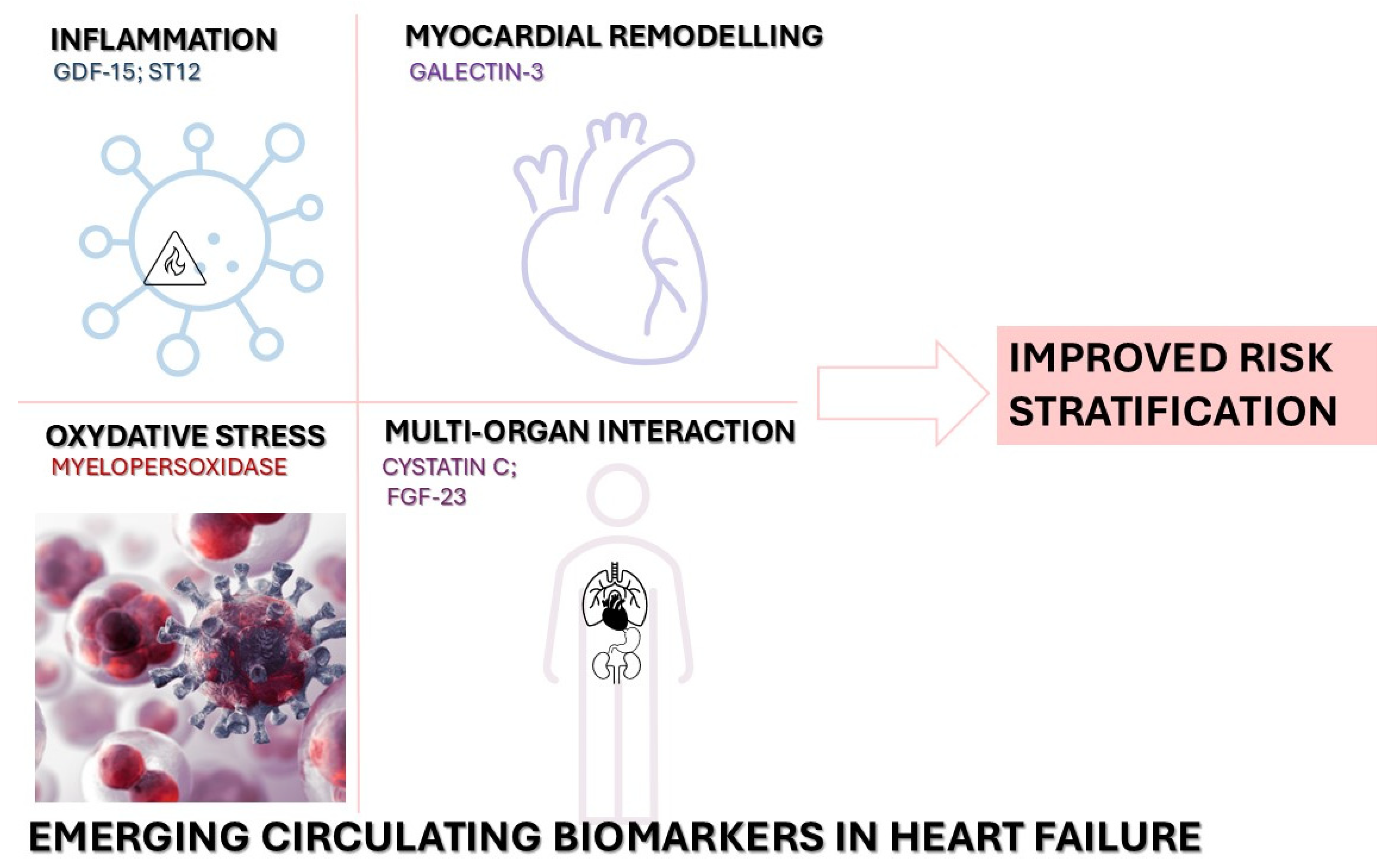
| Biomarker/Strategy | Advantages | Limitations |
|---|---|---|
| Galectin-3 [25] | Reflects fibrosis and inflammation; secreted by activated macrophages/fibroblasts; Linked to adverse remodeling in HFpEF and HFrEF; Independent prognostic marker for mortality and rehospitalization | Influenced by renal dysfunction and other fibrotic diseases, reducing specificity; Limited dynamic change with therapy, so less useful for monitoring response; No universally accepted assay thresholds. |
| Soluble ST2 (sST2) [40] | Marker of myocardial strain and inflammation (IL-33 decoy receptor); Adds prognostic value beyond NP and troponin in acute and chronic HF; Less affected by age, obesity, or renal function than natriuretic peptides. | Assay cost and availability barriers; Cutoffs vary between platforms; no standard thresholds; Elevated in systemic inflammatory states, reducing cardiac specificity. |
| GDF-15 [41,42] | Reflects oxidative stress and mitochondrial dysfunction; Correlates with all-cause mortality, especially in elderly/comorbid HF patients; Provides incremental prognostic information when combined with NP and troponin. | Highly non-specific (elevated in malignancy, renal disease, inflammation); Limited data on serial changes with therapy; Not yet part of routine HF management algorithms. |
| Copeptin [43] | Surrogate for vasopressin release; reflects neurohormonal activation; Prognostic in acute decompensated HF and post-MI HF; Stable peptide, easier to measure than vasopressin. | Rises with any systemic stress (sepsis, stroke), limiting cardiac specificity; Assay variability; no consensus on clinical cutoffs; Role in chronic HF monitoring remains undefined. |
| H-FABP [44] | Early marker of myocardial ischemia; appears rapidly after injury; May aid rapid diagnosis of acute HF in ED settings. | Very short half-life; timing critical; Cross-reactivity with skeletal muscle FABP can confound results; Limited prognostic value in stable, chronic HF. |
| FGF-23 [45,46] | Involved in phosphate regulation; elevated in HF and linked to LV hypertrophy; Associated with adverse outcomes; may identify cardio-renal axis dysfunction. | Strongly influenced by chronic kidney disease; Assay standardization lacking; variable thresholds; Limited evidence for therapy-monitoring utility. |
| Pentraxin-3 (PTX-3) [45,47] | Acute-phase protein reflecting vascular and myocardial inflammation; Predicts CV events, particularly in inflammation-driven HF. | Rises in any acute inflammatory state, limiting specificity; Sparse data on serial changes with HF treatment; No widely adopted assay or interpretive ranges. |
| Neprilysin [48] | Enzyme targeted by ARNIs; circulating levels may reflect neurohormonal balance and response to therapy; Potential dual role as biomarker and therapeutic target. | Assay complexity and lack of standardization; Directly modulated by ARNI therapy, complicating interpretation; Clinical thresholds not established; utility beyond research unproven. |
| Multi-marker strategies [49,50] | Integration of NP, troponin, sST2, Galectin-3, GDF-15, etc., improves risk stratification and prognostic accuracy; Captures multiple pathophysiological axes (hemodynamic stress, fibrosis, inflammation, neurohormonal activation). | Increased cost and logistical complexity; Analytical variability and lack of harmonized panels limit clinical adoption; No consensus on which combinations/algorithms to use in routine care. |
| Acronym/Short Name | Title | Phase | Biomarker/Tool Investigated | Country | Intervention/ Treatment | Population | Primary Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| DCM Precision Medicine Study * | Genetic Testing and Cardiovascular Magnetic Resonance Imaging in Dilated Cardiomyopathy | N/A | Genetic testing, CMR imaging | USA | Genetic testing and CMR imaging | Patients with idiopathic dilated cardiomyopathy | Identification of myocardial scar and etiology | NCT03037632 [116] |
| HERMES * | Effects of Ziltivekimab Versus Placebo on Morbidity and Mortality in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction and Systemic Inflammation | Phase 3 | High-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6) | International | Ziltivekimab (IL-6 inhibitor) | Patients with HFpEF/HFmrEF and systemic inflammation | Cardiovascular death or heart failure hospitalization | NCT05636176 |
| ZEUS * | Effects of Ziltivekimab Versus Placebo on Cardiovascular Outcomes in Participants With Established Atherosclerotic Cardiovascular Disease, Chronic Kidney Disease and Systemic Inflammation | Phase 3 | hsCRP, IL-6 | International | Ziltivekimab | Patients with atherosclerotic cardiovascular disease, chronic kidney disease, and systemic inflammation | Major adverse cardiovascular events (MACE) | NCT05021835 |
| ATTRibute-CM * | Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy | Phase 3 | Transthyretin (TTR) stabilization | International | Acoramidis (TTR stabilizer) | Patients with transthyretin amyloid cardiomyopathy (ATTR-CM) | Composite of all-cause mortality and cardiovascular-related hospitalization | NCT03860935 |
| CUPID 2 † | Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease | Phase 2b | SERCA2a gene therapy | USA | Mydicar (AAV1/SERCA2a gene therapy) | Patients with advanced heart failure | Time to recurrent heart failure-related events | NCT01643330 [117] |
| RESCUE-2 † | Efficacy and Safety of Interleukin-6 Inhibition With Ziltivekimab in Patients at High Risk of Atherosclerotic Events in Japan | Phase 2 | hsCRP, IL-6 | Japan | Ziltivekimab | Patients with chronic kidney disease and systemic inflammation | Reduction in hsCRP levels | NCT04626505 |
| CDR132L † | Safety and Tolerability of CDR132L in Patients With Heart Failure | Phase 1b | miR-132 levels | Germany | CDR132L (antisense oligonucleotide targeting miR-132) | Patients with heart failure | Safety and tolerability | NCT04045405 [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țica, O.; Țica, O. Molecular Diagnostics in Heart Failure: From Biomarkers to Personalized Medicine. Diagnostics 2025, 15, 1807. https://doi.org/10.3390/diagnostics15141807
Țica O, Țica O. Molecular Diagnostics in Heart Failure: From Biomarkers to Personalized Medicine. Diagnostics. 2025; 15(14):1807. https://doi.org/10.3390/diagnostics15141807
Chicago/Turabian StyleȚica, Ovidiu, and Otilia Țica. 2025. "Molecular Diagnostics in Heart Failure: From Biomarkers to Personalized Medicine" Diagnostics 15, no. 14: 1807. https://doi.org/10.3390/diagnostics15141807
APA StyleȚica, O., & Țica, O. (2025). Molecular Diagnostics in Heart Failure: From Biomarkers to Personalized Medicine. Diagnostics, 15(14), 1807. https://doi.org/10.3390/diagnostics15141807








