Abstract
Background/Objectives: White light imaging (WLI) of colonoscopy has a 26% adenoma miss rate. We aimed to evaluate the effectiveness of an additional 30 s (Add-30s) observation of the right-sided colon using a novel system (EVIS X1; Olympus Co.) with texture and color enhancement imaging (TXI). Methods: We reviewed 515 patients who underwent colonoscopy with Add-30s TXI between February 2021 and December 2023 at three affiliated hospitals. After initial right-sided colon observation with WLI, the colonoscope was reinserted into the cecum, and the right-sided colon was re-observed with Add-30s TXI. Adenoma and sessile serrated lesion (SSL) detection rate (ASDR) and adenoma detection rate (ADR) were examined. Multivariate analysis identified factors influencing lesion detection using the Add-30s TXI. The difference in WLI and TXI between the novel and previous scopes was performed using propensity score matching (PSM). The efficacy of WLI with the novel system was compared to that of the previous system. Results: Among the 515 cases, Add-30s TXI observation increased right-sided ADR and ASDR by 7.4% and 9.5%, respectively. The multivariate analysis showed novel scope as an independent factor for adenoma and SSL detection (odds ratio: 2.41, p < 0.01). Right-sided ADR and ASDR for Add-30s TXI were significantly higher in the novel scope than the previous scope (ADR, 25.2% vs. 15.3%; p = 0.04; ASDR, 32.4% vs. 18.9%; p = 0.02). ASDR for WLI observation was significantly higher in the novel system than the previous system (34.8% vs. 25.9%; p < 0.01). Conclusions: Add-30s TXI significantly improved the detection of missed adenomas and SSLs in the right-sided colon.
1. Introduction
The removal of colorectal adenomas reduces the incidence of colorectal cancer (CRC) and CRC-related mortality [1,2,3]. Colonoscopy is an important tool for detecting colorectal adenomas. However, a systematic review reported a 26% miss rate for adenomas when using white light imaging (WLI) (95% confidence interval [CI]: 23–30%) [4]. Poor bowel preparation, right-sided location, flat morphology, small polyp size, and a high prevalence of sessile serrated lesions (SSLs) have been identified as risk factors for missed polyps [4,5,6,7,8,9].
Numerous clinical studies have reported improvements in adenoma detection rates (ADRs) with image-enhanced endoscopy, including narrow-band imaging (NBI), blue laser imaging, and linked-color imaging [10,11,12]. A recent systematic review demonstrated that NBI detected significantly more adenomas than WLI under optimal bowel preparation conditions [13]. In July 2020, a novel endoscopic system incorporating five-color light-emitting diodes (LEDs) (EVIS X1: CV-1500; Olympus Co., Tokyo, Japan) was released globally. In addition to enhancements in WLI and NBI, this system introduced texture and color enhancement imaging (TXI) [14]. TXI is designed to improve lesion visibility by enhancing texture, brightness, and color contrast, making lesions appear redder and more conspicuous. Several studies have shown that TXI offers superior visibility compared to WLI [15,16]. Recently, a multicenter randomized controlled trial (RCT) demonstrated a positive impact of TXI vs. standard high-definition WLI on ADR [17].
Additional observation of the right-sided colon has been shown to reduce missed polyps. A systematic review showed that supplementary forward and retroflexed observations of the right colon after the initial WLI observation increased right-sided ADR by 10% and 6%, respectively [18]. In a previous study, we reported the efficacy of an additional 30 s (Add-30s) NBI observation following standard WLI for reducing missed polyps in the right colon [19]. Notably, in a multicenter RCT, we demonstrated that Add-30s observation of the right-sided colon using TXI was non-inferior to NBI for detecting missed polyps, with both approaches improving ADR by 10.2% and 10.5%, respectively [20].
In the present multicenter study, we aimed to evaluate the effectiveness of Add-30s TXI observation of the right-sided colon in a large cohort. We also assessed the efficacy of the novel endoscopic system in this context.
2. Materials and Methods
This multicenter, retrospective observational study was conducted at three affiliated hospitals in Japan: Nishijin Hospital, Kyoto Prefectural University of Medicine, and the Japanese Red Cross Kyoto Daiichi Hospital. We analyzed 515 patients who underwent Add-30s observation with TXI as a second observation following WLI in the right-sided colon between February 2021 and December 2023. Since we previously reported the effectiveness of Add-30s observation, this method was employed at the discretion of the endoscopist at each institution (Figure 1) [19]. All procedures were performed using either a 290-series scope (PCF-H290AZI, CF-HQ290ZL/I; Olympus Co., Tokyo, Japan) or a novel scope (CF-XZ1200L/I, CF-EZ1500DL/I; Olympus Co., Tokyo, Japan) in combination with the novel system (CV-1500; Olympus Co., Tokyo, Japan).
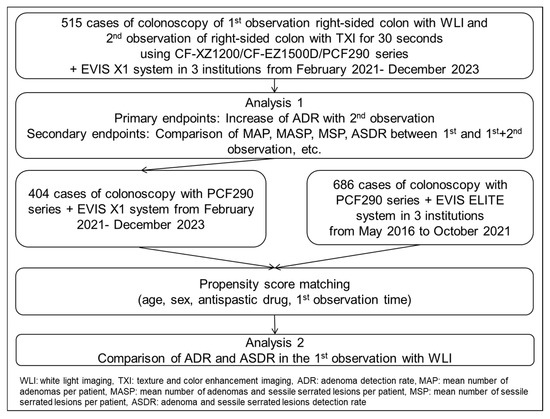
Figure 1.
Study flowchart.
The inclusion criteria were as follows: (1) symptoms such as abdominal pain, constipation, anemia, and hematochezia; (2) surveillance after resection of polyps or cancer; and (3) positive fecal occult blood. Exclusion criteria were as follows: confirmed recurrent lesions with scarring after previous endoscopic mucosal resection (EMR) or polypectomy, T1–T4 colorectal cancers, and prior surgical resection involving the cecum or ascending colon. All examinations were performed by 14 endoscopists.
Regarding the observation method, the cecum and ascending colon were first examined using WLI. Thereafter, the colonoscope was reinserted into the cecum from the hepatic flexure, and the right-sided colon, from the cecum to the ascending colon, was observed using Add-30s TXI (Figure 1). TXI1 was used, as in our previous study, because it provided better color enhancement than TXI2 and demonstrated superior visibility for colorectal lesions [20,21]. The Add-30s observation method involved sufficient insufflation of the right-sided colon to enable a distant view, following a previously reported protocol. The 30 s duration was based on our earlier study (Supplemental Video, Figure 2). Even if the right-sided colon was not completely observed during the 30 s, the second observation was nonetheless completed. Polyp location, morphology, and size were carefully documented to prevent duplicate polyp counts.
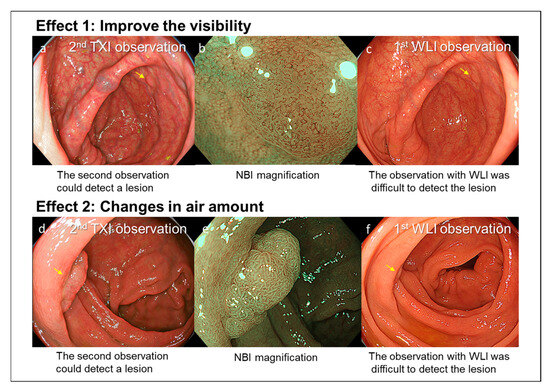
Figure 2.
Case 1: A 2 mm non-polypoid polyp located in the cecum. (a) The lesion was detected during the second observation using TXI, which enhanced polyp visibility (yellow arrow). (b) NBI magnification findings were consistent with a typical adenoma. (c) A movie of the first observation was checked. The lesion was difficult to detect during the first WLI observation (yellow arrow). Case 2: A 10 mm polypoid polyp located in the ascending colon. (d) The lesion was detected during the second observation, adjusting the amount of insufflated air enabled visualization of a polyp hidden behind a mucosal fold (yellow arrow). (e) NBI magnification findings were consistent with a typical adenoma. (f) A movie of the first observation was checked. The lesion was difficult to detect during the first observation (yellow arrow).
The primary endpoint of this study was the increase in ADR with the sAdd-30s TXI observation as the second observation. The secondary endpoints included the mean number of adenomas per patient (MAP), the mean number of adenomas and sessile serrated lesions per patient (MASP), the mean number of sessile serrated lesions per patient (MSP), and adenoma and SSL detection rates (ASDRs) between the first and combined first and second observations. Patient characteristics, such as age, sex, insertion time, bowel preparation, use of antispastic drugs, sedation, endoscopists, scope type, and duration of the first observation, and polyp characteristics, including size, location, morphology, and histology, were analyzed for both observations.
Additionally, these parameters were compared between the adenoma/SSL-detected and non-detected groups during the second observation using univariate and multivariate analyses. The ADRs and ASDRs from the first and second observations were also compared between groups that underwent colonoscopy using the novel and previous scopes. Using propensity score matching (PSM), we matched these two groups in a 1:1 ratio, selecting 111 patients in the novel scope group based on age, sex, and variables with p-values < 0.05 in the univariate analysis including the duration of the first observation time.
Finally, the ADR and ASDR of the first WLI observation using the novel system were compared with those of 686 colonoscopies performed between May 2016 and October 2021 using the previous system (EVIS LUCERA ELITE System, CV-190 [Video system]/CLV-290 [Light source], Olympus Medical Co., Tokyo, Japan) and 290-series scopes to determine whether the system affected detection outcomes (Figure 1). These two groups were also matched using PSM with 402 patients in the new system group at a 1:1 ratio, based on age, sex, and factors with p < 0.05 in univariate analysis (specifically, the use of antispastic drugs and the duration of the first observation).
To evaluate bowel preparation, the Aronchick bowel preparation score was used, which grades bowel preparation as excellent, good, fair, poor, or inadequate [22]. Excellent and good bowel preparation was defined as good in the present study. Midazolam was routinely administered for sedation. Endoscopic diagnosis of all lesions was performed using NBI magnification [23,24], and all polyps diagnosed as neoplastic or SSLs were resected using polypectomy, EMR, or endoscopic submucosal dissection (ESD) in accordance with the Japan Gastroenterological Endoscopy Society guidelines [25,26].
Some polyps that did not require endoscopic resection—such as hyperplastic or inflammatory polyps—were diagnosed based on magnified NBI observation rather than histological assessment due to the retrospective nature of the study. Experts were defined as endoscopists with experience performing more than 1000 colonoscopies and at least 50 withdrawal colonoscopies with TXI, according to previous reports [20].
Polyp size was defined by its maximum diameter and estimated based on comparison with snares or biopsy forceps. A cap was used for all procedures. Polyps were classified as polypoid or non-polypoid according to the Paris classification [27]. Histopathological diagnoses were made using biopsy or resected specimens in accordance with the World Health Organization classification. In this study, intramucosal cancer was categorized as high-grade dysplasia (HGD) [28]. SSLs were classified following the World Health Organization system [29].
Most patients consumed a liquid diet and 10 mL of sodium picosulfate the day before the colonoscopy. On the day of the procedure, they ingested 1.0–2.0 L of a highly concentrated polyethylene glycol solution with ascorbic acid (MoviPrep; EA Pharma Co., Ltd., Tokyo, Japan), followed by >0.5–1.0 L of water.
This retrospective study was conducted in accordance with the principles of the Declaration of Helsinki. After consultation with the Institutional Review Board (IRB) of each participating institution, the Ethics Committee of Kyoto Prefectural University of Medicine provided collective approval for the study (Approval No. ERB-C-1704-5, 5 June 2024). An opt-out of the study to the patients was performed in each hospital using a website or a board in an endoscopic unit, or both. Written informed consent was achieved from all the patients.
3. Statistical Analysis
The results were analyzed using the Mann–Whitney U test and the chi-squared test. Continuous variables, such as polyp size, were analyzed with the Mann–Whitney U test. Multivariate logistic regression analyses were also conducted to identify predictive factors for adenoma/SSL detection during the second observation. All statistical analyses were performed using SPSS software (version 22.0; IBM Japan, Tokyo, Japan). Statistical significance was defined as p < 0.05.
4. Results
Among the 515 cases, the mean age was 67.1 ± 11.0 years, and 60.6% were male (Table 1). The rate of good bowel preparation was 74.2%, and the use of the novel scope was observed in 21.6% of cases.

Table 1.
Clinical characteristics of patients.
Regarding differences in detected lesions between the first WLI observation and the second Add-30s TXI observation, significant differences were noted in polyp size (mean ± SD: 4.8 ± 3.9 mm vs. 3.9 ± 3.9 mm, p < 0.01) and in the proportion of lesions located in the ascending colon (73.6% vs. 84.4%; p = 0.01) (Table 2).

Table 2.
The results of the 1st observation and the 2nd observation.
Comparison of the first observation with the combined first and second observations showed significant increases in ADR (30.3% vs. 37.7%, p < 0.01) and ASDR (38.3% vs. 47.8%, p < 0.01) (Table 3). The increase in ADR was 7.4% (95% CI: 5.4–10.0). Significant differences were also observed in MAP, MASP, and MSP between the two observation strategies.

Table 3.
Comparison between 1st observation and 1st + 2nd observation.
In the analysis of factors related to adenoma/SSL detection, only the use of the novel scope remained significant after PSM in the multivariate analysis (odds ratio: 2.41, 95% CI: 1.47–3.94; p < 0.01) (Table 4).

Table 4.
Comparison of lesion-detected group and non-detection group in 2nd TXI observation.
In the analysis of factors related to ADR, the use of the novel scope also remained significant after PSM in the multivariate analysis (odds ratio: 2.11, 95% CI: 1.25–3.57; p = 0.01) (Table 5).

Table 5.
Comparison of adenoma-detected group and non-detection group in 2nd TXI observation.
Comparison between the novel and previous scopes showed that ADR and ASDR during the second Add-30s TXI observation were significantly higher in the novel scope group than in the previous scope group, according to PSM (ADR: 25.2% vs. 15.3%, p = 0.04; ASDR: 32.4% vs. 18.9%, p = 0.02) (Table 6).

Table 6.
Comparison of lesion detection according to scope type before and after propensity score matching.
Finally, with respect to the endoscopic system type, the ASDR during the first WLI observation was significantly higher in the novel system group than in the previous system group, based on PSM (34.8% vs. 25.9%; p < 0.01) (Table 7).

Table 7.
Comparison of lesion detection according to system (EVIS X1 vs. EVIS LUCERA ELITE) before and after propensity score matching.
5. Discussion
In this multicenter study, we analyzed 515 patients who underwent Add-30s observation with TXI of the right-sided colon. This method increased the right-sided ADR by 7.4% and ASDR by 9.5%. We propose that the Add-30s TXI observation method has two main strengths (Figure 2). First, TXI enhances polyp visibility, thereby facilitating detection. Second, performing a second observation of the right-sided colon allows for the detection of polyps hidden behind mucosal folds, as the amount of insufflated air can be changed between observations.
A multicenter RCT reported that the ADR was 58.9% (221/375) in the TXI group and 42.7% (159/372) in the WLI group [risk ratio (RR): 1.38, 95% CI: 1.20–1.59; p < 0.001] [17]. Sakamoto et al. also reported in a multicenter study that TXI was a significant factor associated with MAP (OR: 1.4, 95% CI: 1.2–1.6; p < 0.001) and ADR (OR: 1.5, 95% CI: 1.0–2.3; p = 0.044), based on multivariate regression analysis [30]. On the other hand, a recent multicenter RCT from Japan reported that ADR was 57.2% and 56.0% in the TXI and WLI groups, respectively, with no statistically significant difference between the two groups. The polyp detection rate and flat polyp detection rate were significantly higher in the TXI group than in the WLI group, which were 82.5% vs. 74.4% (p = 0.003), and 76.5% vs. 70.3% (p = 0.036), respectively [31]. Further analysis is expected for the use of TXI as routine first observation. In the current study, consistent with these findings, TXI contributed to an increased ADR, although our study was a retrospective setting for improving polyp miss. Several studies have shown that colonoscopy is significantly less effective in reducing cancer incidence in the proximal colon compared to the distal colon [32,33]. Missed polyps in the proximal colon may account for this discrepancy, and TXI observation may help to mitigate this issue.
In the current study, polyps detected during the second Add-30s TXI observation were smaller and more frequently located in the ascending colon compared to those detected during the first WLI observation. In our previous RCT, Add-30s TXI observation of the right-sided colon achieved a significantly higher detection rate for polyps in the ascending colon compared to NBI (cecum/ascending colon: 14.3%/85.7% for TXI vs. 34.3%/65.7% for NBI; p < 0.01) [20]. Compared to NBI, TXI offers a brighter view and maintains adequate illumination even under suboptimal bowel preparation, making it potentially advantageous for detecting polyps in the ascending colon.
With WLI, additional observation of the right-sided colon can be performed using either the forward or retroflex view. A systematic review reported that additional forward and retroflex observations increased the right-sided ADR by 10% and 6%, respectively [18]. A recent RCT showed that the ADR for the right-sided colon in the second forward observation and first observation group was 27.1% and 21.6%, respectively (p = 0.042) [34]. Another meta-analysis found that the increase in right-sided colon ADR was lower in the retroflex view group than in the forward view group (8.1% vs. 11.3%; p = 0.04) [35]. However, additional WLI observation typically requires 2–3 min, and retroflex observation is technically challenging for both endoscopists and patients. In contrast, our Add-30s TXI observation achieved a comparable increase in ADR to additional WLI observations, making the short observation time the greatest advantage of this method.
The use of novel scopes resulted in higher ASDR and ADR during the second TXI observation compared to previous scopes. A previous multicenter study similarly demonstrated that the use of a new-generation scope was associated with an increased ADR [36]. The improved brightness and image quality of the novel scope, relative to previous models, may contribute to enhanced lesion detection, even when used with TXI. Furthermore, the new endoscopic system yielded a higher ASDR during WLI observation than the previous system. In our previous study using previous system, the MASP, MSP, and MAP for the first WLI observation of the right-sided colon were 0.43, 0.06, and 0.34, respectively [37]. In the current study using the novel system, the MASP, MSP, and MAP increased to 0.61, 0.14, and 0.50, respectively—higher than previously reported. To the best of our knowledge, system-based comparisons of this nature have not been previously reported; however, the improved resolution and brightness of the new system likely contributed to these findings.
This study has some limitations. First, it was a retrospective observational study. The Add-30s TXI observation primarily revealed minute polyps. There is potential for selection bias, as the cases included were not consecutive, and the decision to perform Add-30s TXI was made by the endoscopists at each hospital. Additionally, some cases included in this study overlapped with those in previous reports [20,37]. The comparison between the first observation and the combined first and second observations may also have been influenced by the polyp count during the first observation. The indication of the colonoscopy including positive fecal occult blood and family history were not examined in this study though they were likely to affect the ADR. Patient characteristics related to insulin resistance, including diabetes mellitus and body mass index, were not examined in this study, although these factors might be associated with right-sided polyps. Furthermore, approximately 20% of polyps were examined endoscopically but not histologically.
In conclusion, our study demonstrated that Add-30s TXI observation of the right-sided colon significantly improved ADR and ASDR. This method is simple to implement and effective in detecting missed polyps.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics15141759/s1, Supplemental Video: The cecum and ascending colon were first examined using WLI. Thereafter, the colonoscope was reinserted into the cecum, and the right-sided colon, from the cecum to the ascending colon, was observed using Add-30s TXI. The lesion was detected during the second observation using TXI, which enhanced polyp visibility.
Author Contributions
Y.I. (Yoshikazu Inagaki) and N.Y. conceived and designed the study. Material preparation, data collection, and analysis were performed by Y.I. (Yoshikazu Inagaki), N.Y., H.H., Y.I. (Yutaka Inada), T.M., T.S., Y.T., R.K., K.I. and O.D. K.K. and Y.I. (Yoshito Itoh) helped design the study. The first draft of the manuscript was written by Y.I. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in compliance with the Declaration of Helsinki. The Institutional Review Boards of Kyoto Prefectural University of Medicine and Jichi Medical University approved the protocol (ERB-C-1704-5, data: 5 June 2024).
Informed Consent Statement
An opt-out of the study to the patients was performed in each hospital using a website or a board in an endoscopic unit, or both. Written informed consent was achieved from all the patients.
Data Availability Statement
The patient data used to support the findings of this study are available from the corresponding author upon request. However, identified patient data are restricted by the institutional review board of Kyoto Prefectural University of Medicine.
Acknowledgments
We thank all members of Nishijin Hospital and the Department of Molecular Gastroenterology and Hepatology at Kyoto Prefectural University of Medicine and Japanese Red Cross Kyoto Daiichi Hospital for their assistance with this study. The authors thank all members of the Kyoto Improvement of Colonoscopy Seminar for their assistance in this study.
Conflicts of Interest
Author Hikaru Hashimoto was employed by the company Department of Gastroenterology, Osaka General Hospital of West Japan Railway Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Zauber, A.G.; O’Brien, M.J.; Ho, M.N.; Gottlieb, L.; Sternberg, S.S.; Waye, J.D.; Bond, J.; Schapiro, M.; Stewart, E.T.; et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 328, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Pan, P.; Xia, T.; Chang, X.; Yang, X.; Guo, L.; Meng, Q.; Yang, F.; Qian, W.; et al. Magnitude, risk factors, and factors associated with adenoma Miss Rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019, 156, 1661–1674.e11. [Google Scholar] [CrossRef]
- Kim, N.H.; Jung, Y.S.; Jeong, W.S.; Yang, H.J.; Park, S.K.; Choi, K.; Park, D.I. Miss rate of colorectal neoplastic polyps and risk factors for missed polyps in consecutive colonoscopies. Intest. Res. 2017, 15, 411–418. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.W.; Kim, Y.S.; Lee, K.J.; Sung, H.; Song, P.H.; Yoon, W.J.; Moon, J.S. Risk factors of missed colorectal lesions after colonoscopy. Medicine 2017, 96, e7468. [Google Scholar] [CrossRef]
- Leufkens, A.M.; van Oijen, M.G.H.; Vleggaar, F.P.; Siersema, P.D. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy 2012, 44, 470–475. [Google Scholar] [CrossRef]
- Zimmermann-Fraedrich, K.; Sehner, S.; Rex, D.K.; Kaltenbach, T.; Soetikno, R.; Wallace, M.; Leung, W.K.; Guo, C.; Gralnek, I.M.; Brand, E.C.; et al. Right-sided location not associated with missed colorectal adenomas in an individual-level reanalysis of tandem colonoscopy studies. Gastroenterology 2019, 157, 660–671.e2. [Google Scholar] [CrossRef]
- Laiyemo, A.O.; Doubeni, C.; Sanderson, A.K., 2nd; Pinsky, P.F.; Badurdeen, D.S.; Doria-Rose, V.P.; Marcus, P.M.; Schoen, R.E.; Lanza, E.; Schatzkin, A.; et al. Likelihood of missed and recurrent adenomas in the proximal versus the distal colon. Gastrointest. Endosc. 2011, 74, 253–261. [Google Scholar] [CrossRef]
- Bisschops, R.; East, J.E.; Hassan, C.; Hazewinkel, Y.; Kamiński, M.F.; Neumann, H.; Pellisé, M.; Antonelli, G.; Bustamante Balen, M.; Coron, E.; et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 1155–1179. [Google Scholar]
- Dos Santos, C.E.O.; Malaman, D.; Pereira-Lima, J.C.; Onófrio, F.Q.; Ribas Filho, J.M. Impact of linked-color imaging on colorectal adenoma detection. Gastrointest. Endosc. 2019, 90, 826–834. [Google Scholar] [CrossRef]
- Leung, W.K.; Tsui, V.W.M.; Mak, L.L.Y.; Cheung, M.K.S.; Hui, C.K.Y.; Lam, C.P.M.; Wong, S.Y.; Liu, K.S.H.; Ko, M.K.L.; To, E.W.P.; et al. Blue-light imaging or narrow-band imaging for proximal colonic lesions: A prospective randomized tandem colonoscopy study. Gastrointest. Endosc. 2023, 98, 813–821. [Google Scholar] [CrossRef]
- Atkinson, N.S.S.; Ket, S.; Bassett, P.; Aponte, D.; De Aguiar, S.; Gupta, N.; Horimatsu, T.; Ikematsu, H.; Inoue, T.; Kaltenbach, T.; et al. Narrow-band imaging for detection of neoplasia at colonoscopy: A meta-analysis of data from individual patients in randomized controlled trials. Gastroenterology 2019, 157, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Sato, T. Txi: Texture and color enhancement imaging for endoscopic image enhancement. J. Healthc. Eng. 2021, 2021, 5518948. [Google Scholar] [CrossRef]
- Okumura, T.; Hotta, K.; Imai, K.; Ito, S.; Kishida, Y.; Takada, K.; Kawaguchi, D.; Mori, Y.; Tanaka, Y.; Tsushima, T.; et al. Efficacy of texture and color enhancement imaging for the visibility and diagnostic accuracy of non-polypoid colorectal lesions. DEN Open 2024, 5, e380. [Google Scholar] [CrossRef]
- Yoshida, N.; Inoue, K.; Dohi, O.; Kobayashi, R.; Tomita, Y.; Hashimoto, H.; Sugino, S.; Hirose, R.; Murakami, T.; Inada, Y.; et al. The analysis of texture and color enhancement imaging for improving the visibility of non-polypoid colorectal lesions. Dis. Dig. Sci. 2022, 67, 5657–5665. [Google Scholar] [CrossRef]
- Antonelli, G.; Bevivino, G.; Pecere, S.; Ebigbo, A.; Cereatti, F.; Akizue, N.; Di Fonzo, M.; Coppola, M.; Barbaro, F.; Walter, B.M.; et al. Texture and color enhancement imaging versus high definition white-light endoscopy for detection of colorectal neoplasia: A randomized trial. Endoscopy 2023, 55, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Bilal, M.; Hamade, N.; Gorrepati, V.S.; Chandrasekar, V.T.; Jegadeesan, R.; Gupta, N.; Bhandari, P.; Repici, A.; Hassan, C.; et al. Increasing adenoma detection rates in the right side of the colon comparing retroflexion with a second forward view: A systematic review. Gastrointest. Endosc. 2019, 89, 453–459.e3. [Google Scholar] [CrossRef]
- Yoshida, N.; Inoue, K.; Yasuda, R.; Hirose, R.; Dohi, O.; Naito, Y.; Murakami, T.; Inada, Y.; Ogiso, K.; Morinaga, Y.; et al. An Additional 30-s Observation of the Right-Sided Colon with Narrow Band Imaging Decreases Missed Polyps: A Pilot Study. Dig. Dis. Sci. 2018, 63, 3457–3464. [Google Scholar] [CrossRef]
- Yoshida, N.; Inagaki, Y.; Inada, Y.; Kobayashi, R.; Tomita, Y.; Hashimoto, H.; Dohi, O.; Hirose, R.; Inoue, K.; Murakami, T.; et al. Additional 30-Second Observation of the Right-Sided Colon for Missed Polyp Detection with Texture and Color Enhancement Imaging Compared with Narrow Band Imaging: A Randomized Trial. Am. J. Gastroenterol. 2024, 119, 539–546. [Google Scholar] [CrossRef]
- Tamai, N.; Horiuchi, H.; Matsui, H.; Furuhashi, H.; Kamba, S.; Dobashi, A.; Sumiyama, K. Visibility evaluation of colorectal lesion using texture and color enhancement imaging with video. DEN Open 2022, 2, e90. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Saltzman, J.R.; Cash, B.D.; Pasha, S.F.; Early, D.S.; Muthusamy, V.R.; Khashab, M.A.; Chathadi, K.V.; Fanelli, R.D.; Chandrasekhara, V.; et al. Bowel preparation before colonoscopy. Gastrointest. Endosc. 2015, 81, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Tanaka, S.; Kudo, S.E.; Saito, S.; Matsuda, T.; Wada, Y.; Fujii, T.; Ikematsu, H.; Uraoka, T.; Kobayashi, N.; et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig. Endosc. 2016, 28, 526–533. [Google Scholar] [CrossRef]
- Yamashina, T.; Takeuchi, Y.; Uedo, N.; Aoi, K.; Matsuura, N.; Nagai, K.; Matsui, F.; Ito, T.; Fujii, M.; Yamamoto, S.; et al. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: A prospective study of diagnostic accuracy. J. Gastroenterol. Hepatol. 2015, 30, 117–123. [Google Scholar] [CrossRef]
- Tanaka, S.; Kashida, H.; Saito, Y.; Yahagi, N.; Yamano, H.; Saito, S.; Hisabe, T.; Yao, T.; Watanabe, M.; Yoshida, M.; et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc. 2015, 27, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Uraoka, T.; Takizawa, K.; Tanaka, S.; Kashida, H.; Saito, Y.; Yahagi, N.; Yamano, H.; Saito, S.; Hisabe, T.; Yao, T.; et al. Guidelines for Colorectal Cold Polypectomy (supplement to “Guidelines for Colorectal Endoscopic Submucosal Dissection/Endoscopic Mucosal Resection”). Dig. Endosc. 2022, 34, 668–675. [Google Scholar] [CrossRef]
- The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 2003, 58, S3–S43. [CrossRef]
- Hamilton, S.R.; Aaltonen, L.A. (Eds.) World Health Organization classification of tumors. In Pathology and Genetics of Tumours of the Digestive System; IARC Press: Lyon, France, 2010; pp. 104–109. [Google Scholar]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Sakamoto, T.; Ikematsu, H.; Tamai, N.; Mizuguchi, Y.; Takamaru, H.; Murano, T.; Shinmura, K.; Sasabe, M.; Furuhashi, H.; Sumiyama, K.; et al. Detection of colorectal adenomas with texture and color enhancement imaging: Multicenter observational study. Dig. Endosc. 2023, 35, 529–537. [Google Scholar] [CrossRef]
- Toyoshima, N.; Mizuguchi, Y.; Takamaru, H.; Nakamura, K.; Kakugawa, Y.; Sakamoto, T.; Shiroyama, M.; Kawagoe, R.; Tsuchiya, K.; Shinmura, K.; et al. The Efficacy of Texture and Color Enhancement Imaging Observation in the Detection of Colorectal Lesions: A Multicenter, Randomized Controlled Trial (deTXIon Study). Gastroenterology 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Shergill, A.K.; Conners, E.E.; McQuaid, K.R.; Epstein, S.; Ryan, J.C.; Shah, J.N.; Inadomi, J.; Somsouk, M. Protective association of colonoscopy against proximal and distal colon cancer and patterns in interval cancer. Gastrointest. Endosc. 2015, 82, 529–537.e1. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Demers, A.A.; Kliewer, E.V.; Mahmud, S.M.; Bernstein, C.N. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010, 139, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.S.Y.; Lee, J.W.J.; Chang, L.C.; Ong, D.E.H.; Chiu, H.M.; Matsuda, T.; Kim, H.S.; Sekiguchi, M.; Leong, R.W.; Ho, A.M.Y.; et al. Two vs one forward view examination of right colon on adenoma detection: An international multicenter randomized trial. Clin. Gastroenterol. Hepatol. 2022, 20, 372–380.e2. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.H.; Lu, Q.; Sun, Y.N.; Deng, K.; Yang, J.L. Retroflexed view for reexamination of the right colon after forward view examination: Systematic review and meta-analysis. Dig. Endosc. 2022, 34, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Gkolfakis, P.; Lazaridis, L.D.; Facciorusso, A.; Antonelli, G.; Hassan, C.; Repici, A.; Sharma, P.; Rex, D.K.; Triantafyllou, K. High-definition colonoscopy for improving adenoma detection: A systematic review and meta-analysis of randomized controlled studies. Gastrointest. Endosc. 2020, 91, 1027–1036. [Google Scholar] [CrossRef]
- Hashimoto, H.; Yoshida, N.; Inagaki, Y.; Fukumoto, K.; Hasegawa, D.; Okuda, K.; Tomie, A.; Yasuda, R.; Morimoto, Y.; Murakami, T.; et al. Additional 30-second observation of the right-sided colon for missed polyp detection with linked color imaging compared with narrow band imaging. Endosc. Int. Open 2024, 12, E1092–E1101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).