Qualitative and Quantitative Analysis of Contrast-Enhanced Ultrasound in the Characterization of Kidney Cancer Subtypes
Abstract
1. Introduction
2. Materials and Methods
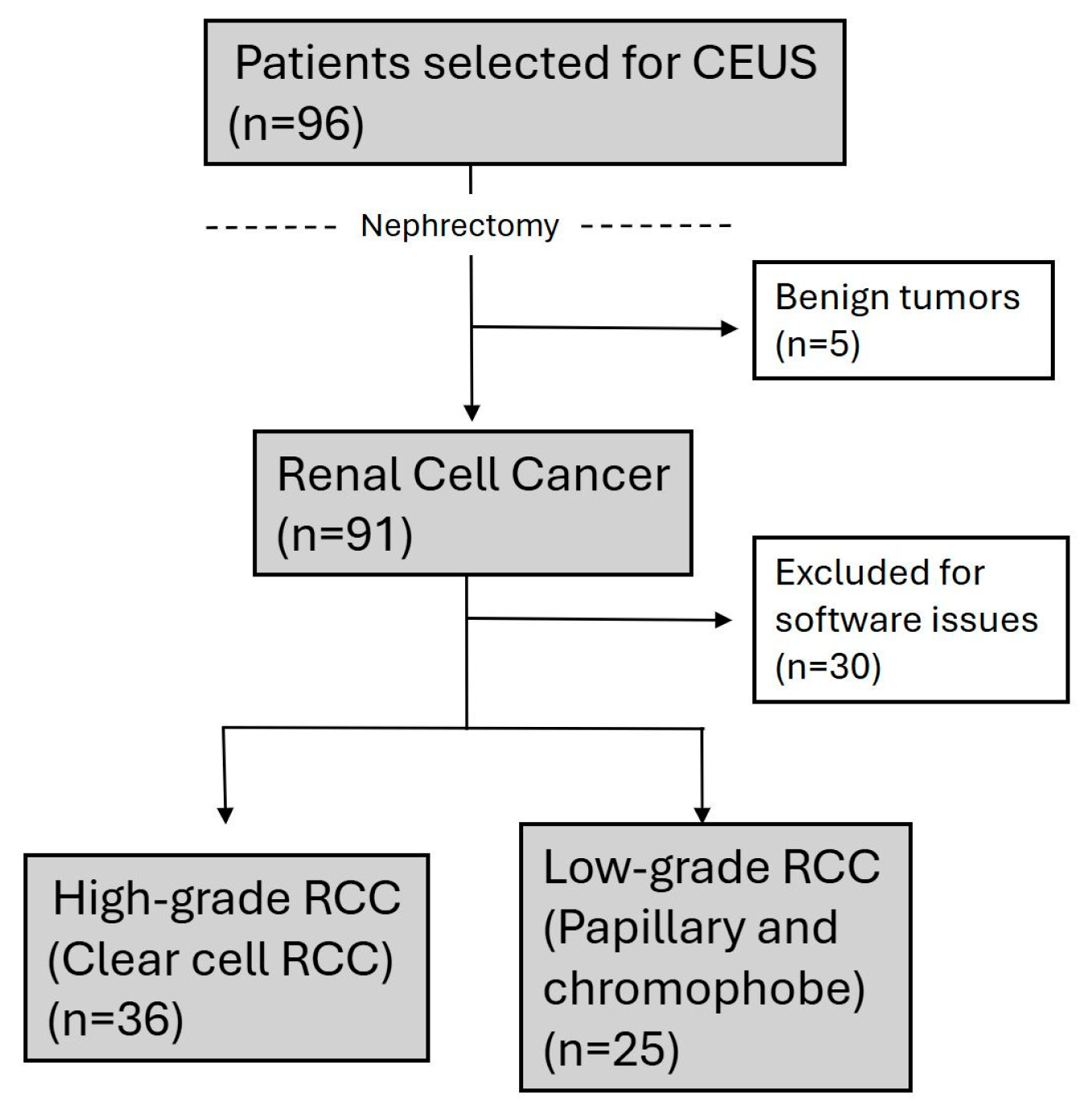
2.1. Study Design and Patient Population
2.2. Image Acquisition and Analysis
2.3. Statistical Analyses
3. Results
3.1. Univariate Analysis
3.2. Binary Logistic Regression Model
4. Discussion
4.1. Qualitative Evaluation
4.2. Quantitative Evaluation
4.3. Limitations and Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEUS | Contrast-Enhanced Ultrasound |
| RCC | Renal Cell Carcinoma |
| ccRCC | Clear Cell Renal Cell Carcinoma |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| US | Ultrasound |
References
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Lyske, J.; Mathew, R.P.; Hutchinson, C.; Patel, V.; Low, G. Multimodality imaging review of focal renal lesions. Egypt. J. Radiol. Nucl. Med. 2021, 52, 14. [Google Scholar] [CrossRef]
- Prasad, S.R.; Humphrey, P.A.; Catena, J.R.; Narra, V.R.; Srigley, J.R.; Cortez, A.D.; Dalrymple, N.C.; Chintapalli, K.N. Common and uncommon histologic subtypes of renal cell carcinoma: Imaging spectrum with pathologic correlation. Radiographics 2006, 26, 1795–1806; discussion 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Paño, B.; Soler, A.; Goldman, D.A.; Salvador, R.; Buñesch, L.; Sebastià, C.; Nicolau, C. Usefulness of multidetector computed tomography to differentiate between renal cell carcinoma and oncocytoma. A model validation. Br. J. Radiol. 2020, 93, 20200064. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, I.; Cadeddu, J.A. How We Do It: Managing the Indeterminate Renal Mass with the MRI Clear Cell Likelihood Score. Radiology 2022, 302, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.S.; Fraum, T.J.; Ballard, D.H.; Hoegger, M.J.; Itani, M.; Rajput, M.Z.; Lanier, M.H.; Cusworth, B.M.; Mehrsheikh, A.L.; Cabrera-Lebron, J.A.; et al. Renal Mass Imaging with MRI Clear Cell Likelihood Score: A User’s Guide. Radiographics 2023, 43, e220209. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Bertolotto, M.; Clevert, D.A.; Correas, J.M.; Drudi, F.M.; Fischer, T.; Gilja, O.H.; Granata, A.; Graumann, O.; Harvey, C.J.; et al. EFSUMB 2020 Proposal for a Contrast-Enhanced Ultrasound-Adapted Bosniak Cyst Categorization—Position Statement. Ultraschall Der Med. Eur. J. Ultrasound 2021, 42, 154–166. (In English) [Google Scholar] [CrossRef] [PubMed]
- Herms, E.; Weirich, G.; Maurer, T.; Wagenpfeil, S.; Preuss, S.; Sauter, A.; Heck, M.; Gärtner, A.; Hauner, K.; Autenrieth, M.; et al. Ultrasound-based “CEUS-Bosniak” classification for cystic renal lesions: An 8-year clinical experience. World J. Urol. 2023, 41, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Jenssen, C.; Correas, J.M.; Safai Zadeh, E.; Bertolotto, M.; Ignee, A.; Dong, Y.; Cantisani, V.; Dietrich, C.F. CEUS Bosniak Classification-Time for Differentiation and Change in Renal Cyst Surveillance. Cancers 2023, 15, 4709. [Google Scholar] [CrossRef] [PubMed]
- Gerst, S.; Hann, L.E.; Li, D.; Gonen, M.; Tickoo, S.; Sohn, M.J.; Russo, P. Evaluation of renal masses with contrast-enhanced ultrasound: Initial experience. AJR Am. J. Roentgenol. 2011, 197, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Rübenthaler, J.; Negrão de Figueiredo, G.; Mueller-Peltzer, K.; Clevert, D.A. Evaluation of renal lesions using contrast-enhanced ultrasound (CEUS); a 10-year retrospective European single-centre analysis. Eur. Radiol. 2018, 28, 4542–4549. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, I.; Jung, F.; Jung, E.M. Contrast-enhanced ultrasound (CEUS) and perfusion imaging using VueBox®. Clin. Hemorheol. Microcirc. 2021, 78, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Huang, B.J.; Xue, L.Y.; Fan, P.L.; Wang, W.P. Differentiation of Renal Tumor Histotypes: Usefulness of Quantitative Analysis of Contrast-Enhanced Ultrasound. AJR Am. J. Roentgenol. 2015, 205, W335–W342. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wei, C.; Li, Y.; Lu, Q.; Zhang, W.; Hu, B. Contrast-Enhanced Ultrasonography with Quantitative Analysis allows Differentiation of Renal Tumor Histotypes. Sci. Rep. 2016, 6, 35081. [Google Scholar] [CrossRef] [PubMed]
- Dipinto, P.; Canale, V.; Minelli, R.; Capuano, M.A.; Catalano, O.; Di Pierro, G.B.; Anceschi, U.; Perdonà, S.; Tufano, A. Qualitative and quantitative characteristics of CEUS for renal cell carcinoma and angiomyolipoma: A narrative review. J. Ultrasound 2024, 27, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Qiu, H.; Ou, B.; Wu, J.; Zhao, X.; Luo, B. Evaluation of contrast-enhanced ultrasound for predicting tumor grade in small (≤4 cm) clear cell renal cell carcinoma: Qualitative and quantitative analysis. Clin. Hemorheol. Microcirc. 2024, 88, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.Y.; Lu, Q.; Huang, B.J.; Li, Z.; Li, C.X.; Wen, J.X.; Wang, W.P. Papillary renal cell carcinoma and clear cell renal cell carcinoma: Differentiation of distinct histological types with contrast—Enhanced ultrasonography. Eur. J. Radiol. 2015, 84, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, N.; Zhao, P.; Wang, Y.; Song, Q.; Song, L.; Li, Q.; Luo, Y. Contrast-enhanced ultrasound (CEUS) of benign and malignant renal tumors: Distinguishing CEUS features differ with tumor size. Cancer Med. 2023, 12, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Leonardo, C.; Di Bella, C.; Lucarelli, G.; Dolcetti, V.; Dipinto, P.; Proietti, F.; Flammia, R.S.; Anceschi, U.; Perdonà, S.; et al. Qualitative Assessment of Contrast-Enhanced Ultrasound in Differentiating Clear Cell Renal Cell Carcinoma and Oncocytoma. J. Clin. Med. 2023, 12, 3070. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Drudi, F.M.; Angelini, F.; Polito, E.; Martino, M.; Granata, A.; Di Pierro, G.B.; Kutrolli, E.; Sampalmieri, M.; Canale, V.; et al. Contrast-Enhanced Ultrasound (CEUS) in the Evaluation of Renal Masses with Histopathological Validation-Results from a Prospective Single-Center Study. Diagnostics 2022, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Huang, G.; Moloo, Z.; Girgis, S.; Patel, V.H.; Low, G. Multimodality Imaging Characteristics of the Common Renal Cell Carcinoma Subtypes: An Analysis of 544 Pathologically Proven Tumors. J. Clin. Imaging Sci. 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, T.K.; Ahn, H.J.; Kim, C.S.; Kim, K.R.; Cho, K.S. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am. J. Roentgenol. 2002, 178, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.; Rosenkrantz, A.B.; Pedrosa, I. MRI phenotype in renal cancer: Is it clinically relevant? Top. Magn. Reson. Imaging 2014, 23, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, D.; Zhu, J.; Wang, N.; Liu, L.; Nie, F. Application value of contrast-enhanced ultrasound in the preoperative evaluation of renal cell carcinoma histological classification and RENAL score. Quant. Imaging Med. Surg. 2024, 14, 9444–9458. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hu, Z.; Liu, Y.; Ding, J.; Han, P.; Jing, X.; Kan, Y. Dynamic contrast-enhanced ultrasound characteristics of renal tumors: VueBox™ quantitative analysis. Clin. Hemorheol. Microcirc. 2023, 85, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Correas, J.M.; Cui, X.W.; Dong, Y.; Havre, R.F.; Jenssen, C.; Jung, E.M.; Krix, M.; Lim, A.; Lassau, N.; et al. EFSUMB Technical Review—Update 2023: Dynamic Contrast-Enhanced Ultrasound (DCE-CEUS) for the Quantification of Tumor Perfusion. Ultraschall Der Med. Eur. J. Ultrasound 2024, 45, 36–46. (In English) [Google Scholar] [CrossRef] [PubMed]
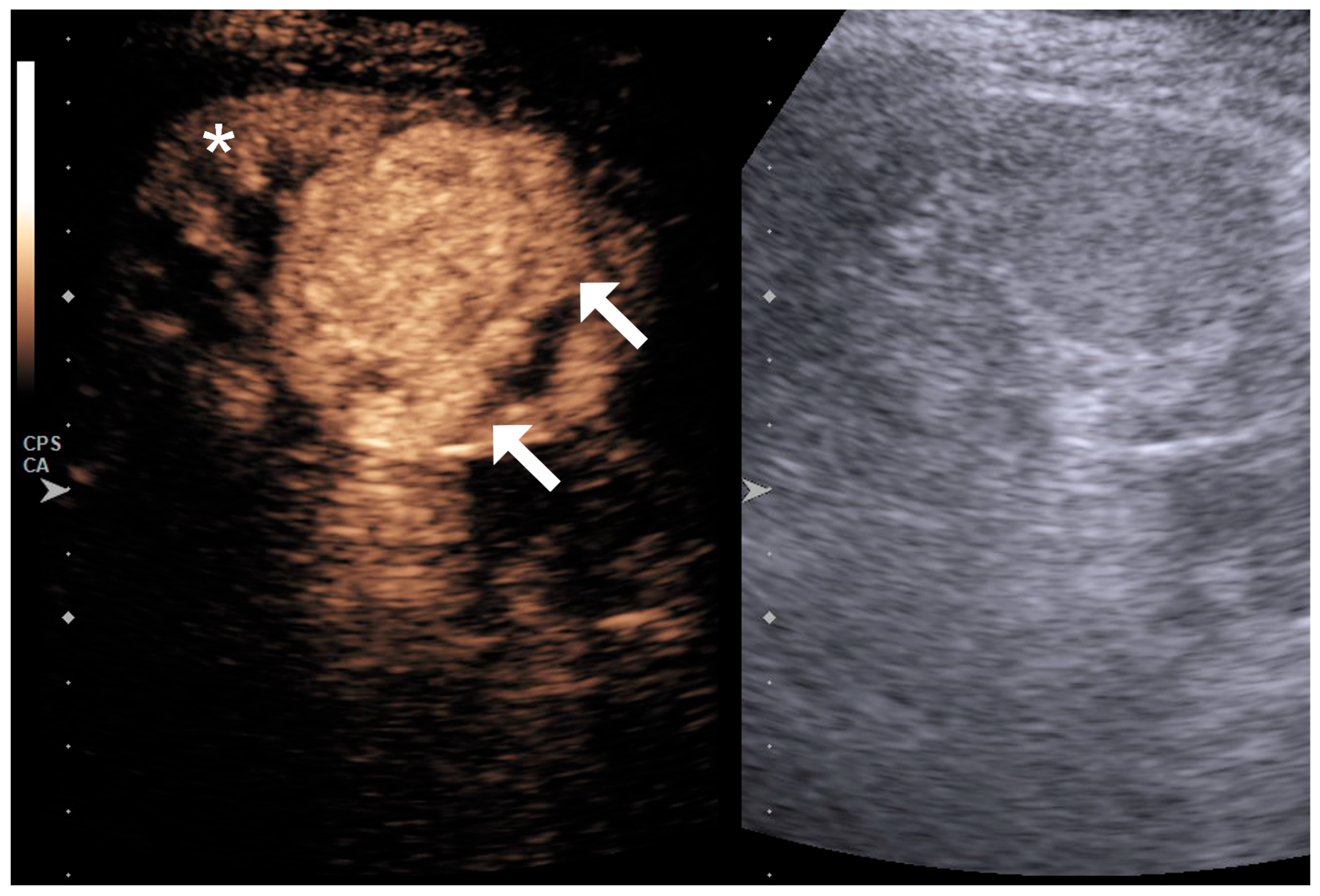
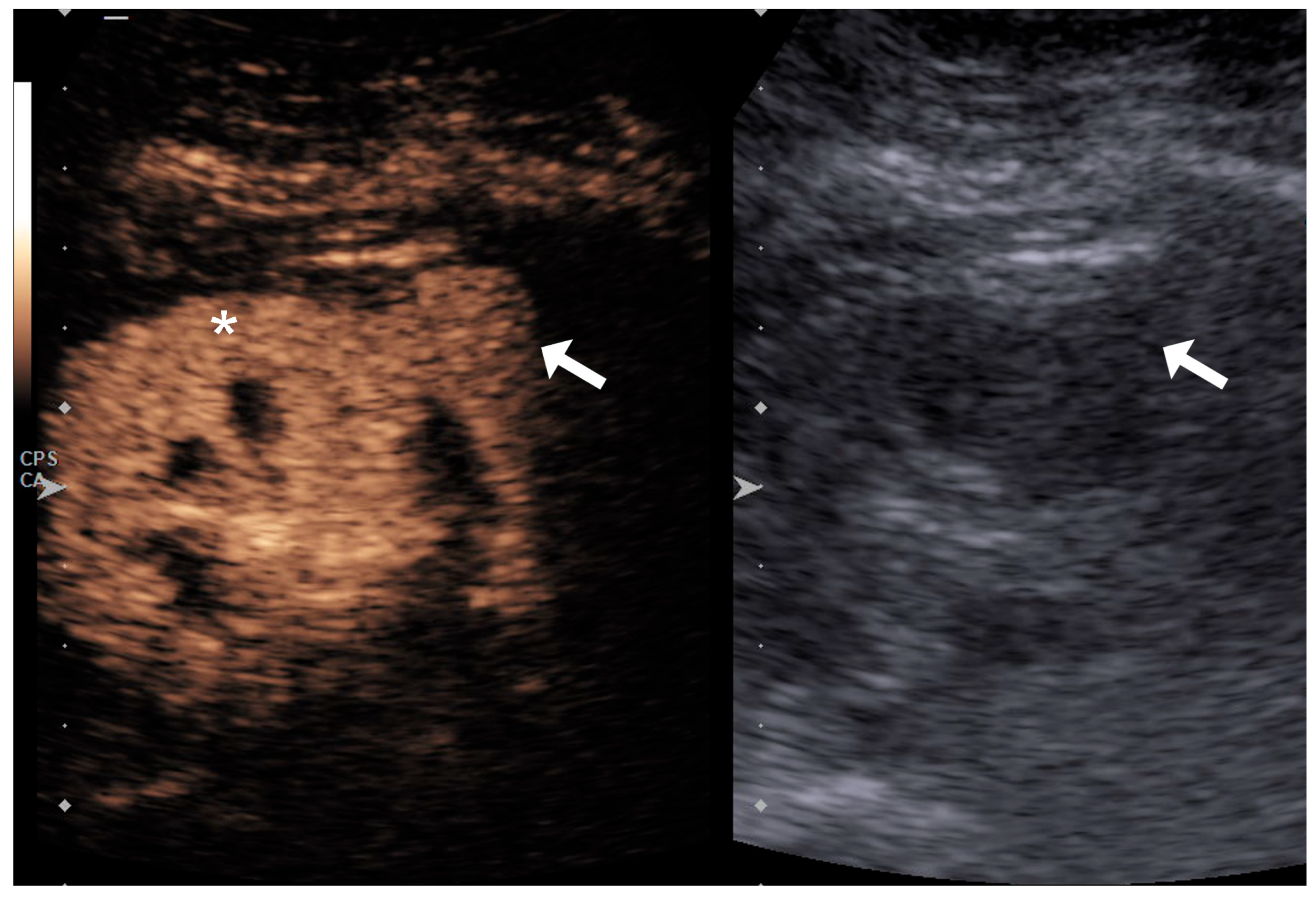
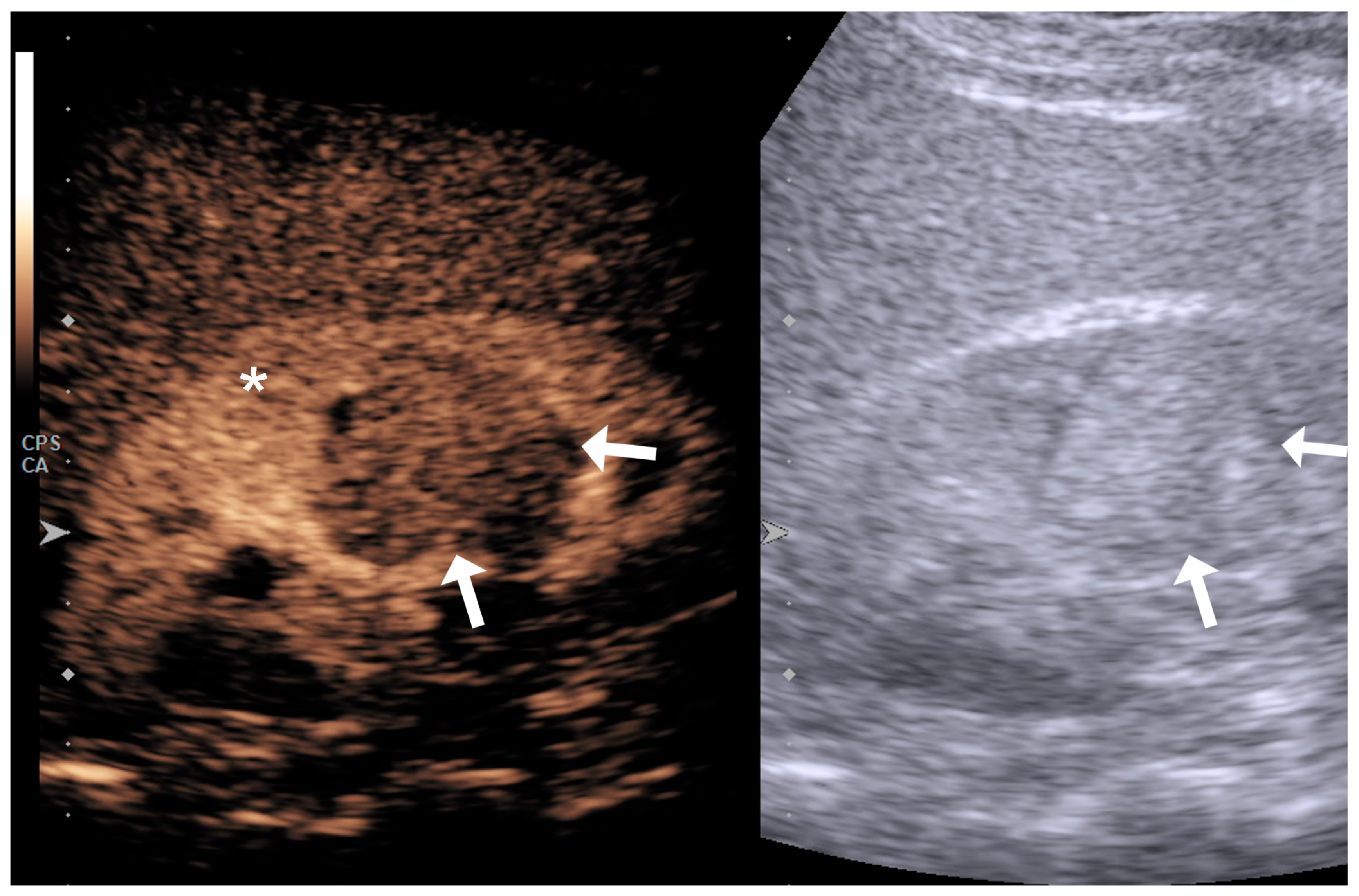


| Variable | RCC Low Grade (n = 25) | RCC High Grade (n = 36) | p Value |
|---|---|---|---|
| Age (median, IQR) | 65.00 (56.00, 74.00) | 60.00 (51.50, 66.00) | 0.159 |
| Male Sex (N, %) | 17 (68.0%) | 28 (77.8%) | 0.393 |
| Localization (N, %) | 0.535 | ||
| Superior | 8 (32.0%) | 8 (22.2%) | |
| Medium | 6 (24.0%) | 13 (36.1%) | |
| Inferior | 11 (44.0%) | 15 (41.7%) | |
| Exophytic (N, %) | 19 (76.0%) | 25 (69.4%) | 0.574 |
| Size (median, IQR) | 3.60 (2.50, 6.50) | 5.45 (3.90, 6.15) | 0.129 |
| Variable | RCC Low Grade (n = 25) | RCC High Grade (n = 36) | p Value |
|---|---|---|---|
| Heterogeneous (N,%) | 12 (48.0%) | 30 (83.3%) | 0.003 ** |
| Enhancement (N,%) | 3 (12.0%) | ||
| Hypo | 20 (80.0%) | 6 (16.7%) | 0.000 ** |
| Iso | 3 (12.0%) | 7 (19.4%) | 0.505 |
| Hyper | 2 (8.0%) | 23 (63.9%) | 0.000 ** |
| Pseudocapsule sign (N,%) | 6 (24.0%) | 14 (38.9%) | 0.223 |
| Quantitative Parameters (Median, IR) | RCC Low Grade (n = 25) | RCC High Grade (n = 36) | p Value |
|---|---|---|---|
| PE1 | 4.83 × 107 (1.77 × 107, 8.41 × 107) | 1.69 × 108 (6.90 × 107, 5.37 × 108) | 0.001 ** |
| WiAUC1 | 1.56 × 108 (6.19 × 107, 2.88 × 108) | 7.71 × 108 (3.01 × 108, 2.23 × 109) | 0.000 ** |
| RT1 | 5561.50 (4070.50, 7768.50) | 7141.00 (5448.00, 9912.00) | 0.052 |
| mTTl1 | 23,277.00 (13,674.00, 46,630.00) | 27,244.00 (17,809.00, 46,651.00) | 0.564 |
| TTP1 | 9161.00 (7362.00, 11,124.00) | 12,044.00 (8361.00, 14,223.00) | 0.086 |
| WiR1 | 1.04 × 107 (5.46 × 106, 2.05 × 107) | 5.79 × 107 (9.05 × 106, 1.65 × 108) | 0.009 ** |
| WiPI1 | 3.06 × 107 (1.13 × 107, 5.38 × 107) | 1.03 × 108 (4.44 × 107, 3.36 × 108) | 0.000 ** |
| WoAUC1 | 2.77 × 108 (9.73 × 107, 4.54 × 108) | 1.74 × 109 (6.53 × 108, 3.45 × 109) | 0.000 ** |
| WiWoAUC1 | 4.33 × 108 (1.63 × 108, 7.20 × 108) | 2.35 × 109 (9.25 × 108, 5.62 × 109) | 0.000 ** |
| FT1 | 10,473.00 (9674.00, 19,684.00) | 14,608.00 (11,801.00, 22,050.00) | 0.077 |
| WoR1 | 4.78 × 106 (2.52 × 106, 8.06 × 106) | 2.35 × 107 (3.06 × 106, 5.57 × 107) | 0.040 * |
| QOF1 | 66,164.50 (31,111.00, 85,726.00) | 74,451.00 (54,181.00, 83,754.00) | 0.483 |
| Area1 | 0.18 (0.13, 0.30) | 0.25 (0.14, 0.37) | 0.257 |
| PE3 | 3.07 × 107 (9.90 × 106, 6.68 × 107) | 7.60 × 107 (3.73 × 107, 2.09 × 108) | 0.001 ** |
| WiAUC3 | 1.23 × 108 (4.30 × 107, 2.84 × 108) | 3.95 × 108 (1.82 × 108, 1.23 × 109) | 0.005 ** |
| RT3 | 7059.00 (5215.00, 8917.00) | 7814.00 (5549.00, 11,651.00) | 0.380 |
| MTTl3 | 24,305.00 (13,870.00, 51,630.00) | 37,267.00 (21,873.00, 100,282.00) | 0.253 |
| TTP3 | 6.20 × 106 (2.44 × 106, 1.11 × 107) | 2.21 × 107 (5.26 × 106, 4.66 × 107) | 0.008 ** |
| WiR3 | 1.95 × 107 (6.38 × 106, 4.21 × 107) | 4.80 × 107 (2.55 × 107, 1.34 × 108) | 0.001 ** |
| WiPI3 | 1.85 × 108 (4.93 × 107, 4.16 × 108) | 6.85 × 108 (2.85 × 108, 2.15 × 109) | 0.004 ** |
| WoAUC3 | 3.33 × 108 (7.95 × 107, 7.57 × 108) | 1.12 × 109 (5.40 × 108, 3.45 × 109) | 0.002 ** |
| WiWoAUC3 | 14,010.00 (10,619.00, 19,822.00) | 15,360.00 (10,163.00, 21,718.00) | 0.708 |
| FT3 | 2.45 × 106 (1.14 × 106, 4.27 × 106) | 7.20 × 106 (1.85 × 106, 1.61 × 107) | 0.005 ** |
| WoR3 | 2.48 × 106 (1.69 × 106, 4.88 × 106) | 8.09 × 106 (2.21 × 106, 1.62 × 107) | 0.014 * |
| QOF3 | 8.62 × 104 (5.73 × 104, 9.61 × 104) | 9.01 × 104 (6.84 × 104, 9.54 × 104) | 0.752 |
| Area3 | 0.007 (0.004, 0.011) | 0.010 (0.007, 0.015) | 0.036 * |
| (PE1-PE2)/100 | 14.77 (−85.46, 91.05) | 107.72 (13.87, 315.71) | 0.002 ** |
| Variable | B | S.E. | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) |
|---|---|---|---|---|---|---|---|
| CEUS enhancement: Hypo (ref.) | 19 | 2 | 0 | ||||
| CEUS enhancement: Iso | 2.051 | 0.832 | 6.073 | 1 | 0.014 | 7.778 | [1.522, 39.754] |
| CEUS enhancement: Hyper | 3.646 | 0.872 | 17.491 | 1 | 0.000 | 38.333 | [6.941, 211.694] |
| Constant | −1.204 | 0.465 | 6.690 | 1 | 0.010 | 0.300 | |
| Cut-off Probability | Sensitivity | Specificity | Youden Index | Total Accuracy | |||
| p = 0.465 | 83.3% | 80.0% | 0.633 | 81.9% | |||
| Variable | B | S.E. | Wald | df | Sig. | Exp (B) | 95% CI for Exp (B) |
|---|---|---|---|---|---|---|---|
| CEUS heterogeneous: Yes | 1.827 | 0.966 | 3.579 | 1 | 0.059 | 6.214 | [0.936, 41.245] |
| CEUS enhancement: Hypo (ref.) | 9.391 | 2 | 0.009 | ||||
| CEUS enhancement: Iso (ref.) | 1.902 | 1.124 | 2.862 | 1 | 0.091 | 6.697 | [0.740, 60.615] |
| CEUS enhancement: Hyper | 3.209 | 1.057 | 9.214 | 1 | 0.002 | 24.747 | [3.117, 196.475] |
| logWoAUC1 | 1.384 | 0.690 | 4.019 | 1 | 0.045 | 3.989 | [1.031, 15.431] |
| Constant | −14.682 | 6.284 | 5.459 | 1 | 0.019 | 0.000042 | |
| Cut-off Probability | Sensitivity | Specificity | Youden Index | Total Accuracy | |||
| p = 0.649 | 82.4% | 91.7% | 0.741 | 86.2% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vas, D.; Paño, B.; Soler-Perromat, A.; Corominas, D.; Salvador, R.; Sebastià, C.; Buñesch, L.; Nicolau, C. Qualitative and Quantitative Analysis of Contrast-Enhanced Ultrasound in the Characterization of Kidney Cancer Subtypes. Diagnostics 2025, 15, 1795. https://doi.org/10.3390/diagnostics15141795
Vas D, Paño B, Soler-Perromat A, Corominas D, Salvador R, Sebastià C, Buñesch L, Nicolau C. Qualitative and Quantitative Analysis of Contrast-Enhanced Ultrasound in the Characterization of Kidney Cancer Subtypes. Diagnostics. 2025; 15(14):1795. https://doi.org/10.3390/diagnostics15141795
Chicago/Turabian StyleVas, Daniel, Blanca Paño, Alexandre Soler-Perromat, Daniel Corominas, Rafael Salvador, Carmen Sebastià, Laura Buñesch, and Carlos Nicolau. 2025. "Qualitative and Quantitative Analysis of Contrast-Enhanced Ultrasound in the Characterization of Kidney Cancer Subtypes" Diagnostics 15, no. 14: 1795. https://doi.org/10.3390/diagnostics15141795
APA StyleVas, D., Paño, B., Soler-Perromat, A., Corominas, D., Salvador, R., Sebastià, C., Buñesch, L., & Nicolau, C. (2025). Qualitative and Quantitative Analysis of Contrast-Enhanced Ultrasound in the Characterization of Kidney Cancer Subtypes. Diagnostics, 15(14), 1795. https://doi.org/10.3390/diagnostics15141795





