Optimal Number of Needle Punctures in EUS-FNA/B with ROSE for Solid Pancreatic Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. EUS-FNA/B Procedure
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Final Diagnosis and Patient Characteristics
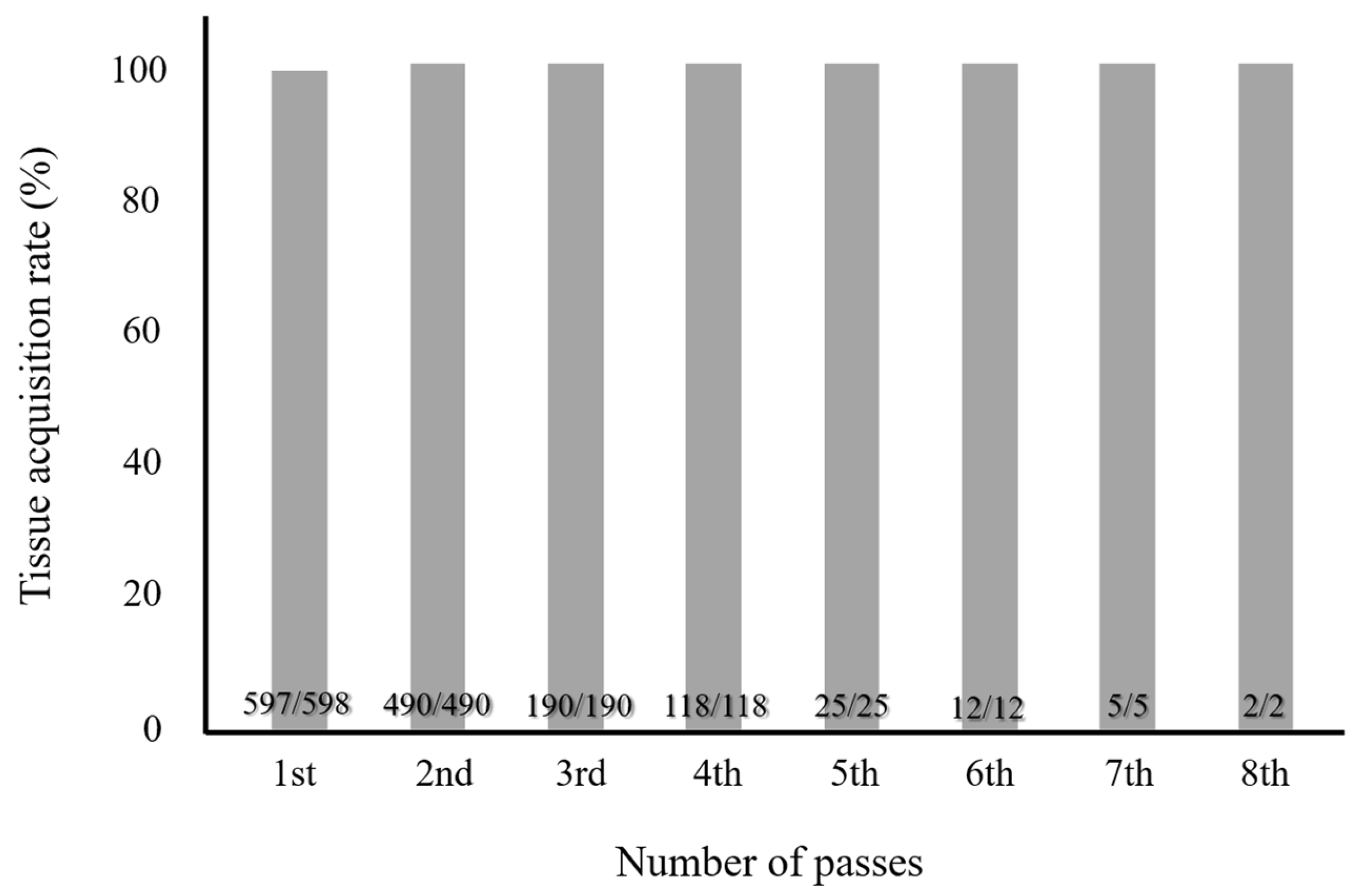
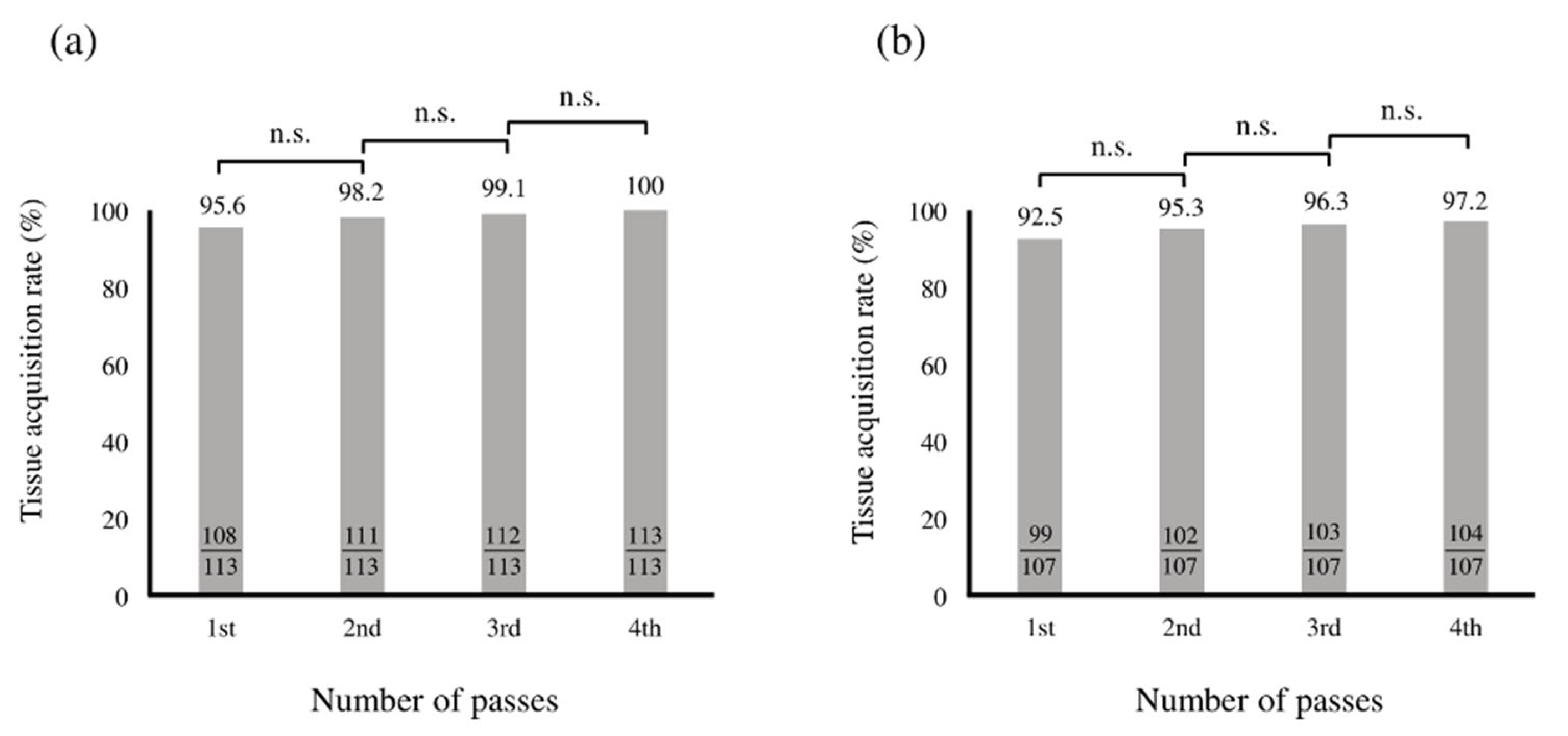
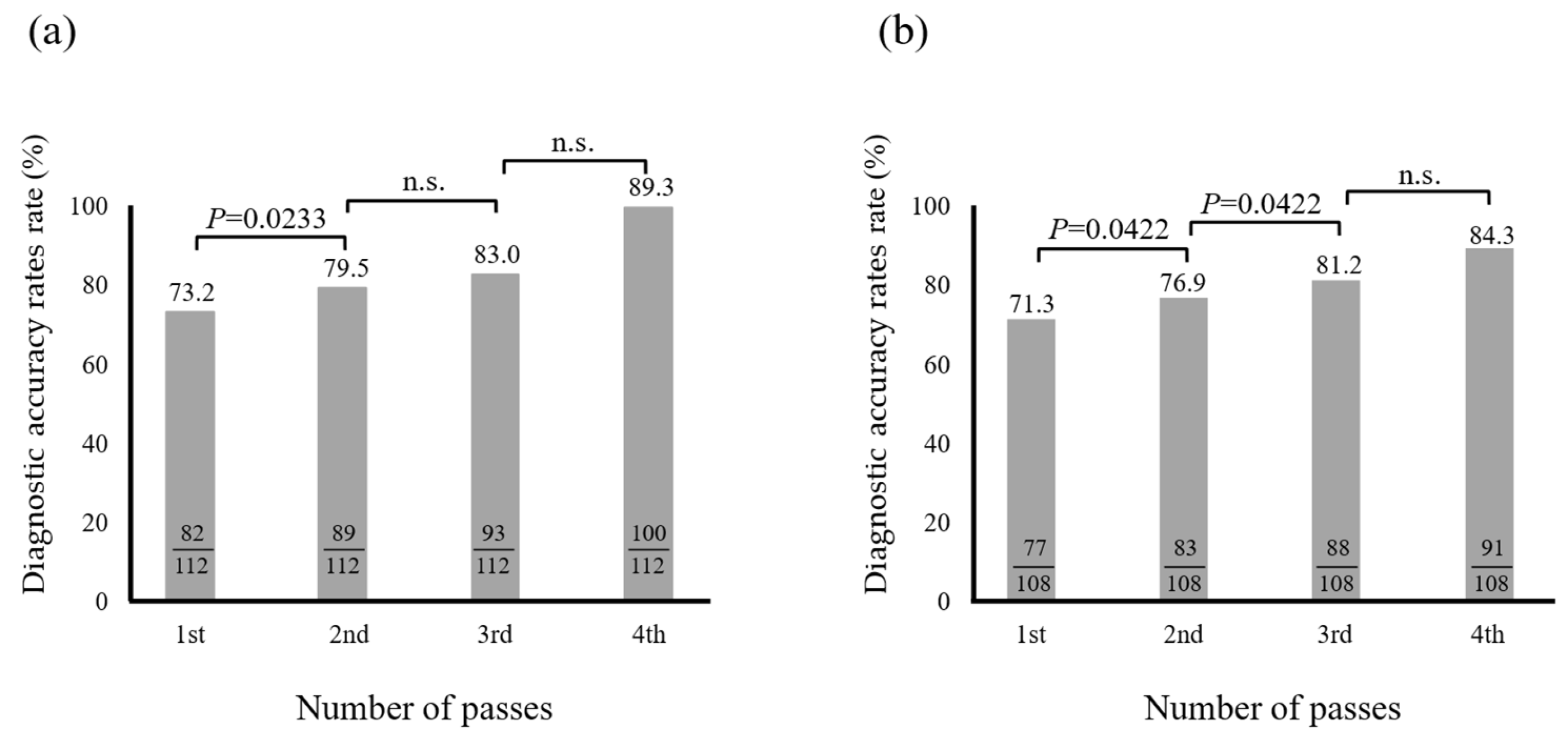
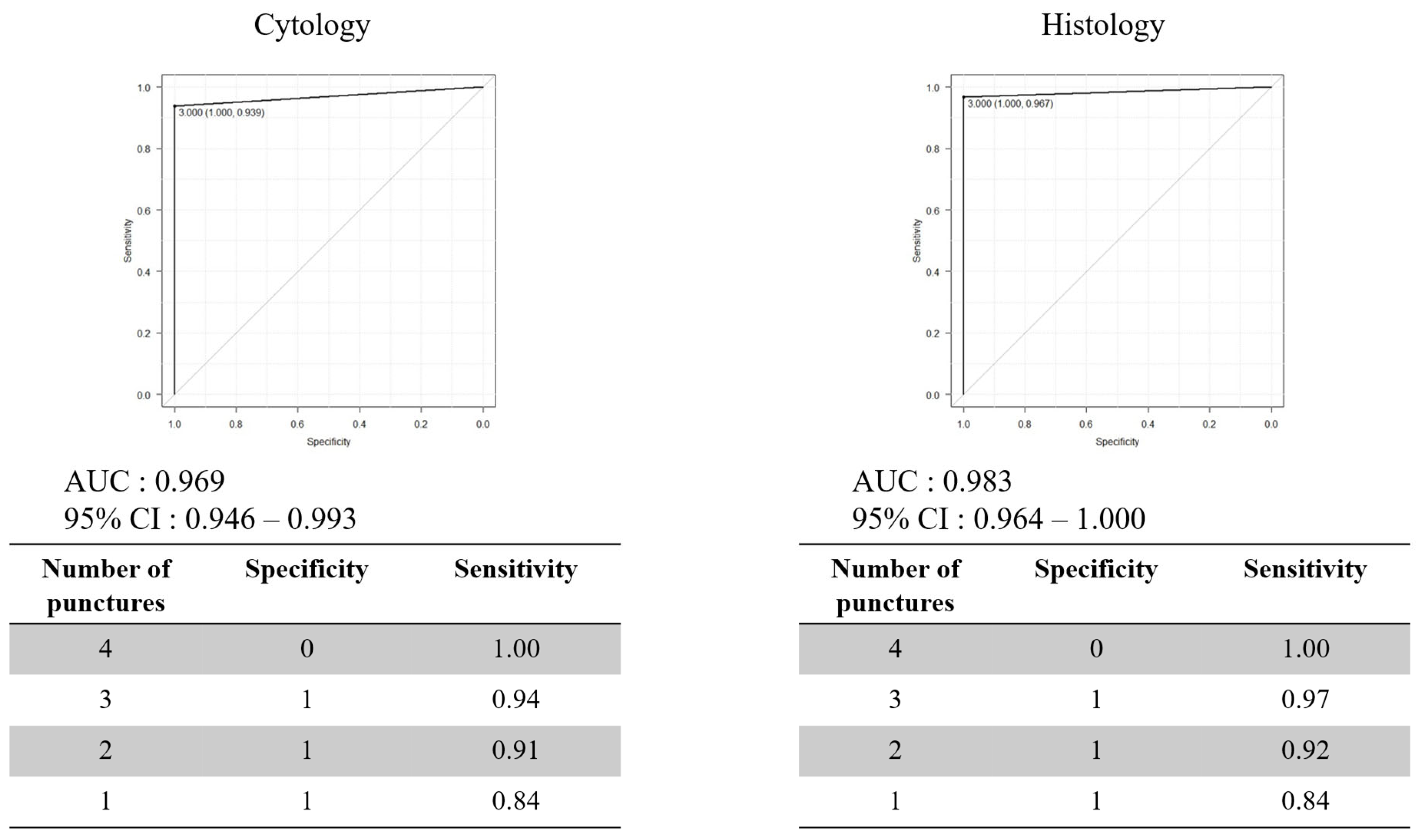
3.2. Optimal Number of Needle Punctures with EUS-FNB
3.3. Factors Associated with Diagnostic Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. CA: A Cancer Cancer Stat. J. Clin. 2022, 72, 7–33. [Google Scholar]
- Cancer Research Foundation: Cancer Statistics 2021. Available online: https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2021.pdf (accessed on 30 June 2025).
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Puli, S.R.; Bechtold, M.L.; Buxbaum, J.L.; Eloubeidi, M.A. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas 2013, 42, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Qu, C.; Liang, S.; Zeng, B. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: A systematic review and Meta-Analysis. Sci. Rep. 2016, 6, 22978. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Grimm, I.S.; Ali, B.; Nollan, R.; Tombazzi, C.; Ismail, M.K.; Baron, T.H. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc. Int. Open 2017, 5, E363–E375. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Xu, X.; Li, P. A meta-analysis comparing endoscopic ultrasound-guided fine-needle aspiration with endoscopic ultrasound-guided fine-needle biopsy. J. Clin. Gastroenterol. 2022, 56, 668–678. [Google Scholar] [CrossRef]
- van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar] [CrossRef]
- Facciorusso, A.; Bajwa, H.S.; Menon, K.; Buccino, V.R.; Muscatiello, N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc. Ultrasound 2020, 9, 167–174. [Google Scholar] [CrossRef]
- Facciorusso, A.; Bajwa, H.S.; Menon, K.; Buccino, V.R.; Muscatiello, N. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2019, 90, 893–903.e7. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhou, Q.Y.; Fan, B. Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e0207. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Chen, Y.; Jia, R.; Zhang, X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: A meta-analysis. Medicine 2017, 96, e7452. [Google Scholar] [CrossRef] [PubMed]
- Renelus, B.D.; Jamorabo, D.S.; Boston, I.; Briggs, W.M.; Poneros, J.M. Endoscopic ultrasound-guided fine needle biopsy needles provide higher diagnostic yield compared to endoscopic ultrasound-guided fine needle aspiration needles when sampling solid pancreatic lesions: A meta-analysis. Clin. Endosc. 2021, 54, 261–268. [Google Scholar] [CrossRef]
- Park, J.K.; Kang, K.J.; Oh, C.R.; Lee, J.K.; Lee, K.T.; Jang, K.T.; Park, S.M.; Lee, K.H. Evaluating the minimal specimens from endoscopic ultrasound-guided fine-needle aspiration in pancreatic masses. Medicine 2016, 95, e3740. [Google Scholar] [CrossRef]
- Polkowski, M.; Jenssen, C.; Kaye, P.; Carrara, S.; Deprez, P.; Gines, A.; Fernández-Esparrach, G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European society of gastrointestinal endoscopy (ESGE) technical guideline-march 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhang, Y.; Chen, Q.; Sun, B.; Deng, Z.; Shan, H.; Dou, L.; Wang, J.; Li, Y.; Yang, X.; et al. Analysis of fine-needle biopsy vs fine-needle aspiration in diagnosis of pancreatic and abdominal masses: A Prospective, multicenter, randomized controlled trial. Clin. Gastroenterol. Hepatol. 2018, 16, 1314–1321. [Google Scholar] [CrossRef]
- Tian, L.; Tang, A.L.; Zhang, L.; Liu, X.W.; Li, J.B.; Wang, F.; Shen, S.R.; Wang, X.Y. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: A prospective comparison study. Surg. Endosc. 2018, 32, 3533–3539. [Google Scholar] [CrossRef]
- Imaoka, H.; Sasaki, M.; Hashimoto, Y.; Watanabe, K.; Miyazawa, S.; Shibuki, T.; Mitsunaga, S.; Ikeda, M. Impact of endoscopic ultrasuound-guided tissue acquisition on decision-making in precision medicine for pancreatic cancer: Beyond diagnosis. Diagnostics. 2021, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Oppong, K.W.; Bekkali, N.L.H.; Leeds, J.S.; Johnson, S.J.; Nayar, M.K.; Darné, A.; Egan, M.; Bassett, P.; Haugk, B. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses; a randomized crossover study. Endoscopy 2020, 52, 454–461. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, E.; Jalaj, S.; Grimm, I.S.; Baron, T.H. Impact of EUS-guided fine-needle biopsy sampling with a new core needle on the need for onsite cytopathologic assessment: A preliminary study. Gastrointest. Endosc. 2016, 84, 1040–1046. [Google Scholar] [CrossRef]
- Crinò, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. Endoscopic ultrasound-guided fine-needle biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesions: A randomized controlled non-inferiority trial. Gastroenterology 2021, 161, 899–909. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; Takeyama, Y.; et al. Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: A synopsis. Int. J. Clin. Oncol. 2023, 28, 493–511. [Google Scholar] [CrossRef]
- Wani, S.; Mullady, D.; Early, D.S.; Rastogi, A.; Collins, B.; Wang, J.F.; Marshall, C.; Sams, S.B.; Yen, R.; Rizeq, M.; et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am. J. Gastroenterol. 2015, 110, 1429–1439. [Google Scholar] [CrossRef]
- Chong, C.C.N.; Lakhtakia, S.; Nguyen, N.; Hara, K.; Chan, W.K.; Puri, R.; Almadi, M.A.; Ang, T.L.; Kwek, A.; Yasuda, I.; et al. Endoscopic ultrasound-guided tissue acquisition with or without macroscopic on-site evaluation: Randomized controlled trial. Endoscopy 2020, 52, 856–863. [Google Scholar] [CrossRef]
- Wong, T.; Pattarapuntakul, T.; Netinatsunton, N.; Ovartlarnporn, B.; Sottisuporn, J.; Chamroonkul, N.; Sripongpun, P.; Jandee, S.; Kaewdech, A.; Attasaranya, S.; et al. Diagnostic performance of endoscopic ultrasound-guided tissue acquisition by EUS-FNA versus EUS-FNB for solid pancreatic mass without ROSE: A retrospective study. World. J. Surg. Oncol. 2022, 20, 215. [Google Scholar] [CrossRef]
- Kaneko, J.; Ishiwatari, H.; Sasaki, K.; Satoh, T.; Sato, J.; Matsubayashi, H.; Yabuuchi, Y.; Kishida, Y.; Yoshida, M.; Sayo, I.; et al. Macroscopic on-site evaluation of biopsy specimens for accurate pathological diagnosis during EUS-guided fine needle biopsy using 22-G Franseen needle. Endosc Ultrasound. 2020, 9, 385–391. [Google Scholar] [PubMed]
- Kudo, T.; Kawakami, H.; Hayashi, T.; Yasuda, I.; Mukai, T.; Inoue, H.; Katanuma, A.; Kawakubo, K.; Ishiwatari, H.; Doi, S.; et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014, 80, 1030–1037.e1. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Hamada, T.; Hakuta, R.; Sato, T.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Mizuno, S.; Kogure, H.; et al. A Meta-analysis of Slow Pull versus Suction for Endoscopic Ultrasound-Guided Tissue Acquisition. Gut Liver. 2021, 15, 625–633. [Google Scholar] [CrossRef]
- Ishikawa, T.; Hayakawa, M.; Suzuki, H.; Ohno, E.; Mizutani, Y.; Iida, T.; Fujishiro, M.; Kawashima, H.; Hotta, K. Development of a Novel Evaluation Method for Endoscopic Ultrasound-Guided Fine-Needle Biopsy in Pancreatic Diseases Using Artificial Intelligence. Diagnostics 2022, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, J.M.; Hawa, F.; Grantham, T.; Lester, J.; Carpenter, E.S.; Mendoza-Ladd, A.; Wani, S.; Machicado, J.D. Effect of the number of passes on diagnostic performance of EUS fine-needle biopsy of solid pancreatic masses: A systematic review and meta-analysis. Gastrointest. Endosc. 2024, 100, 595–604. [Google Scholar] [CrossRef]
- Shah, T.; Zfass, A.M. Accuracy of EUS-FNA in Solid Pancreatic Lesions: Sometimes Size Does Matter. Dig. Dis. Sci. 2019, 64, 1734–1735. [Google Scholar] [CrossRef]

| Type | Diagnosis | n (%) |
|---|---|---|
| Malignant | Pancreatic cancer | 419 (70.1) |
| Neuroendocrine neoplasm | 37 (6.2) | |
| Metastatic pancreatic tumor | 21 (3.5) | |
| Intraductal papillary mucinous carcinoma | 12 (2.0) | |
| Solid pseudopapillary neoplasm | 5 (0.8) | |
| Lymphoma | 5 (0.8) | |
| Others | 3 (0.5) | |
| Mucinous cystic neoplasm | 1 (0.2) | |
| Benign | Autoimmune pancreatitis | 37 (6.2) |
| Chronic pancreatitis | 20 (3.3) | |
| Nonspecific inflammation | 14 (2.3) | |
| Others | 14 (2.3) | |
| Serous cyst neoplasm | 7 (1.2) | |
| Intraductal papillary mucinous neoplasm | 3 (0.5) |
| Characteristics | n = 598 |
|---|---|
| Age, years | 69 (15–91) |
| Male, sex | 344 (57.5) |
| Lesion location | |
| Head | 291 (48.7) |
| Body | 123 (20.6) |
| Tail | 145 (24.2) |
| Head/body | 9 (1.5) |
| Body/tail | 22 (3.7) |
| Uncinate | 8 (1.3) |
| Tumor size, mm | 25.5 (6.8–107.8) |
| Number of passes | 2 (1–8) |
| n (%) | |
|---|---|
| Cytology | 554 (93.4) |
| Histology | 540 (91.4) |
| Cytology ± Histology | 576 (96.3) |
| Cytology | Histology | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| Sensitivity | 73.3 | 80 | 83.8 | 90.5 | 70.3 | 81.2 | 87.1 | 87.1 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPN | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| NPV | 20 | 29 | 29.2 | 41.2 | 18.9 | 26.9 | 39 | 35 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| (a) | ||||
| Age ≥ 70 years | 1.7 (0.5–5.3) | 0.394 | 1.6 (0.48–5.4) | 0.439 |
| Male | 0.2 (0.06–0.85) | 0.028 | 2.1 (0.05–0.81) | 0.023 |
| Malignant | 1.5 (0–Inf) | 0.99 | 6.2 (0–Inf) | 0.994 |
| Diameter ≥ 10 mm | 1.6 (0–Inf) | 0.99 | 9.3 (0–Inf) | 0.996 |
| Location (head) | 0.8 (0.3–2.4) | 0.67 | 6.7 (0.2–2.2) | 0.5 |
| (b) | ||||
| Age ≥ 70 years | 1.0 (0.4–2.6) | 1.0 | 1.2 (0.4–3.4) | 0.731 |
| Male | 0.7 (0.3–1.9) | 0.498 | 0.8 (0.3–2.2) | 0.607 |
| Malignant | 0.5 (0.06–3.9) | 0.476 | 1.1 (0.2–5.3) | 0.952 |
| Diameter ≥ 10 mm | 1.4 (0.14–13) | 0.79 | 1.7 (0.2–18) | 0.665 |
| Location (head) | 1.3 (0.5–3.6) | 0.558 | 1.2 (0.4–3.4) | 0.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, N.; Kawakami, H.; Ozono, Y.; Hatada, H.; Ogawa, S.; Sekiguchi, S.; Noguchi, H.; Sato, Y. Optimal Number of Needle Punctures in EUS-FNA/B with ROSE for Solid Pancreatic Lesions. Diagnostics 2025, 15, 1692. https://doi.org/10.3390/diagnostics15131692
Uchiyama N, Kawakami H, Ozono Y, Hatada H, Ogawa S, Sekiguchi S, Noguchi H, Sato Y. Optimal Number of Needle Punctures in EUS-FNA/B with ROSE for Solid Pancreatic Lesions. Diagnostics. 2025; 15(13):1692. https://doi.org/10.3390/diagnostics15131692
Chicago/Turabian StyleUchiyama, Naomi, Hiroshi Kawakami, Yoshinori Ozono, Hiroshi Hatada, Soichiro Ogawa, Satoshi Sekiguchi, Hiroshi Noguchi, and Yuichiro Sato. 2025. "Optimal Number of Needle Punctures in EUS-FNA/B with ROSE for Solid Pancreatic Lesions" Diagnostics 15, no. 13: 1692. https://doi.org/10.3390/diagnostics15131692
APA StyleUchiyama, N., Kawakami, H., Ozono, Y., Hatada, H., Ogawa, S., Sekiguchi, S., Noguchi, H., & Sato, Y. (2025). Optimal Number of Needle Punctures in EUS-FNA/B with ROSE for Solid Pancreatic Lesions. Diagnostics, 15(13), 1692. https://doi.org/10.3390/diagnostics15131692






