A New Staining Method Using Methionyl-tRNA Synthetase 1 Antibody for Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology of Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
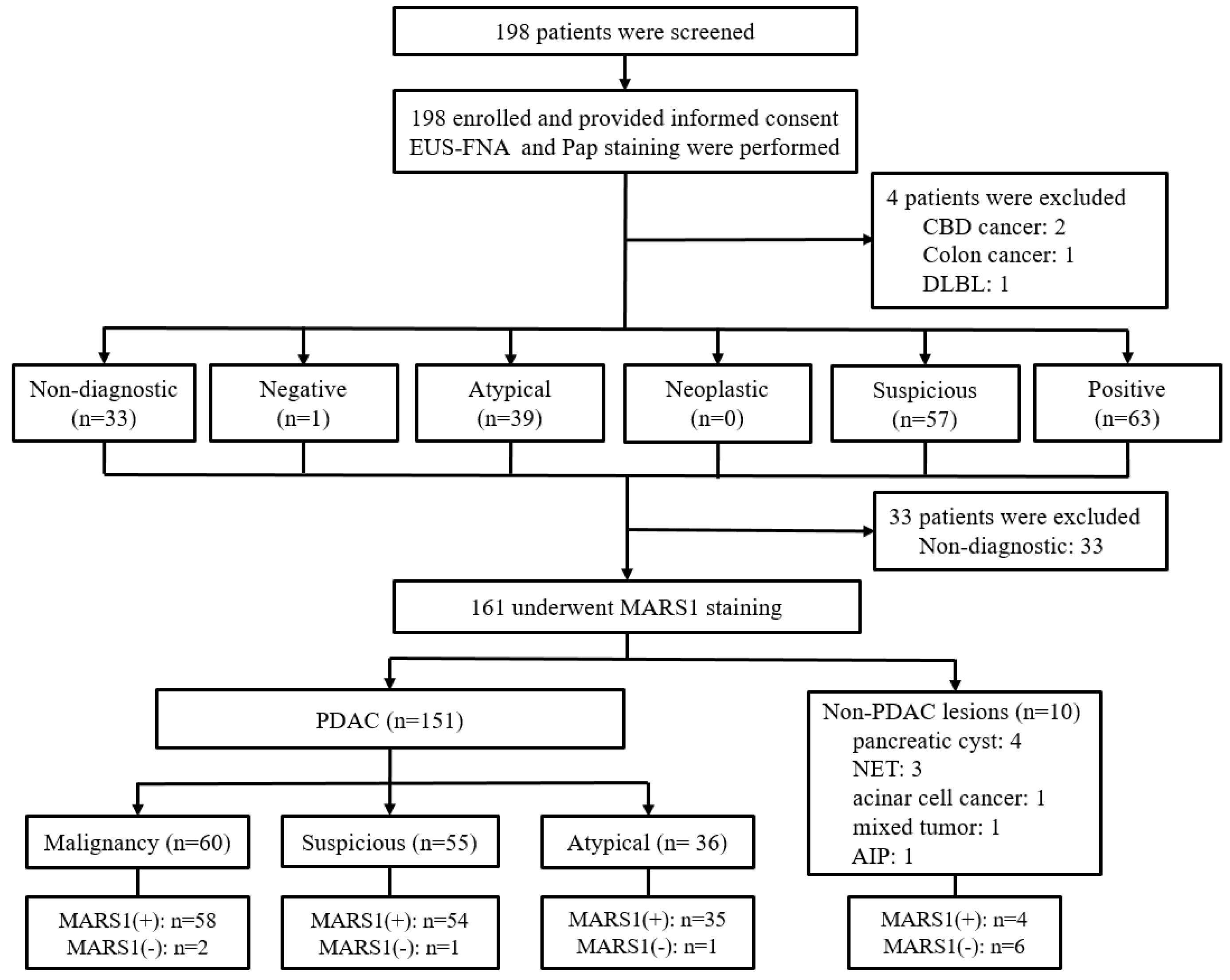
2.1. Study Design and Participants
2.2. MARS1 Immunohistochemical Staining in Surgical Specimens
2.3. EUS-FNA Cytology Samples
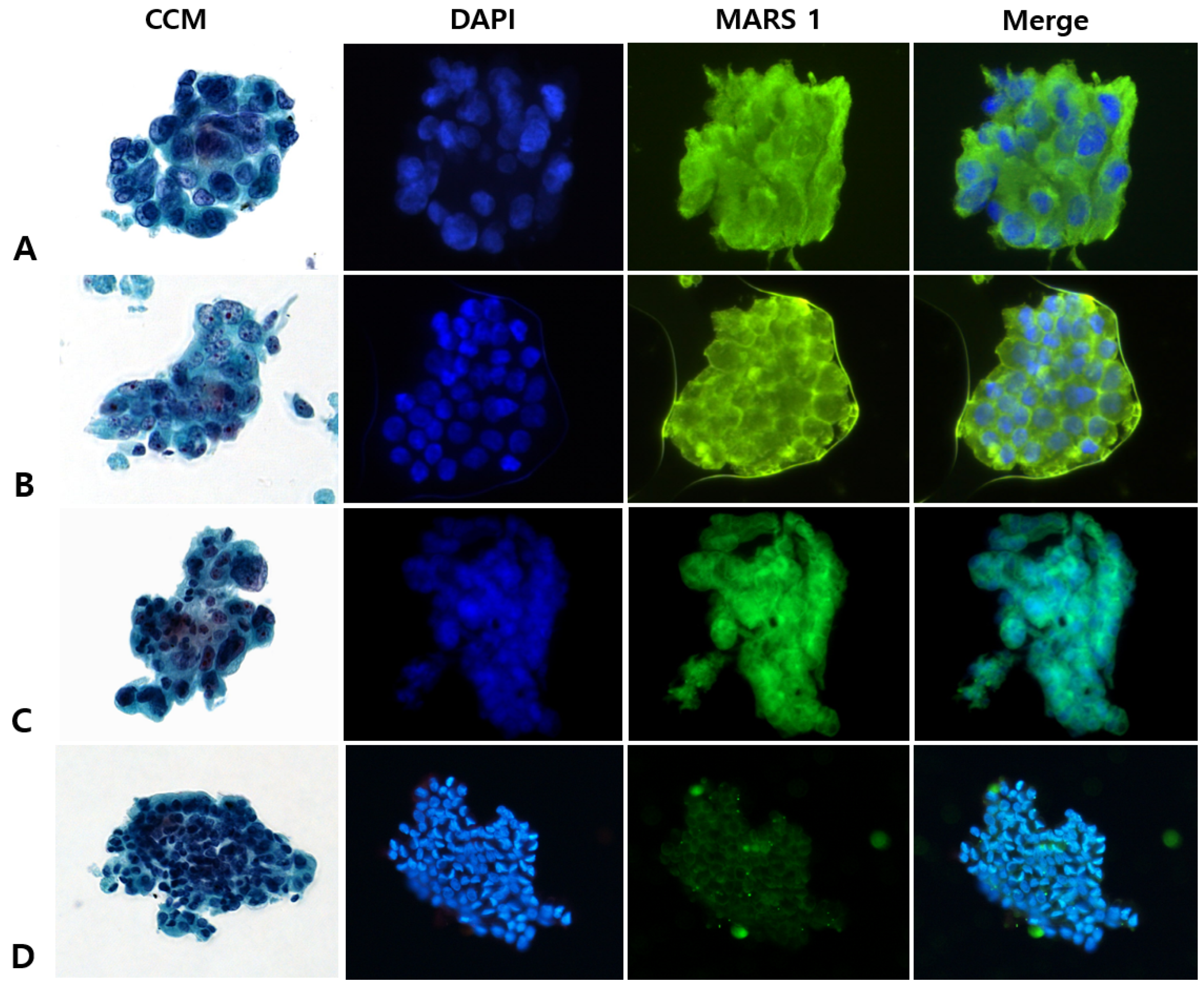
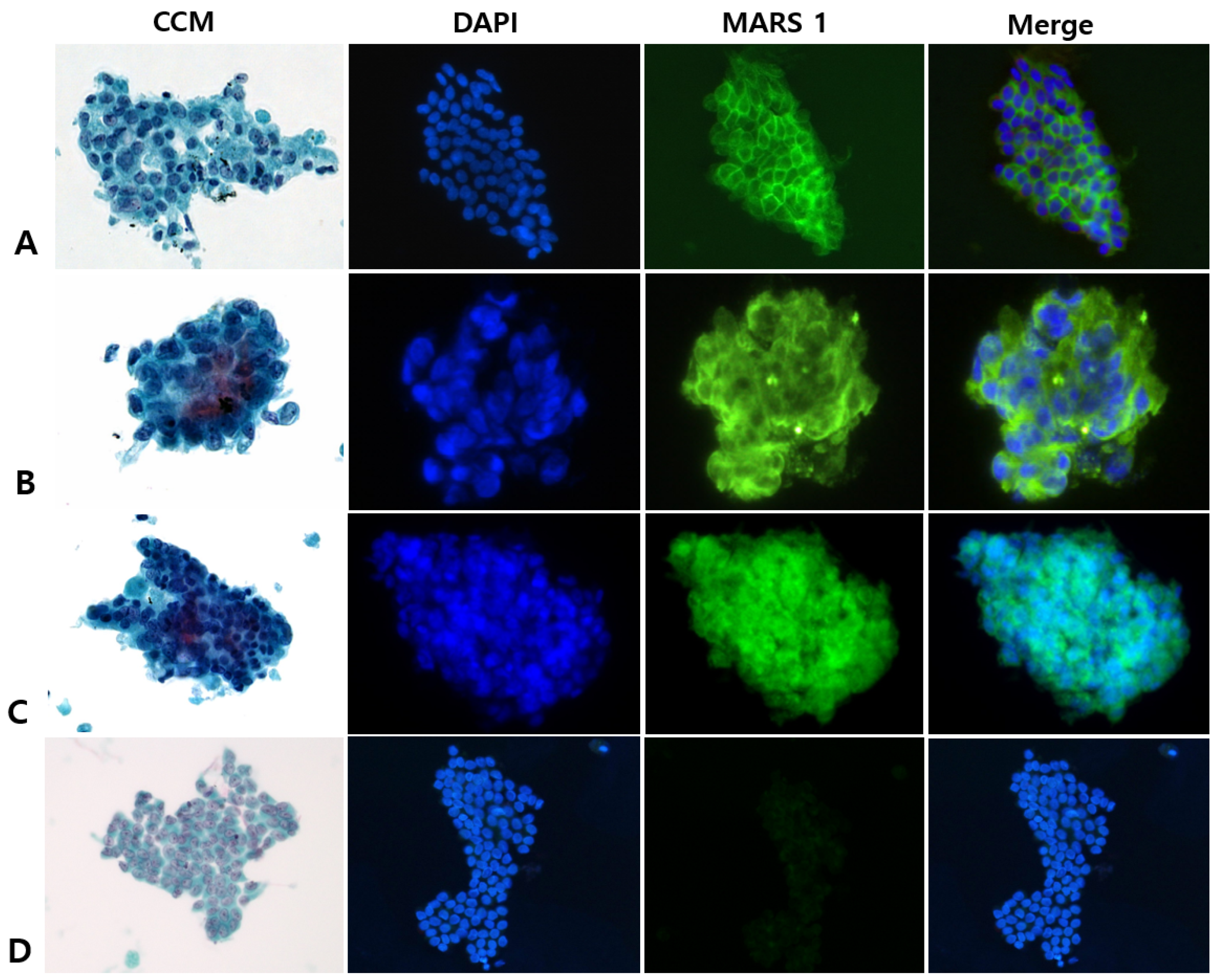
2.4. MARS1 Immunofluorescence Staining of Cytology Specimens
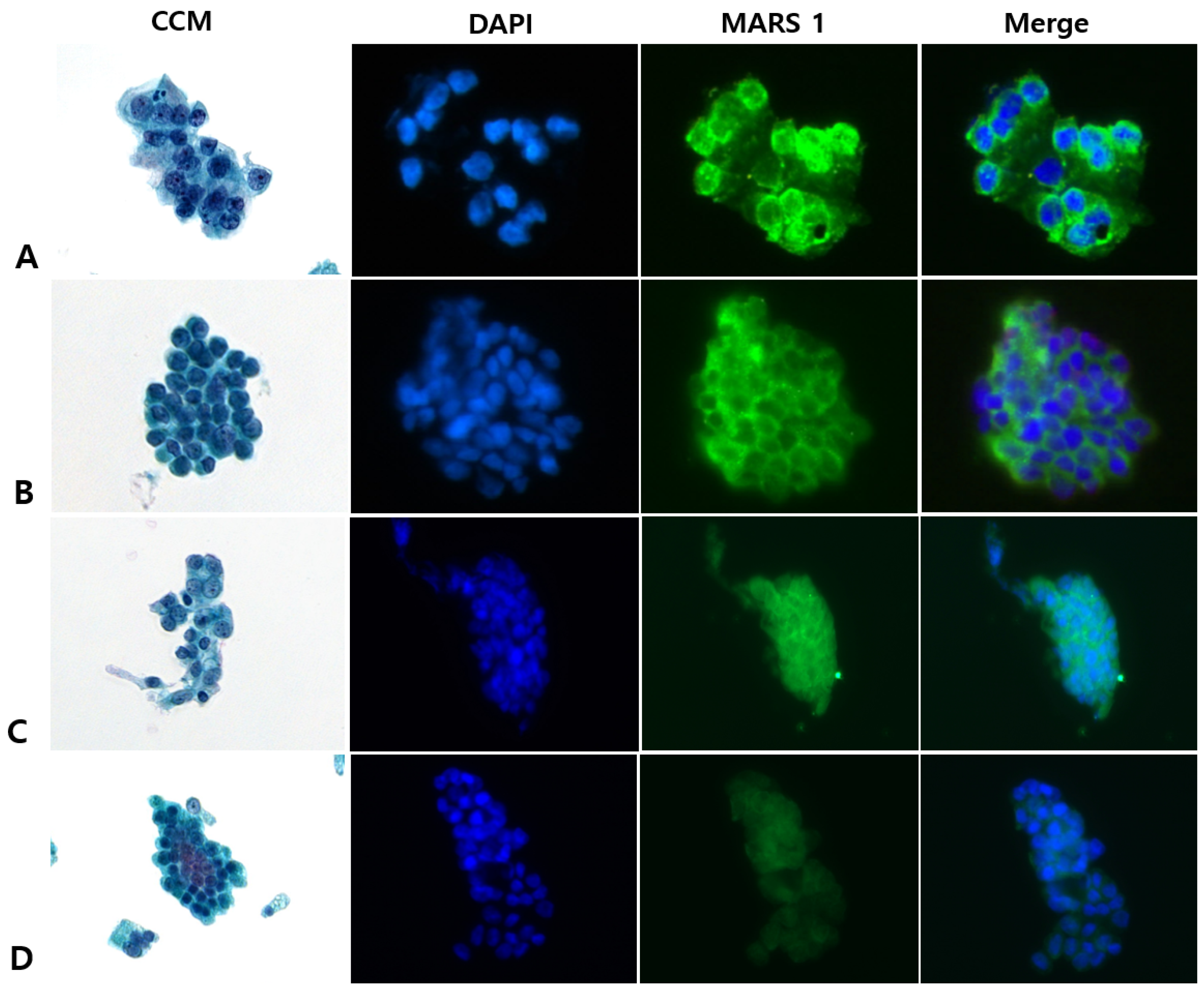
2.5. Interpreting CCM (Pap) and MARS1 IF Stains
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. CCM Versus MARS1 IF Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIMP3 | ARS-interacting multifunctional protein-3 |
| AIMP2-DX2 | ARS-interacting multifunctional protein-lacking exon 2 |
| ARS | Aminoacyl-tRNA synthetase |
| CCM | Conventional cytological staining method |
| CDK4 | Cyclin-dependent kinase 4 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EUS-FNA | Endoscopic ultrasound-guided fine-needle aspiration |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| IMP3 | Insulin-like growth factor II mRNA binding protein3 |
| MARS1 | Methionyl-tRNA synthetase 1 |
| NPV | Negative predictive value |
| NET | Neuroendocrine tumor |
| NSCLC | Non-small-cell lung cancer |
| Pap | Papanicolaou |
| PDAC | Pancreatic ductal adenocarcinoma |
| PBST | Phosphate-buffered saline with Tween |
| PPV | Positive predictive value |
| ROSE | Rapid on-site evaluation |
| RT | Room temperature |
| S100P | S100 calcium-binding protein P |
| TBST | Tris-buffered saline containing 0.05% Tween 20 |
| tRNA | Transfer RNA |
Appendix A
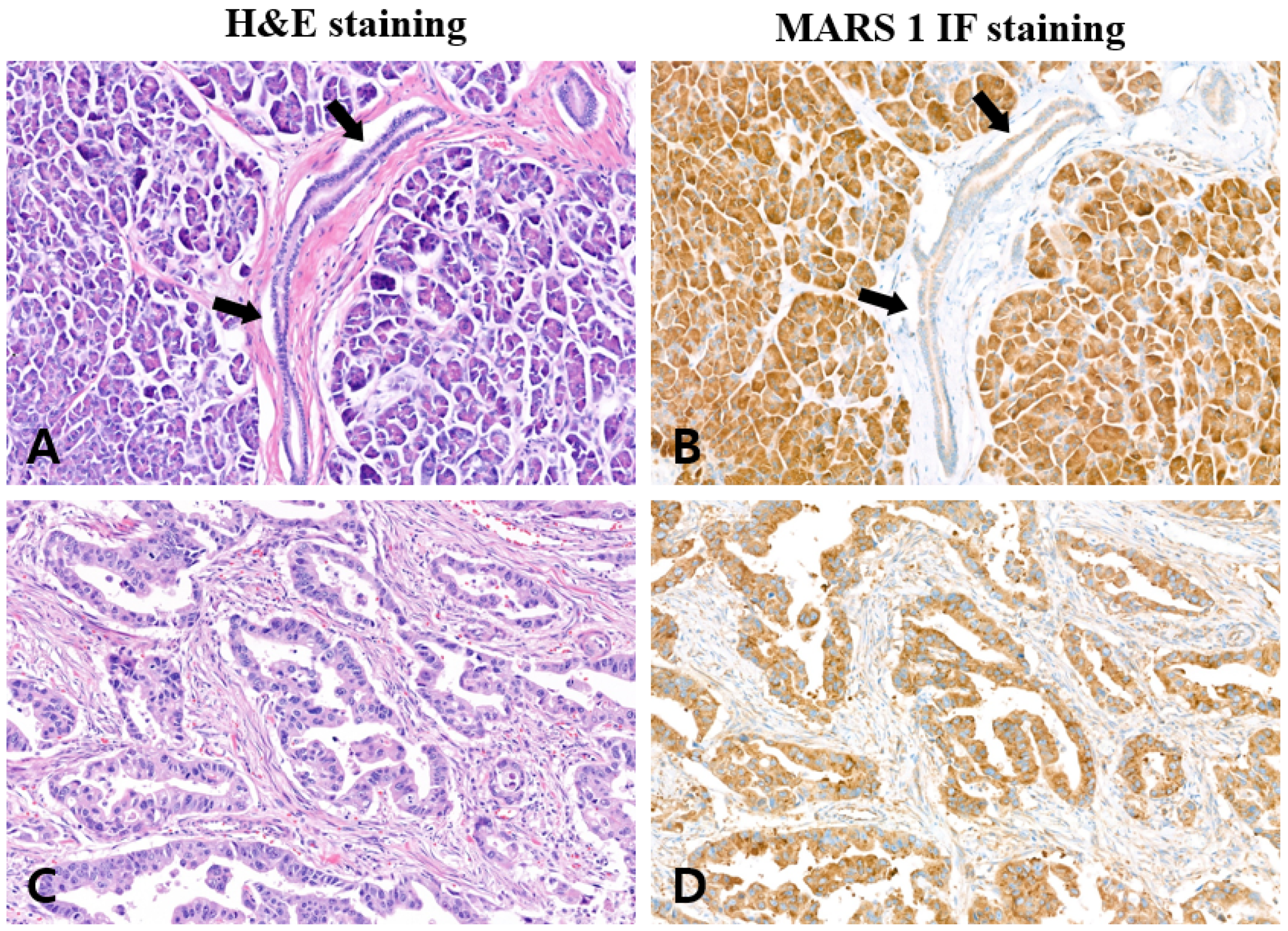
Appendix A.1. MARS1 IHC Staining of Surgical Specimens
| Pathologic Diagnosis | Specimen No. | MARS1 Immunostaining | Positive Rate (%) † | |
|---|---|---|---|---|
| Positive | Negative | |||
| Pancreatic ductal adenocarcinoma | 10 | 10 | 0 | 100 |
| Normal pancreatic tissue | 10 | 0 | 10 | 00 |
Appendix B
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Storm, A.C.; Lee, L.S. Endoscopic ultrasound-guided techniques for diagnosing pancreatic mass lesions: Can we do better? World J. Gastroenterol. 2016, 22, 8658–8669. [Google Scholar] [CrossRef]
- Layfield, L.J.; Schmidt, R.L.; Hirschowitz, S.L.; Olson, M.T.; Ali, S.Z.; Dodd, L.L. Significance of the diagnostic categories “atypical” and “suspicious for malignancy” in the cytologic diagnosis of solid pancreatic masses. Diagn. Cytopathol. 2014, 42, 292–296. [Google Scholar] [CrossRef]
- Sun, B.; Yang, X.; Ping, B.; He, Y.; Zhang, Z. Impact of inconclusive endoscopic ultrasound-guided fine-needle aspiration results in the management and outcome of patients with solid pancreatic masses. Dig. Endosc. 2015, 27, 130–136. [Google Scholar] [CrossRef]
- Kim, T.; Lee, K.; Nahm, J.H.; Kim, E.Y.; Lee, S.H.; Chang, Y.S. Methionyl-trna synthetase and aminoacyl-trna synthetases interacting multi-functional protein-lacking exon 2 as potential diagnostic biomarkers for lung cancer. Am. J. Cancer Res. 2021, 11, 3135–3144. [Google Scholar]
- Kim, S.; You, S.; Hwang, D. Aminoacyl-trna synthetases and tumorigenesis: More than housekeeping. Nat. Rev. Cancer 2011, 11, 708–718. [Google Scholar] [CrossRef]
- Forus, A.; Florenes, V.A.; Maelandsmo, G.M.; Fodstad, O.; Myklebost, O. The protooncogene chop/gadd153, involved in growth arrest and DNA damage response, is amplified in a subset of human sarcomas. Cancer Genet. Cytogenet. 1994, 78, 165–171. [Google Scholar] [CrossRef]
- Nilbert, M.; Rydholm, A.; Mitelman, F.; Meltzer, P.S.; Mandahl, N. Characterization of the 12q13-15 amplicon in soft tissue tumors. Cancer Genet. Cytogenet. 1995, 83, 32–36. [Google Scholar] [CrossRef]
- Palmer, J.L.; Masui, S.; Pritchard, S.; Kalousek, D.K.; Sorensen, P.H.B. Cytogenetic and molecular genetic analysis of a pediatric pleomorphic sarcoma reveals similarities to adult malignant fibrous histiocytoma. Cancer Genet. Cytogenet. 1997, 95, 141–147. [Google Scholar] [CrossRef]
- Reifenberger, G.; Ichimura, K.; Reifenberger, J.; Elkahloun, A.G.; Meltzer, P.S.; Collins, V.P. Refined mapping of 12q13-q15 amplicons in human malignant gliomas suggests cdk4/sas and mdm2 as independent amplification targets. Cancer Res. 1996, 56, 5141–5145. [Google Scholar]
- Kim, E.Y.; Jung, J.Y.; Kim, A.; Kim, K.; Chang, Y.S. Methionyl-trna synthetase overexpression is associated with poor clinical outcomes in non-small cell lung cancer. BMC Cancer 2017, 17, 467. [Google Scholar] [CrossRef]
- Kwon, N.H.; Fox, P.L.; Kim, S. Aminoacyl-trna synthetases as therapeutic targets. Nat. Rev. Drug Discov. 2019, 18, 629–650. [Google Scholar] [CrossRef]
- Kushner, J.P.; Boll, D.; Quagliana, J.; Dickman, S. Elevated methionine-trna synthetase activity in human colon cancer. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1976, 153, 273–276. [Google Scholar] [CrossRef]
- Jang, S.I.; Nahm, J.H.; Lee, S.Y.; Cho, J.H.; Do, M.Y.; Park, J.S.; Lee, H.S.; Yang, J.; Kong, J.; Jung, S.; et al. Prediction of prognosis in pancreatic cancer according to methionyl-trna synthetase 1 expression as determined by immunohistochemical staining. Cancers 2023, 15, 5413. [Google Scholar] [CrossRef]
- Tannapfel, A.; Benicke, M.; Katalinic, A.; Uhlmann, D.; Kockerling, F.; Hauss, J.; Wittekind, C. Frequency of p16(ink4a) alterations and k-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut 2000, 47, 721–727. [Google Scholar] [CrossRef]
- Lee, K.; Oh, M.; Lee, K.S.; Cha, Y.J.; Chang, Y.S. Is methionyl-trna synthetase applicable as a diagnostic marker for lung cancer in bronchial ultrasound-guided brushing cells? Diagnostics 2021, 11, 1830. [Google Scholar] [CrossRef]
- Jang, S.I.; Nahm, J.H.; Kwon, N.H.; Jeong, S.; Lee, T.H.; Cho, J.H.; Kwon, C.I.; Kim, D.U.; Kim, J.M.; Cho, H.D.; et al. Clinical utility of methionyl-trna synthetase 1 immunostaining in cytologic brushings of indeterminate biliary strictures: A multicenter prospective study. Gastrointest. Endosc. 2021, 94, 733–741.e734. [Google Scholar] [CrossRef]
- Pitman, M.B.; Layfield, L.J. Guidelines for pancreaticobiliary cytology from the papanicolaou society of cytopathology: A review. Cancer Cytopathol. 2014, 122, 399–411. [Google Scholar] [CrossRef]
- Lisotti, A.; Frazzoni, L.; Fuccio, L.; Serrani, M.; Cominardi, A.; Bazzoli, F.; Fusaroli, P. Repeat eus-fna of pancreatic masses after nondiagnostic or inconclusive results: Systematic review and meta-analysis. Gastrointest. Endosc. 2020, 91, 1234–1241.e1234. [Google Scholar] [CrossRef]
- Fusaroli, P.; Napoleon, B.; Gincul, R.; Lefort, C.; Palazzo, L.; Palazzo, M.; Kitano, M.; Minaga, K.; Caletti, G.; Lisotti, A. The clinical impact of ultrasound contrast agents in eus: A systematic review according to the levels of evidence. Gastrointest. Endosc. 2016, 84, 587–596.e510. [Google Scholar] [CrossRef]
- Bang, J.Y.; Magee, S.H.; Ramesh, J.; Trevino, J.M.; Varadarajulu, S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy 2013, 45, 445–450. [Google Scholar] [CrossRef]
- Mukai, S.; Itoi, T.; Ashida, R.; Tsuchiya, T.; Ikeuchi, N.; Kamada, K.; Tanaka, R.; Umeda, J.; Tonozuka, R.; Fukutake, N.; et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for eus-fna of solid pancreatic masses (with videos). Gastrointest. Endosc. 2016, 83, 1210–1217. [Google Scholar] [CrossRef]
- Aadam, A.A.; Wani, S.; Amick, A.; Shah, J.N.; Bhat, Y.M.; Hamerski, C.M.; Klapman, J.B.; Muthusamy, V.R.; Watson, R.R.; Rademaker, A.W.; et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc. Int. Open 2016, 4, E497–E505. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hawes, R.; Varadarajulu, S. A meta-analysis comparing procore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016, 48, 339–349. [Google Scholar] [CrossRef]
- Facciorusso, A.; Stasi, E.; Di Maso, M.; Serviddio, G.; Ali Hussein, M.S.; Muscatiello, N. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 gauge needles: A meta-analysis. United Eur. Gastroenterol. J. 2017, 5, 846–853. [Google Scholar] [CrossRef]
- The ASGE Standards of Practice Committee; Machicado, J.D.; Sheth, S.G.; Chalhoub, J.M.; Forbes, N.; Desai, M.; Ngamruengphong, S.; Papachristou, G.I.; Sahai, V.; Nassour, I.; et al. American society for gastrointestinal endoscopy guideline on the role of endoscopy in the diagnosis and management of solid pancreatic masses: Summary and recommendations. Gastrointest. Endosc. 2024, 100, 786–796. [Google Scholar] [CrossRef]
- Chong, C.C.; Pittayanon, R.; Pausawasdi, N.; Bhatia, V.; Okuno, N.; Tang, R.S.; Cheng, T.Y.; Kuo, Y.T.; Oh, D.; Song, T.J.; et al. Consensus statements on endoscopic ultrasound-guided tissue acquisition. Guidelines from the asian endoscopic ultrasound group. Dig. Endosc. 2024, 36, 871–883. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Qu, C.; Liang, S.; Zeng, B.; Luo, Z. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22978. [Google Scholar] [CrossRef]
- Gkolfakis, P.; Crino, S.F.; Tziatzios, G.; Ramai, D.; Papaefthymiou, A.; Papanikolaou, I.S.; Triantafyllou, K.; Arvanitakis, M.; Lisotti, A.; Fusaroli, P.; et al. Comparative diagnostic performance of end-cutting fine-needle biopsy needles for eus tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2022, 95, 1067–1077.e1015. [Google Scholar] [CrossRef]
- van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar] [CrossRef]
- Levine, I.; Trindade, A.J. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J. Gastroenterol. 2021, 27, 4194–4207. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, Z.E.; Wang, H.L. Utility of immunohistochemistry in the pancreatobiliary tract. Arch. Pathol. Lab. Med. 2015, 139, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, N.E.; Tahoun, N.S.; Ismail, Y.M. The role of s100p and imp3 in the cytologic diagnosis of pancreatic adenocarcinoma. J. Egypt. Natl. Cancer Inst. 2016, 28, 229–234. [Google Scholar] [CrossRef]
- Chiba, M.; Imazu, H.; Kato, M.; Ikeda, K.; Arakawa, H.; Kato, T.; Sumiyama, K.; Homma, S. Novel quantitative analysis of the s100p protein combined with endoscopic ultrasound-guided fine needle aspiration cytology in the diagnosis of pancreatic adenocarcinoma. Oncol. Rep. 2017, 37, 1943–1952. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Chen, G. Diagnostic value of mesothelinin pancreatic cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 4000–4007. [Google Scholar]
- Sierzega, M.; Mlynarski, D.; Tomaszewska, R.; Kulig, J. Semiquantitative immunohistochemistry for mucin (muc1, muc2, muc3, muc4, muc5ac, and muc6) profiling of pancreatic ductal cell adenocarcinoma improves diagnostic and prognostic performance. Histopathol. 2016, 69, 582–591. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, G.; Wang, B.; Li, S.B.; Ni, K.; Wang, C.Y.; Ren, J.Y.; Zhang, S.; Dai, Y.F. High methionyl-trna synthetase expression predicts poor prognosis in patients with breast cancer. J. Clin. Pathol. 2020, 73, 803–812. [Google Scholar] [CrossRef]
| Characteristic | N (%) |
|---|---|
| Total number of patients | 161 |
| Age, y (mean ± SD) | 65.6 ± 10.3 |
| Sex, n (M:F) | 82:79 |
| Diagnosis, n (%) | |
| Pancreatic ductal adenocarcinoma | 151 (93.8) |
| Pancreatic cyst | 4 (2.5) |
| Intraductal pancreatic muninous neoplasm | 1 |
| Mucinous cystic neoplasm | 1 |
| Serous cystadenoma | 1 |
| Solid papillary neoplasm | 1 |
| Other pancreatic lesions | 6 (3.7) |
| Pancreatic neuroendocrine tumor | 3 |
| Mixed acinar-neuroendocrine carcinoma | 1 |
| Acinar cell carcinoma | 1 |
| Autoimmune pancreatitis | 1 |
| Diagnosis | Pap Staining † | MARS1 Immunostaining * | |
|---|---|---|---|
| Positive | Negative | ||
| PDAC (n = 151) | malignancy (n = 60) | 58 | 2 |
| suspicious (n = 55) | 54 | 1 | |
| atypical (n = 36) | 35 | 1 | |
| Pancreatic cyst (n = 4) | |||
| IPMN (n = 1) | suspicious (n = 1) | 1 | 0 |
| MCN (n = 1) | atypical (n = 1) | 0 | 1 |
| SCA (n = 1) | suspicious (n = 1) | 0 | 1 |
| SPN (n = 1) | atypical (n = 1) | 1 | 0 |
| Other pancreatic lesions (n = 6) | |||
| NET (n = 3) | malignancy (n = 2) | 0 | 2 |
| suspicious (n = 1) | 1 | 0 | |
| Acinar cell carcinoma (n = 1) | malignancy (n = 1) | 0 | 1 |
| Mixed AC-NEC (n = 1) ‡ | atypical (n = 1) | 1 | 0 |
| AIP (n = 1) | negative (n = 1) | 0 | 1 |
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Conventional cytology method | 79.1 (72.6–85.5) | 37.5 (39.5–71.1) | 96.0 (92.6–99.0) | 8.6 (0–17.8) | 77.0 (70.5–83.5) |
| MARS1 immunostaining | 97.4 (94.8–99.9) | 50 (15.4–84.6) | 97.4 (94.9–99.9) | 50 (15.4–84.6) | 95.0 (91.7–98.4) |
| p value * | <0.0001 | 0.6506 | 0.4030 | 0.0166 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.I.; Lee, S.Y.; Nahm, J.H.; Cho, J.H.; Jo, J.H.; Jung, C.M.; Lim, B.J.; Lim, J.H.; Kim, H.S.; Lee, S.Y.; et al. A New Staining Method Using Methionyl-tRNA Synthetase 1 Antibody for Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology of Pancreatic Cancer. Diagnostics 2025, 15, 1783. https://doi.org/10.3390/diagnostics15141783
Jang SI, Lee SY, Nahm JH, Cho JH, Jo JH, Jung CM, Lim BJ, Lim JH, Kim HS, Lee SY, et al. A New Staining Method Using Methionyl-tRNA Synthetase 1 Antibody for Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology of Pancreatic Cancer. Diagnostics. 2025; 15(14):1783. https://doi.org/10.3390/diagnostics15141783
Chicago/Turabian StyleJang, Sung Ill, See Young Lee, Ji Hae Nahm, Jae Hee Cho, Jung Hyun Jo, Chan Min Jung, Beom Jin Lim, Jin Hong Lim, Hyung Sun Kim, Su Yun Lee, and et al. 2025. "A New Staining Method Using Methionyl-tRNA Synthetase 1 Antibody for Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology of Pancreatic Cancer" Diagnostics 15, no. 14: 1783. https://doi.org/10.3390/diagnostics15141783
APA StyleJang, S. I., Lee, S. Y., Nahm, J. H., Cho, J. H., Jo, J. H., Jung, C. M., Lim, B. J., Lim, J. H., Kim, H. S., Lee, S. Y., Hong, I. Y., Kim, S., & Lee, D. K. (2025). A New Staining Method Using Methionyl-tRNA Synthetase 1 Antibody for Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology of Pancreatic Cancer. Diagnostics, 15(14), 1783. https://doi.org/10.3390/diagnostics15141783







