Abstract
Background: Multidrug-resistant bacteria (MDRB) represent a significant challenge in intensive care units (ICUs), as they limit treatment options, prolong hospital stays, and escalate healthcare costs. Respiratory ICUs are particularly affected due to the high prevalence of chronically ill patients with recurrent infections. Understanding the impact of culture positivity and MDRB on clinical outcomes and readmission rates is essential for enhancing patient care and addressing the growing burden of antimicrobial resistance. Methods: This retrospective study was conducted in a specialized respiratory ICU at a tertiary care hospital between 1 January 2019, and 1 January 2020. A total of 695 ICU admissions were analyzed, with patients grouped based on readmission status and culture results. Demographic, clinical, and laboratory data were reviewed. Statistical analyses were performed using appropriate tests, with p-values ≤ 0.05 considered statistically significant. Results: Among the 519 unique patients, 65 experienced ICU readmissions. Male patients were significantly more likely to be readmitted (p = 0.008). Culture positivity was predominantly observed in respiratory samples, with Klebsiella spp. identified as the most common pathogen. MDRB prevalence exceeded 60% in both groups, significantly prolonging ICU stays (p = 0.013). However, no significant differences in survival rates were observed between MDRB-positive and MDRB-negative groups. Notably, patients with readmissions had lower C-reactive protein (CRP) levels both during admission and at discharge compared to non-readmitted patients (p = 0.004). This paradox may reflect a subclinical inflammatory response associated with bacterial colonization rather than active infection, particularly in patients with chronic respiratory diseases. Conclusions: MDRB infections and culture positivity are key contributors to prolonged ICU stays, resulting in increased healthcare costs. Implementing effective strategies to manage MDRB infections is critical for improving outcomes in respiratory ICUs and reducing associated risks. This study underscores the growing burden of MDRB and highlights the importance of enhanced antimicrobial stewardship in respiratory ICUs.
1. Introduction
Patients admitted to intensive care units (ICUs) are at high risk of infections due to their critical conditions, which often require intensive treatments and invasive procedures. In these patients, infections caused by multidrug-resistant (MDR) bacteria frequently arise as a consequence of extensive antibiotic use. These bacteria significantly limit treatment options, extend hospital stays, and increase mortality rates [1].
Antibiotic resistance has emerged as a growing threat to global healthcare systems. The increasing prevalence of MDR bacteria not only compromises patient outcomes but also imposes substantial financial burdens on healthcare services. Identifying effective strategies to combat these infections in ICUs is, therefore, of paramount importance [2]. However, the prolonged and widespread use of broad-spectrum antibiotics to treat MDR infections accelerates the emergence of resistant bacteria [3].
Culture positivity is a vital biomarker for assessing the risk of infection among ICU patients. Prolonged exposure to treatments such as invasive procedures and mechanical ventilation facilitates the colonization of Gram-negative bacteria and MDR organisms. Patients with culture positivity are at higher risk of developing severe infections and often require readmission. Additionally, culture positivity plays a crucial role in the early detection of pathogens, which aids in preventing infections and guiding treatment strategies [4].
This study aims to investigate the relationships between culture positivity, antibiotic resistance, associated risk factors, readmission rates, and clinical outcomes in patients admitted to respiratory ICUs. By evaluating the impact of culture positivity and antibiotic resistance on patient prognosis, this study seeks to identify effective strategies for managing infections and improving clinical outcomes.
One of the innovative aspects of this study lies in its comprehensive evaluation of culture positivity and multidrug-resistant bacteria (MDRB) in respiratory ICU patients, not only in terms of infection burden but also in relation to readmission rates and survival outcomes. Furthermore, the interpretation of culture positivity as indicative of both active infection and colonization—supported by laboratory parameters—offers a novel perspective for infection control strategies. In this regard, the study moves beyond microbiological outcomes alone and adopts a holistic approach by integrating multiple clinical variables that influence patient management and ICU processes.
2. Materials and Methods
This study was conducted in compliance with the ethical standards of the institutional and national research committee and adhered to the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from all participants included in the study, along with consent to publish the study findings.
The study was designed as a retrospective, cross-sectional investigation and conducted in a tertiary care hospital specializing in pulmonary medicine. Ethics approval was obtained from the Clinical Research Ethics Committee of the University of Health Sciences, Ankara Sanatorium Training and Research Hospital (approval date: 13 November 2024, approval number: 2024-BÇEK/164). The study population consisted of patients admitted to the pulmonary intensive care unit (ICU) between 1 January 2019, and 1 January 2020. All included patients had at least one primary respiratory comorbidity that justified their ICU admission. Patients were not enrolled in a specific follow-up program; however, their survival status after discharge was assessed via the national healthcare database.
Demographic data, including gender, age, comorbidity history, intubation history, previous culture results, and past ICU admissions, were collected from the hospital database. For cases where complete information was unavailable, the national patient database was consulted, especially when patients had been admitted to other healthcare centers prior to the current ICU admission. Additional information, such as pneumonia at admission, pulmonology consultations, source of ICU admission (e.g., outpatient clinic, emergency ward, inpatient ward, or another ICU), non-invasive mechanical ventilation (NIMV) use, and antibiotic regimens, was extracted from ICU records. Antibiotherapy regimens were categorized into three groups based on respiratory severity: 1—combinations involving cephalosporins, penicillins, macrolides, or fluoroquinolones; 2—antipseudomonal combinations; and 3—carbapenems and other broad-spectrum regimens. Pathogen-specific treatments, such as antifungals, antivirals, and methicillin-resistant Staphylococcus aureus (MRSA) treatments, were recorded separately.
Culture results were considered positive for a specific bacterium regardless of the sample origin, as long as the result was not deemed contaminated. Samples were classified into respiratory (e.g., endotracheal aspiration, orofacial sampling, sputum), urinary, or hematological (e.g., catheter and blood cultures) categories. For patients with multiple positive cultures, the most resistant sample was reported. If the same resistance pattern was reported across multiple samples, the earliest reported result was used. Cultures were classified as multidrug-resistant if at least one result indicated resistance to commonly used regimens. Rare culture samples (less than 1% of cases) were excluded from the statistical analysis.
Survival duration was calculated from the date of the initial ICU admission. For patients with repeated ICU admissions, survival was assessed based on the initial admission. ICU mortality (exitus) was defined as death during the ICU stay, regardless of whether it occurred during the initial or subsequent admission. ICU length of stay was defined as the time from admission to discharge or, for deceased patients, to the time of death during the initial admission. Durations of repeated admissions were excluded from statistical evaluation.
Laboratory markers were collected at different time points. Results obtained at admission were considered baseline values. Follow-up values were recorded as part of routine weekly tests, with the first follow-up conducted 3–4 days after admission and the second follow-up 6–8 days after admission. For cases with additional tests due to clinical necessity, values closest to these time points were used. Laboratory results at or near discharge were accepted as the third and final follow-up values.
Statistical Analysis
All collected data were initially organized and reviewed in Microsoft Excel to ensure accuracy and completeness. Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 25.0, IBM Corp., Armonk, NY, USA). Descriptive analyses were conducted, with results presented as mean and standard deviation for normally distributed data or as median and percentiles for non-normally distributed data. Parametric distribution was evaluated using Q-Q plot analysis.
For comparisons, Chi-square tests and independent samples t-tests were used for nominal and scale variables with parametric distributions, respectively. For non-parametric data, the Mann–Whitney U test was applied. Chi-square analyses excluded parameters with subgroup counts below expected thresholds to ensure reliable comparisons. Statistical significance was set at p ≤ 0.05.
To establish a valid model for linear bivariate regression analysis with a Type 1 error rate of 0.05 and 90% power, a minimum of 190 patients was required, assuming an equal allocation ratio between groups. The study design, however, anticipated a sample size at least twice this number to allow for more robust subgroup evaluations.
3. Results
A total of 695 patient admissions were evaluated for the study. Of these, 24 records were excluded: 10 patients due to confirmed outlier data for laboratory markers, 4 patients due to hospital system limitations resulting in incomplete demographic information, and 10 patients due to indeterminate culture results that prevented an exact diagnosis. These 24 excluded patients were not included in any statistical analyses or tables. Ultimately, 519 unique patient records were included in the analysis, with an additional 152 records evaluated for readmissions. Among these 152 records, 65 unique patients accounted for 87 repeat admissions. Patients were first grouped based on their readmission status into “readmitted” (at least one readmission) and “no readmission”. Subsequently, patients were reclassified based on culture results and multidrug resistance (MDR) (Figure 1).
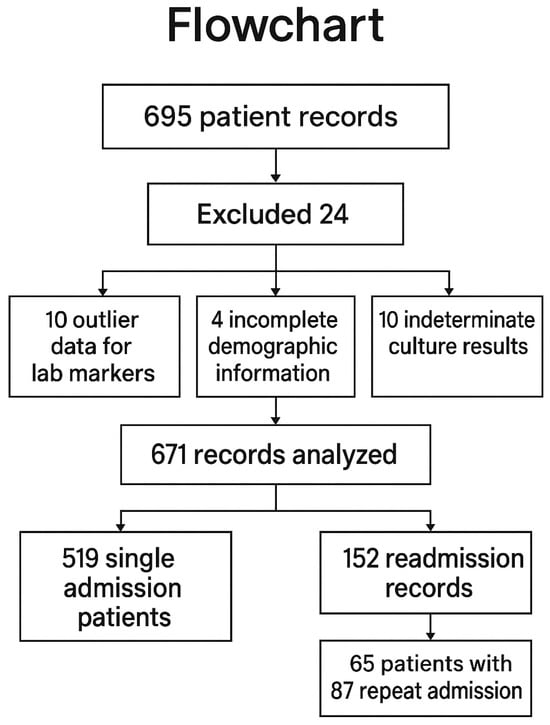
Figure 1.
Flowchart of the study.
Table 1 presents the demographic and clinical characteristics of the study population. The mean age of patients with readmissions and those without was 69.6 (±11.48) and 69.97 (±11.9) years, respectively. Male patients were more prevalent in the readmission group (n = 48, 73.8%) compared to the no-readmission group (n = 294, 56.6%), and this gender difference was statistically significant (p = 0.008). Cardiovascular disease was the most common comorbidity, affecting 60% (n = 39) of readmitted patients and 66.4% (n = 344) of non-readmitted patients. Neurological comorbidities and malignancy were rare, with 7.7% (n = 40) of non-readmitted patients and 1.6% (n = 1) of readmitted patients affected. Pneumonia at admission was present in approximately one-third of patients in both groups. Emphysema was observed in less than 1% of patients, while bronchiectasis was more frequent in the readmission group (6.2%) compared to the no-readmission group (3.3%).

Table 1.
Demographic parameters, comorbidities, and treatment regimen.
More than half of ICU admissions in both groups originated from other ICUs, but patients with readmissions were more likely to be admitted from emergency wards compared to those without readmissions (46.2% vs. 33%, p = 0.038). Intubation history within the past year was similar between the groups (12.3% vs. 11%), as was the need for non-invasive mechanical ventilation (NIMV) (79% in the readmission group vs. 67.9% in the no-readmission group, p = 0.072). The most common first-line treatment for both groups was a combination of cephalosporins with macrolides or fluoroquinolones (44.6% vs. 43.9%), followed by antipseudomonal regimens (32.3% vs. 24.5%), with no statistically significant difference in treatment preferences (p = 0.389) (Table 1). Additional treatment regimens, such as antifungal and antiviral therapies, were required in less than 5% of patients in both groups. MRSA-targeted treatment was used in 4.6% of the readmission group and 7.3% of the no-readmission group.
As shown in Table 2, culture positivity and MDR status were analyzed in relation to ICU outcomes. Positive culture samples were most frequently derived from respiratory sources (57.7% in the readmission group vs. 59.8% in the no-readmission group), followed by urinary (38.5% vs. 33.7%) and hematological samples, with no significant difference between groups (p = 0.426). Patients with multiple positive cultures (34.6% vs. 38.5%) and those with MDR cultures (65.4% vs. 61.5%) were similar between groups. Klebsiella spp. was the most common isolate in both groups (23.1% vs. 21.1%), followed by Candida spp. (23.1% vs. 11.1%) and Pseudomonas spp. (15.4% vs. 11.1%). No significant differences in overall culture results were observed between the groups (p = 0.240). However, culture positivity within six months prior to ICU admission was more frequent in the readmission group (12.5%, n = 8) compared to the no-readmission group (3.7%, n = 11, p = 0.033). Resistance patterns among these cultures were similar between the groups.

Table 2.
Culture results and survival evaluation.
The median ICU length of stay was 8 days [5,6,7,8,9,10,11] for the readmission group and 7 days [5,6,7,8,9,10,11] for the no-readmission group. Survival duration was significantly longer in the readmission group (median 238 days) compared to the no-readmission group (median 59 days, p = 0.047, log-rank Mantel–Cox survival analysis). ICU mortality (exitus) did not differ significantly between the groups (Table 2).
3.1. MDR vs. Non-MDR Comparison
Table 3 provides a comparison of MDR patterns with demographic and clinical parameters of the study population. No significant differences were observed between patients with MDR and non-MDR cultures regarding age (72.54 vs. 70.21 years), gender distribution, or comorbidities. Admission origin was also similar, with the majority of patients in both groups admitted from other ICUs (75.9% vs. 78.9%). Intubation history and NIMV requirements were comparable (24.1% vs. 19.7%, p = 0.449; 77% vs. 67.6%, p = 0.15). Among culture subgroups, Acinetobacter spp. (18.4% vs. 3.8%) and Klebsiella spp. (28.6% vs. 8.9%) were significantly more prevalent in the MDR group, while Candida spp. was predominantly found in the non-MDR group (32.9% vs. 0.7%, p = 0.001). The median ICU length of stay was longer for the MDR group (10 days, 6–17) compared to the non-MDR group (8 days, 5–12, p = 0.013). Survival duration did not differ significantly between MDR and non-MDR groups (30 days, 12–134 vs. 41 days, 16–147, p = 0.739, Figure 2 and Figure 3) (Table 3).

Table 3.
Comparison of multidrug resistance and demographic parameters.
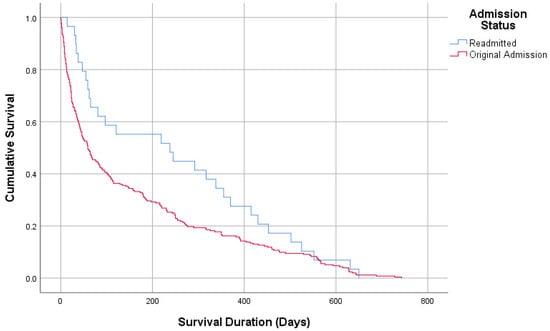
Figure 2.
Survival analysis according to admission.
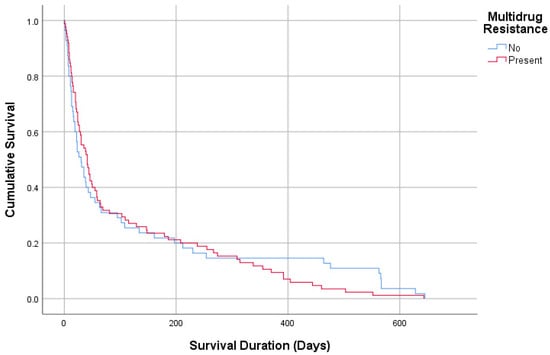
Figure 3.
Survival analysis according to multidrug resistance.
3.2. Laboratory Values
Table 4 summarizes the laboratory and readmission-related parameters. Admission laboratory parameters, including white blood cell (WBC) count, neutrophil count (NEU), C-reactive protein (CRP), and procalcitonin (PRC), did not differ significantly between the readmission and no-readmission groups. However, readmitted patients had significantly lower CRP levels at admission (21 mg/dL, 11.5–60.6) compared to non-readmitted patients (35 mg/dL, 14.04–93.28, p = 0.023). Similarly, at the final follow-up, CRP levels remained lower in the readmission group (12 mg/dL) compared to the no-readmission group (28.06 mg/dL, p = 0.004). WBC counts were also significantly lower at the final follow-up in the readmission group (8.60 × 109/L) compared to the no-readmission group (9.61 × 109/L, p = 0.041). No other significant differences were noted in the first and second follow-up evaluations.

Table 4.
Laboratory results during admission and follow-up.
For MDR and non-MDR comparisons, no significant differences were observed in WBC, NEU, CRP, or PRC levels during admission or follow-up evaluations (Table 4).
4. Discussion
4.1. Gender and Admission Source as Readmission Predictors
In our study, we aimed to reveal the differences in clinical, laboratory, and infectious processes between patients with ICU readmissions and those admitted for the first time. Some of our findings were particularly striking and may contribute to the existing literature. For instance, we observed that male gender was significantly associated with ICU readmissions (p = 0.008). A gender-based ICU study conducted by Blecha et al. in 2020 reported that male patients were more likely to undergo tracheostomy, receive dialysis, and spend significantly more time on mechanical ventilation compared to female patients [5]. Based on these findings, it can be inferred that male patients admitted to the ICU are subjected to more invasive procedures and have a higher likelihood of ICU readmissions compared to females.
Similarly, Ponzoni et al. (2017) investigated the risk factors associated with ICU readmissions and found that patients who were admitted to the ICU as part of a planned postoperative transfer had lower rates of readmission compared to those admitted from general wards or emergency departments [6]. Consistent with this literature, our study also found that patients admitted from the emergency department had significantly higher rates of ICU readmissions during their hospital stay (p = 0.038).
In the groups with and without ICU readmissions, no significant differences were found in the need for non-invasive or invasive mechanical ventilation. However, contrary to the literature, the survival durations of patients with ICU readmissions were significantly higher. In Ponzoni’s study, ICU readmissions were associated with significantly higher ICU mortality, 90-day mortality, and overall hospital mortality [6]. Similarly, a study by Hanberger et al. demonstrated that infections caused by difficult-to-treat pathogens, such as Pseudomonas aeruginosa, Acinetobacter spp., and MRSA, were generally associated with higher ICU and hospital mortality rates compared to other infections. Furthermore, high antibiotic resistance (HighABR) was shown to be significantly more prevalent in ICUs [4,7]. Other ICU studies indicate that the absence of appropriate empirical treatments for these pathogens is linked to an increase in attributable mortality [7,8,9,10].
4.2. Microbiological Profile and Pathogen Distribution
Our patient population predominantly consisted of a relatively homogeneous group with respiratory pathologies. Chronic and/or acute exacerbations of diseases such as COPD, IPF, asthma, and lung cancer were prominent, all of which could lead to ICU readmissions. We believe that the effective treatment and stabilization of exacerbations under ICU conditions extended survival. However, no significant differences in 2-year mortality rates were found between the groups.
In both the readmitted and non-readmitted groups, the most frequently isolated microorganisms were from respiratory tract samples. In both groups, Klebsiella spp. was the leading pathogen, exceeding 20% of isolates, followed by Candida spp. and Pseudomonas spp. When examining the rates of multidrug-resistant bacteria (MDRB), we found that MDRB prevalence exceeded 60% in both groups. Analyzing MDRBs revealed that Klebsiella spp. was dominant in both groups, followed by Acinetobacter spp. A study conducted by Ibrahim et al. in Libya showed that out of 197 swabs collected from ICU patients, staff, and equipment, the most commonly isolated microorganisms were Acinetobacter spp. (44%) and Klebsiella spp. (40%) [11]. Moreover, a large multicenter study involving 57 ICUs across 24 countries identified Klebsiella spp. as the leading MDRB in patients with bacteremia, while Acinetobacter spp. was prominent in the extensively drug-resistant (XDR) profile [12].
Indeed, the prevalence of Acinetobacter spp. strains has increased in recent years, with these pathogens becoming resistant to broad-spectrum antibiotics commonly used in routine care. Additionally, they have the capacity to develop new resistance mechanisms during treatment [13,14]. Similarly, a study by Hasanzade et al. involving 287 respiratory ICU patients reported that 55.1% of cultured isolates were Gram-negative bacteria, while 25.1% were Gram-positive bacteria. Among these isolates, Acinetobacter spp. showed resistance to over 95% of antibiotics except colistin [15].
4.3. Clinical Impact of MDRB on ICU Outcomes
In our study, patients with ICU readmissions had higher positive culture rates within the past six months compared to non-readmitted patients. As previously noted, considering that more than 50% of these cultures were isolated from respiratory tract samples, this finding can be attributed to acute infectious exacerbations of respiratory system diseases or uncontrolled chronic infections.
When comparing patients with MDRB-positive and MDRB-negative cultures, as expected, ICU stays were significantly longer in the MDRB-positive group compared to the MDRB-negative group (p = 0.013), consistent with trends observed in ICUs worldwide. However, no significant difference was found in survival durations between the two groups. A 2020 study from Taiwan analyzed 60,317 ICU patients and compared 279 patients with MDRB-positive cultures to an equal number of patients without MDRB. The study found that ICU length of stay increased by 13%, overall hospital costs were 26% higher for the MDRB group, and healthcare service (8%), medication (26.9%), and bed (10.3%) costs were significantly elevated. Despite these differences, there was no significant difference in mortality rates between MDRB and non-MDRB groups [16].
Both the literature and our study demonstrate that patients infected with MDRB incur prolonged ICU stays and increased treatment costs, significantly impacting healthcare policies and decision-making. In Turkey, high rates of antibiotic resistance have compelled healthcare administrators to take measures to curb the prescription of unnecessary and inappropriate antibiotics, even at the primary care level.
4.4. Laboratory Biomarkers and the Role of Colonization
One of the intriguing findings in our study pertained to laboratory parameters. We observed that ICU admission and discharge CRP levels in the ICU readmission group were significantly lower than in the non-readmission group (p = 0.004). Similarly, white blood cell counts at ICU admission were also lower in the readmission group (p = 0.041). In contrast, most studies in the literature show high CRP and white blood cell levels at ICU discharge with significantly increased rates of ICU readmission [17,18]. Procalcitonin is acknowledged as a more specific biomarker than C-reactive protein for detecting bacterial inflammation [19]. However, procalcitonin did not differ in our study groups.
Considering that the readmitted group in our study predominantly consisted of patients with chronic diseases experiencing acute exacerbations and given the significantly higher culture positivity rates within the past six months compared to the non-readmission group, it is plausible that a substantial proportion of MDRB-positive cultures in this group represent colonization rather than active infection. This may explain the statistically lower CRP levels and longer survival durations in the readmission group.
According to the literature, the prevalence of MDRB colonization in ICU patients is reported to be 25.3%, with an incidence of 45.14% [18].
Recent studies reinforce the clinical importance of colonization in ICU settings, particularly in the context of MDR bacteria. Both Heath et al. and a recent observational study by Gracheva et al. emphasize that the primary focus in colonized patients should be on prevention rather than treatment. The latter also highlights that monomicrobial culture positivity is more likely to progress to infection compared to polymicrobial findings, suggesting that not all colonization carries the same risk [20,21]. Therefore, colonization is by no means clinically irrelevant. On the contrary, it is of high importance—but preventing it before it develops and accurately distinguishing it from active infection when present are crucial steps in avoiding unnecessary and excessive antibiotic use.
4.5. Future Research Directions
Our study offers a multidimensional perspective on the clinical impact of culture positivity and multidrug-resistant bacteria (MDRB) in patients admitted to respiratory intensive care units. The observation that MDRB prevalence was high in both readmitted and non-readmitted patients, despite similar mortality rates, suggests that colonization may play a more prominent role than active infection in this population. This finding challenges conventional infection control strategies and underscores the need to revisit diagnostic criteria that distinguish clinically significant infections from mere colonization. Furthermore, while MDRB positivity was associated with prolonged ICU stays, it did not correspond with increased mortality, indicating that these organisms may impose a greater burden on healthcare resources rather than directly affecting patient survival. The lower CRP and WBC levels observed in readmitted patients, along with their longer survival times, support the hypothesis that culture positivity in this group may reflect chronic colonization and subsequent exacerbations rather than acute, life-threatening infections. However, although colonization may not always require immediate antimicrobial treatment, it still warrants careful monitoring and individualized clinical evaluation, especially in patients with frequent exacerbations or immunocompromised status. Future research should focus on developing predictive models incorporating laboratory trends, colonization history, and readmission patterns to improve antimicrobial stewardship and optimize ICU resource utilization.
5. Limitations of the Study
The primary limitation of our study is its retrospective design. Furthermore, since the study was conducted in a relatively homogeneous patient group, with a significant proportion of culture positivity results derived from the respiratory system, the findings may not be generalizable to all ICU patients.
6. Conclusions
Infectious outbreaks that result in substantial patient mortality within a short time frame have always received well-deserved attention in the medical community. However, bacterial infections that are challenging to treat—encountered daily by physicians during inpatient ward rounds or outpatient visits—not only account for significant annual patient mortality but also impose a heavy financial burden on healthcare systems.
In ICUs, infectious disease specialists and intensivists are increasingly discussing cases where no effective antibiotics remain for the microorganism affecting a patient. Patients with prolonged ICU stays, prior exposure to multiple antibiotic treatments, and chronic diseases colonized by multidrug-resistant microorganisms pose a significant risk to newly admitted ICU patients. These scenarios are fostering a shift in perspective among intensivists, where ICU admissions may be perceived as causing more harm than benefit in certain cases.
This study underscores the growing visibility of what has long been the “hidden part of the iceberg”. Addressing these challenges urgently requires targeted healthcare policies and interventions to mitigate the escalating impact of multidrug-resistant bacteria in ICUs.
Author Contributions
Conceptualization, O.M. and M.Y.; methodology, D.Ç. and M.A.; software, K.E. and O.M.; validation, M.Ö.C., A.K. and Z.N.Ş.; formal analysis, S.G. and Y.T.G.; investigation, O.M. and M.Y.; resources, Z.N.Ş.; data curation, M.Y. and D.Ç.; writing—original draft preparation, O.M., M.Y. and K.E.; writing—review and editing, O.M.; visualization, K.E.; supervision, Y.T.G. and S.G.; project administration, M.Y.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics approval was obtained from the Clinical Research Ethics Committee of the University of Health Sciences, Ankara Sanatorium Training and Research Hospital (approval date: 13 November 2024, approval number: 2024-BÇEK/164).
Informed Consent Statement
Informed consent was obtained from all participants included in the study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Yusuf Tuğrul Şipit for his helpful comments and support.
Conflicts of Interest
The authors declare that they have no conflict of interest regarding the publication of this article.
References
- Collignon, P.J.; McEwen, S.A. One Health: Its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Nanao, T.; Nishizawa, H.; Fujimoto, J. Empiric antimicrobial therapy in the intensive care unit based on the risk of multidrug-resistant bacterial infection: A single-centre case-control study of blood culture results in Japan. Antimicrob. Resist. Infect. Control 2023, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.S.; da Mata, C.P.S.M.; Dos Santos, U.R.; de Araújo, C.A.; Leite, E.M.M.; de Carvalho, L.D.; Vidigal, P.G.; Vieira, C.D.; dos Santos-Key, S.G. Association between multidrug-resistant bacteria and outcomes in intensive care unit patients: A non-interventional study. Front. Public Health 2024, 11, 1297350. [Google Scholar] [CrossRef] [PubMed]
- Hanberger, H.; Antonelli, M.; Holmbom, M.; Lipman, J.; Pickkers, P.; Leone, M.; Rello, J.; Sakr, Y.; Walther, S.M.; Vanhems, P.; et al. Infections, antibiotic treatment and mortality in patients admitted to ICUs in countries considered to have high levels of antibiotic resistance compared to those with low levels. BMC Infect. Dis. 2014, 14, 513. [Google Scholar] [CrossRef]
- Blecha, S.; Zeman, F.; Specht, S.; Pfefferle, A.; Placek, S.; Karagiannidis, C.; Bein, T. Invasiveness of treatment is gender-dependent in intensive care: Results from a retrospective analysis of 26,711 cases. Anesth. Analg. 2020, 132, 1677–1683. [Google Scholar] [CrossRef]
- Ponzoni, C.R.; Corrêa, T.D.; Filho, R.R.; Serpa Neto, A.; Assunção, M.S.; Pardini, A.; Schettino, G.P.P. Readmission to the intensive care unit: Incidence, risk factors, resource use, and outcomes. A retrospective cohort study. Ann. Am. Thorac. Soc. 2017, 14, 1312–1319. [Google Scholar] [CrossRef]
- Lodise, T.P., Jr.; Patel, N.; Kwa, A.; Graves, J.; Furuno, J.P.; Graffunder, E.; Lomaestro, B.; McGregor, J.C. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: Impact of delayed appropriate antibiotic selection. Antimicrob. Agents Chemother. 2007, 51, 3510–3515. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Rafailidis, P.I.; Zouglakis, G.; Morfou, P. Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J. Antimicrob. Chemother. 2006, 57, 1251–1254. [Google Scholar] [CrossRef]
- Shorr, A.F.; Micek, S.T.; Kollef, M.H. Inappropriate therapy for methicillin-resistant Staphylococcus aureus: Resource utilization and cost implications. Crit. Care Med. 2008, 36, 2335–2340. [Google Scholar] [CrossRef]
- Ibrahim, K.; Thwood, D.; Elgheriani, H.; Salem, M.; Elgadiym, Z.; Zaghdani, A.; Alhudiri, I.; Habibi, A.; Elfahem, A.; Belaid, S.; et al. Prevalence of multidrug-resistant bacteria in intensive care units at Tripoli University Hospital, Tripoli, Libya. Libyan J. Med. 2024, 19, 2348235. [Google Scholar] [CrossRef]
- El-Sokkary, R.; Uysal, S.; Erdem, H.; Kullar, R.; Pekok, A.; Amer, F.; Grgić, S.; Carevic, B.; El-Kholy, A.; Liskova, A.; et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: An international ID-IRI survey. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Al Atrouni, A.; Rafei, R.; Dabboussi, F.; Hamze, M.; Osman, M. Molecular mechanisms of antimicrobial resistance in Acinetobacter baumannii, with a special focus on its epidemiology in Lebanon. J. Glob. Antimicrob. Resist. 2018, 15, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kopterides, P. Risk factors for the isolation of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: A systematic review of the literature. J. Hosp. Infect. 2006, 64, 7–15. [Google Scholar] [CrossRef]
- Hasanzade, H.; Gürün Kaya, A.; Çiledağ, A.; Erol, S.; Çiftçi, F.; Güriz, H.; Kaya, A. The distribution of microorganisms and antibiotic resistance profile in pulmonary critical care unit patients: A single-centre study. Tuberk. Toraks 2021, 69, 9–20. [Google Scholar] [CrossRef]
- Su, L.; Chen, I.; Tang, Y.; Lee, J.; Liu, J. Increased financial burdens and lengths of stay in patients with healthcare-associated infections due to multidrug-resistant bacteria in intensive care units: A propensity-matched case-control study. PLoS ONE 2020, 15, e0233265. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Dobb, G.; Lee, K.; Towler, S.; Webb, S. C-reactive protein concentration as a predictor of intensive care unit readmission: A nested case-control study. J. Crit. Care 2006, 21, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ho, K. Procalcitonin concentrations as a predictor of unexpected readmission and mortality after intensive care unit discharge: A retrospective cohort study. J. Crit. Care 2016, 33, 240–244. [Google Scholar] [CrossRef]
- Oussayeh, I.; Moussaid, F.; Traoré, A.O.; Touiti, A.; Elkhayari, M.; Soraa, N.; Hachimi, A. Epidemiology, risk factors, and outcomes of multidrug-resistant bacteria colonization in a Moroccan medical intensive care unit. PAMJ Clin. Med. 2021, 5, 33. [Google Scholar] [CrossRef]
- Pazarli, A.C.; Koseoglu, H.I.; Doruk, S.; Sahin, S.; Etikan, I.; Celikel, S.; Berktas, B. Procalcitonin: Is it a predictor of noninvasive positive pressure ventilation necessity in acute chronic obstructive pulmonary disease exacerbation? J. Res. Med. Sci. 2012, 17, 1047–1051. [Google Scholar] [PubMed] [PubMed Central]
- Heath, M.R.; Fan, W.; Leu, C.S.; Gomez-Simmonds, A.; Lodise, T.; Freedberg, D.E. Gut colonization with multidrug resistant organisms in the intensive care unit: A systematic review and meta-analysis. Crit. Care 2024, 28, 211. [Google Scholar] [CrossRef]
- Gracheva, A.S.; Kuzovlev, A.N.; Salnikova, L.E. Observational Study of Microbial Colonization and Infection in Neurological Intensive Care Patients Based on Electronic Health Records. Biomedicines 2025, 13, 858. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).