A Stepwise Anatomy-Based Protocol for Total Laparoscopic Hysterectomy: Educational Tool with Broad Clinical Utility
Abstract
1. Introduction
2. Patients and Methods
2.1. Preoperative Protocol
2.2. Operative Setup
2.3. Surgical Technique—Standardized Stepwise TLH Protocol
- 1.
- Right pelvic sidewall procedures
- -
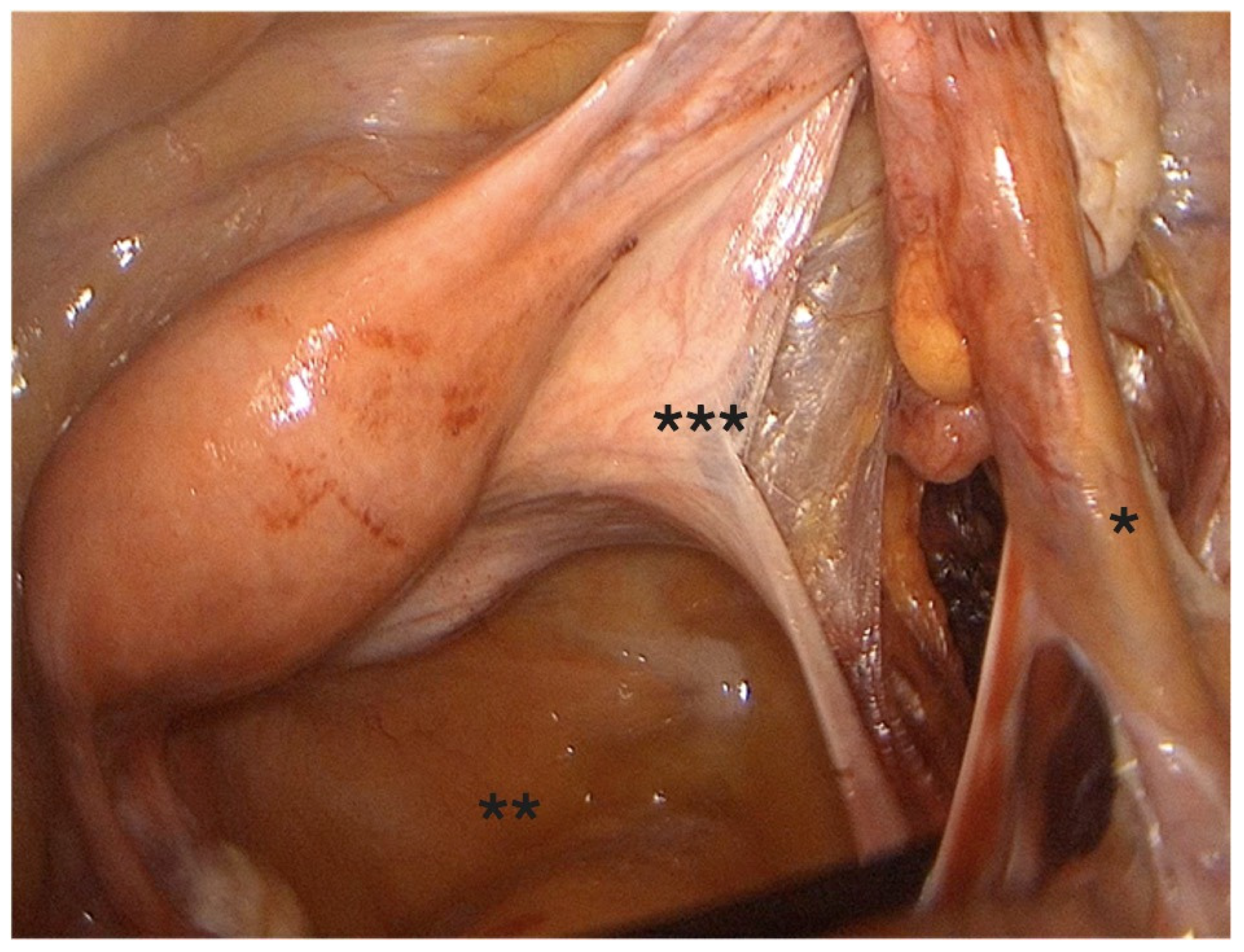
- Coagulation and transection of the round ligament laterally, away from adnexal and iliac vessels (Figure 1).
- -
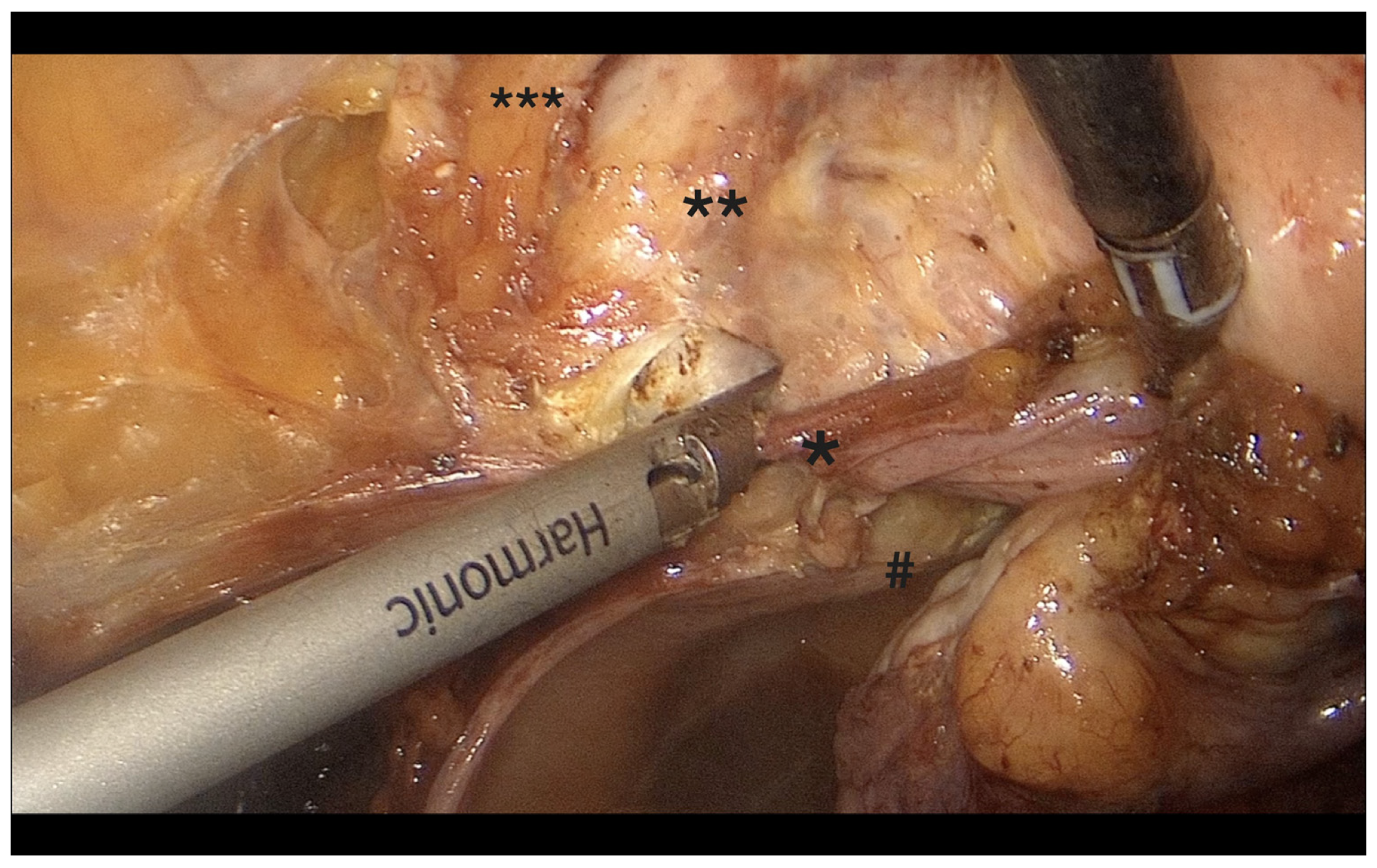
- Retroperitoneal dissection was performed for identification of the obliterated umbilical artery and ligation of the uterine artery at its origin from the internal iliac artery, under direct visualization and lateral displacement of the ureter (Figure 2). The uterine artery was first coagulated using bipolar energy and then transected with ultrasonic shears to ensure complete vessel obliteration and hemostasis.
- -
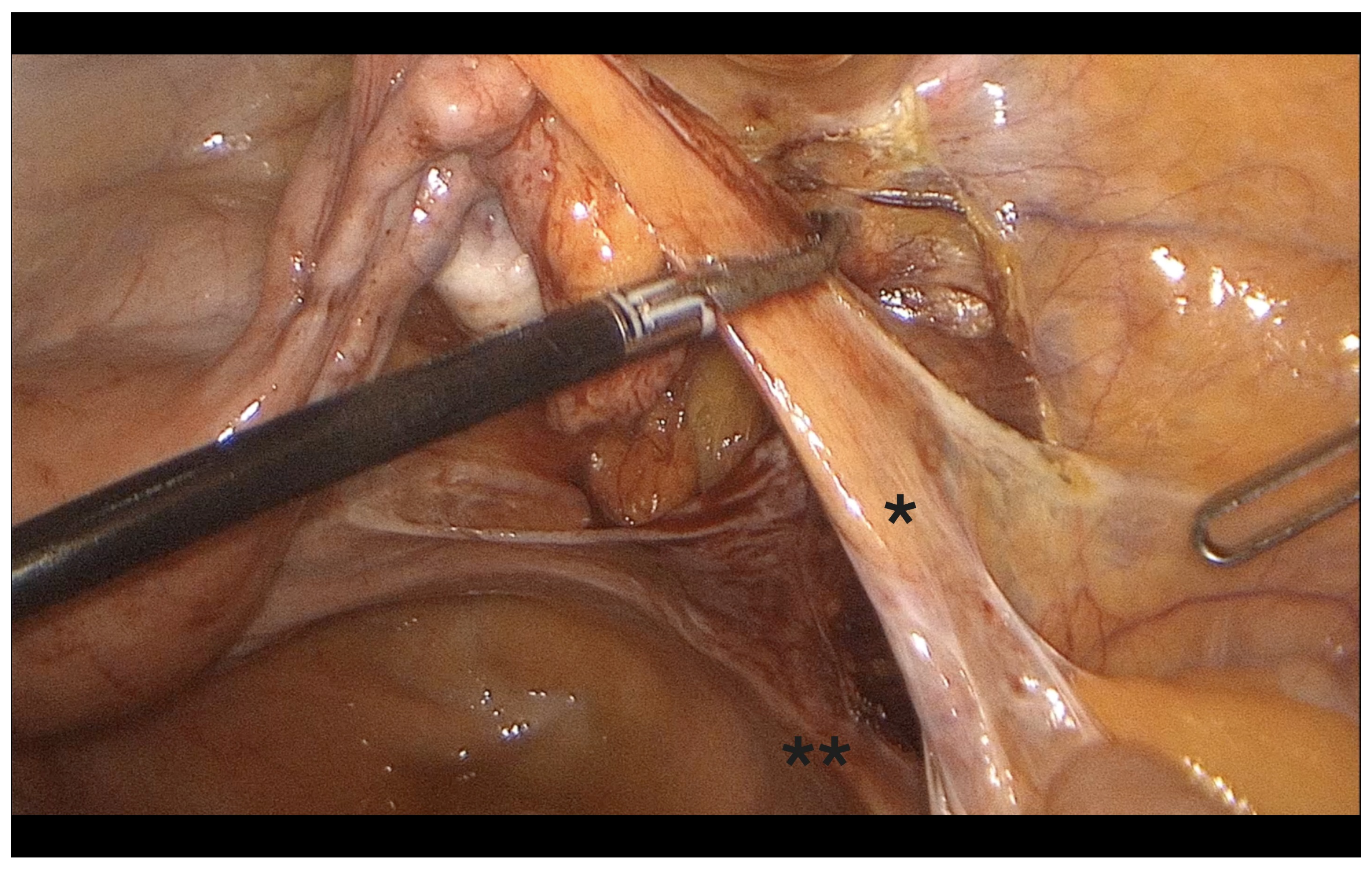
- Fenestration of the broad ligament above the ureter (Figure 3).
- -
- Transection of the mesosalpinx and ovarian ligament (or infundibulopelvic ligament in case of adnexectomy, Figure 4).
- 2.
- Left pelvic sidewall procedures
- -
- Same steps as on the right side.
- 3.
- Bladder mobilization
- -
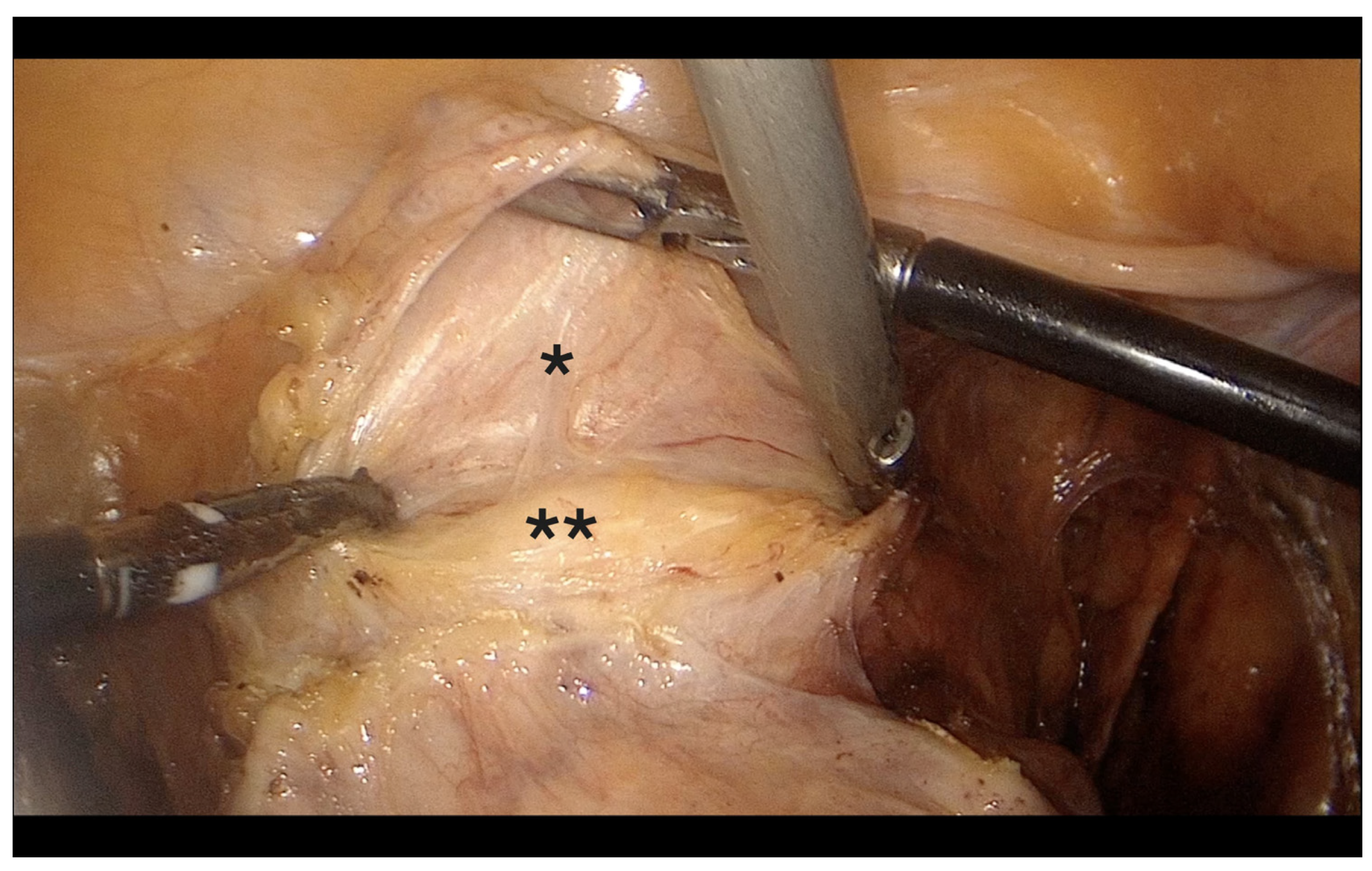
- Opening of the anterior broad ligament fold; vesicouterine space opened with careful blunt dissection, mobilizing the bladder ~2–3 cm caudally (Figure 5). In patients with prior cesarean section or anterior adhesions, bilateral paravesical space development facilitated safe identification of the vesicouterine plane and minimized bladder injury risk.
- 4.
- Posterior peritoneum and uterosacral ligament dissection
- -
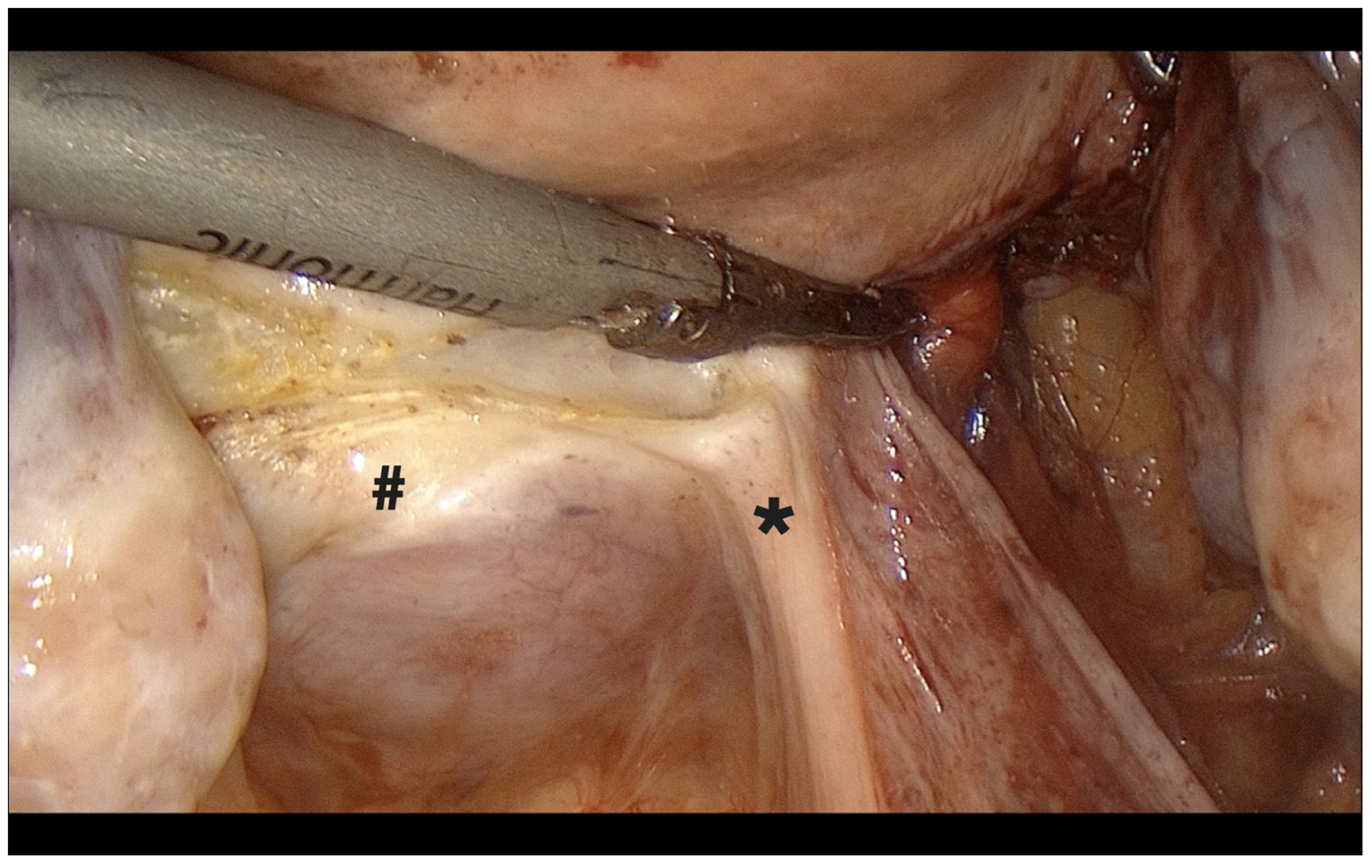
- After rotating the optic, uterosacral ligaments were transected under guidance from the uterine manipulator (Figure 6).
- 5.
- Cardinal ligament and distal uterine artery transection
- -
- Performed with perpendicular dissection under direct visualization of the manipulator, beginning on the right side (Figure 7).
- 6.
- Colpotomy
- -
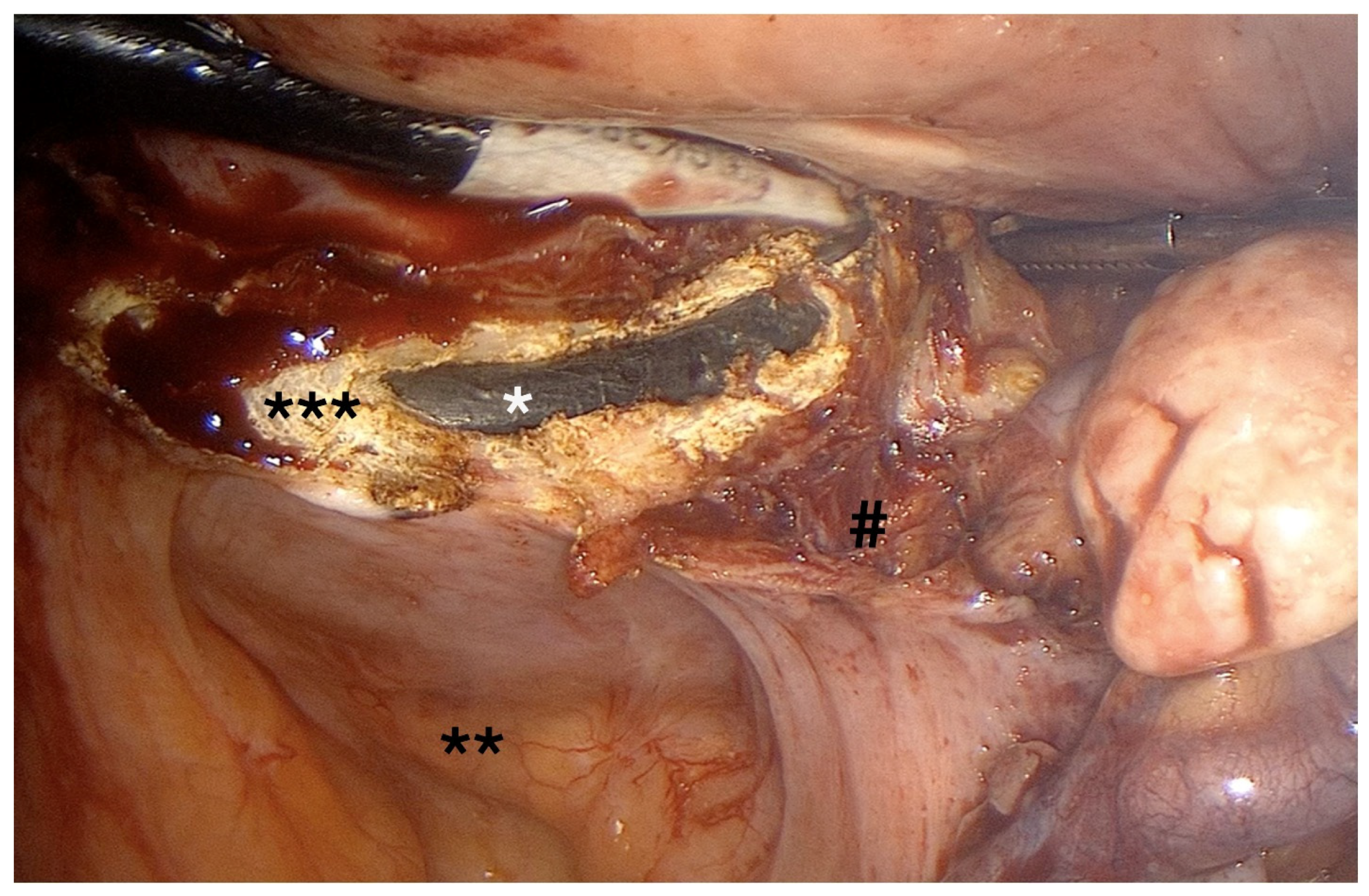
- Initiated medially on the posterior vaginal wall and extended circumferentially around the cervix, with optic adjustments for visualization (Figure 8).
- 7.
- Uterus extraction
- -
- Removed vaginally or by intra-abdominal or vaginal morcellation depending on uterine size.
- 8.
- Vaginal cuff closure
- -
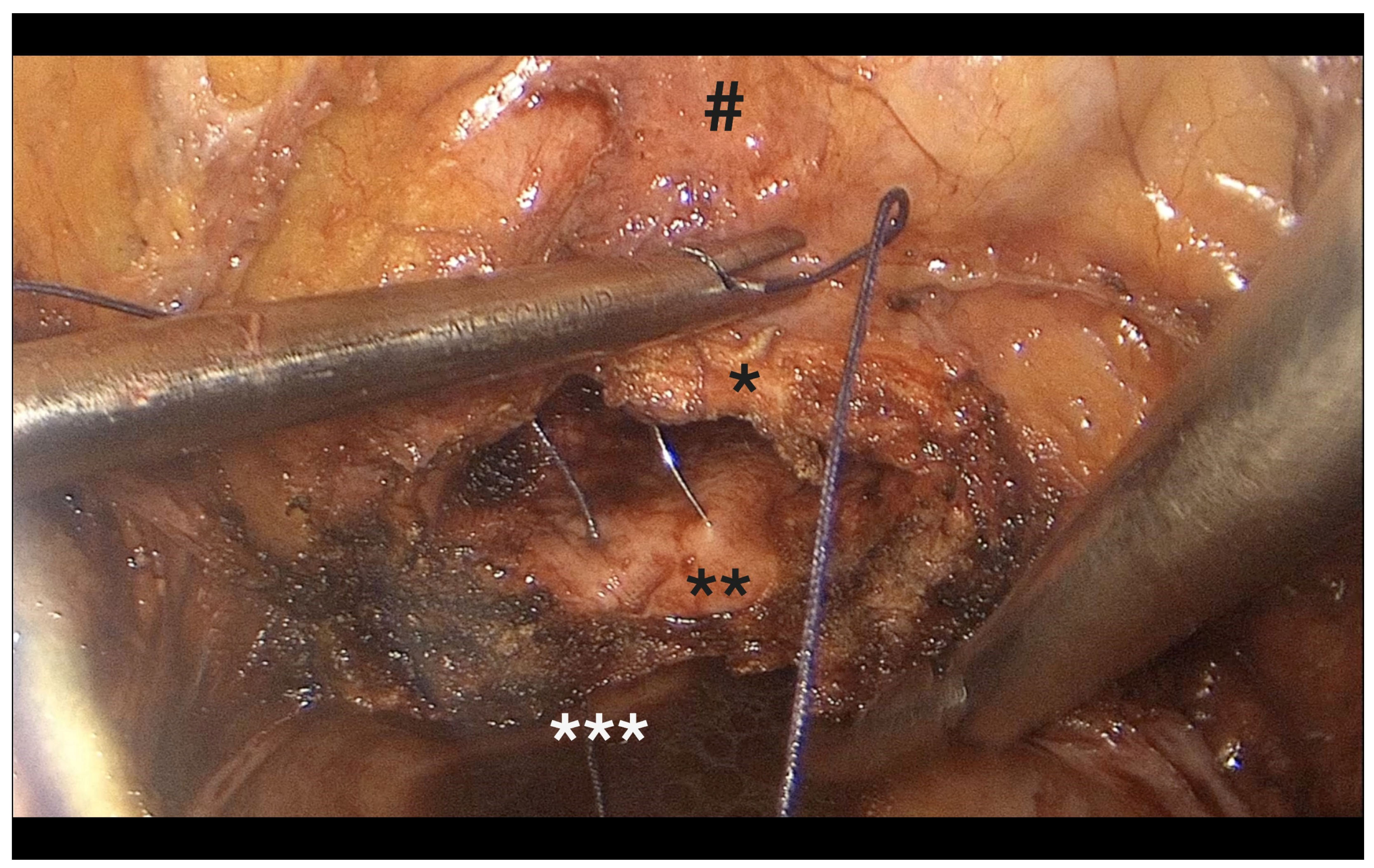
- Performed laparoscopically, incorporating mucosa, vesicovaginal fascia, and both uterosacral ligaments (Figure 9).
2.4. Postoperative Management
2.5. Outcomes and Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Indications and Intraoperative Details
3.3. Intraoperative and Postoperative Complications
4. Discussion
4.1. General Significance of Surgical Standardization in TLH
4.2. Anatomical and Technical Rationale for Each Surgical Step
4.3. Comparison of Our Clinical Outcomes with International Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perino, A.; Cucinella, G.; Venezia, R.; Castelli, A.; Cittadini, E. Total Laparoscopic Hysterectomy versus Total Abdominal Hysterectomy: Assessment of the Learning Curve. Hum. Reprod. 1999, 14, 2996–3000. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, J.I.; Suzuki, Y. Total Laparoscopic Hysterectomy: 10 Steps Toward a Successful Procedure. Rev. Obstet. Gynecol. 2009, 2, 57–65. [Google Scholar]
- Sandberg, E.M.; Hehenkamp, W.J.K.; Geomini, P.M.; Janssen, P.F.; Jansen, F.W.; Twijnstra, A.R.H. Laparoscopic hysterectomy for benign indications: Clinical practice guideline. Arch. Gynecol. Obstet. 2017, 296, 709–718. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlan, K.A.; Emeney, P.L.; Frank, M.I.; Milanfar, L.C.; Sten, M.S.; Uthman, K.F. Total Laparoscopic Hysterectomy: Making It Safe and Successful for Obese Patients. JSLS J. Soc. Laparosc. Robot. Surg. 2021, 25, e2020.00087. [Google Scholar] [CrossRef]
- Uccella, S.; Malzoni, M.; Cromi, A.; Seracchioli, R.; Ciravolo, G.; Fanfani, F.; Shakir, F.; Alletti, S.G.; Legge, F.; Berretta, R.; et al. Laparoscopic vs transvaginal cuff closure after total laparoscopic hysterectomy: A randomized trial. Am. J. Obstet. Gynecol. 2018, 219, 480.e1–480.e12. [Google Scholar]
- Jeung, I.C.; Baek, J.M.; Park, E.K.; Lee, H.N.; Kim, C.J.; Park, T.C.; Lee, Y.S. A prospective comparison of vaginal stump suturing techniques during total laparoscopic hysterectomy. Arch. Gynecol. Obstet. 2010, 282, 645–651. [Google Scholar] [CrossRef]
- Uccella, S.; Garzon, S.; Lanzo, G.; Gallina, D.; Bosco, M.; Porcari, I.; Gueli-Alletti, S.; Cianci, S.; Franchi, M.; Zorzato, P.C. Uterine Artery Closure at the Origin vs at the Uterus Level in Total Laparoscopic Hysterectomy: A Randomized Controlled Trial. Acta Obstet. Gynecol. Scand. 2021, 100, 1840–1848. [Google Scholar] [CrossRef]
- Abo-Hashem, U.; Rashed, R.; El-Bassioune, W.; Abdou, H. Laparoscopic Hysterectomy with Prior Uterine Artery Ligation versus Conventional Laparoscopic Hysterectomy. Int. J. Med. Arts 2021, 3, 1879–1883. [Google Scholar] [CrossRef]
- Alletti, S.G.; Restaino, S.; Finelli, A.; Ronsini, C.; Lucidi, A.; Scambia, G.; Fanfani, F. Step-by-Step Total Laparoscopic Hysterectomy with Uterine Arteries Ligation at the Origin. J. Minim. Invasive Gynecol. 2020, 27, 22–23. [Google Scholar] [CrossRef]
- Moratalla-Bartolomé, E.; Lázaro-de-la-Fuente, J.; López-Carrasco, I.; Cabezas-López, E.; Carugno, J.; Sancho-Sauco, J.; Pelayo-Delgado, I. Surgical impact of bilateral transient occlusion of uterine and utero-ovarian arteries during laparoscopic myomectomy. Sci. Rep. 2024, 14, 7044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wattiez, A. The Learning Curve of Total Laparoscopic Hysterectomy: Comparative Analysis of 1647 Cases. J. Am. Assoc. Gynecol. Laparosc. 2002, 9, 339–345. [Google Scholar] [CrossRef]
- López, C.C.; Ríos, J.F.L.; González, Y.; Vásquez-Trespalacios, E.M.; Serna, D.; Arango, A.; Cifuentes, C.; Vásquez, R.; Castañeda, J.D.; Almanza, L.A.; et al. Barbed Suture versus Conventional Suture for Vaginal Cuff Closure in Total Laparoscopic Hysterectomy: Randomized Controlled Clinical Trial. J. Minim. Invasive Gynecol. 2019, 26, 1104–1109. [Google Scholar] [CrossRef]
- Working Group of ESGE. Surgical Steps of Total Laparoscopic Hysterectomy: Part 1: Benign Disease by the European Society for Gynaecological Endoscopy (ESGE). Facts Views Vis. Obgyn. 2019, 11, 103–110. [Google Scholar]
- Luchristt, D.; Brown, O.; Kenton, K.; Bretschneider, C.E. Trends in Operative Time and Outcomes in Minimally Invasive Hysterectomy from 2008 to 2018. Am. J. Obstet. Gynecol. 2021, 224, 202.e1–202.e12. [Google Scholar] [CrossRef]
- Madhvani, K.; Garcia, S.F.; Fernandez-Felix, B.M.; Zamora, J.; Carpenter, T.; Khan, K.S. Predicting Major Complications in Patients Undergoing Laparoscopic and Open Hysterectomy for Benign Indications. CMAJ 2022, 194, E1306–E1317. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.J.; Cook, E.F.; Cohen, S.L. Risk of Complication at the Time of Laparoscopic Hysterectomy: A Prediction Model Built from the National Surgical Quality Improvement Program Database. Am. J. Obstet. Gynecol. 2020, 223, 555.e1–555.e7. [Google Scholar] [CrossRef] [PubMed]
- Ibeanu, O.A.; Chesson, R.R.; Echols, K.T.; Nieves, M.; Busangu, F.; Nolan, T.E. Urinary Tract Injury during Hysterectomy Based on Universal Cystoscopy. Obstet. Gynecol. 2009, 113, 6–10. [Google Scholar] [CrossRef]
- Ala-Nissilä, S.; Laurikainen, E.; Mäkinen, J.; Jokimaa, V. Vaginal Cuff Dehiscence is Observed in a Higher Rate after Total Laparoscopic Hysterectomy Compared with Other Types of Hysterectomy. Acta Obstet. Gynecol. Scand. 2019, 98, 44–50. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.; Bae, H.S.; Lee, J.K.; Lee, N.W.; Song, J.Y. Evaluation of Risk Factors of Vaginal Cuff Dehiscence after Hysterectomy. Obstet. Gynecol. Sci. 2014, 57, 136–143. [Google Scholar] [CrossRef]
- Göl, M.; Kızılyar, A. Comparison of Two Different Laparoscopic Hysterectomies: Laparoscopic Hysterectomy vs. Total Laparoscopic Hysterectomy. J. Turk. Ger. Gynecol. Assoc. 2010, 11, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Malzoni, M.; Perniola, G.; Perniola, F.; Imperato, F. Optimizing the Total Laparoscopic Hysterectomy Procedure for Benign Uterine Pathology. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]


| Step No. | Step Name | Brief Description | Key Anatomical/Technical Points |
|---|---|---|---|
| 1 | Right pelvic sidewall dissection | Round ligament transection, retroperitoneal entry, uterine artery ligation, fenestration of the broad ligament above the ureter, and transection of the mesosalpinx and ovarian ligament (or infundibulopelvic ligament in case of adnexectomy) | Uterine artery ligated at origin from internal iliac artery; ureter identified and protected; broad ligament fenestration improves exposure; IP ligament handled cautiously to avoid ureteral injury |
| 2 | Left pelvic sidewall dissection | Same as step 1, mirrored | Symmetric approach ensures consistent ureteral visualization |
| 3 | Bladder mobilization | Vesicouterine space developed via anterior peritoneal fold dissection | Blunt dissection; paravesical space used in scarred patients |
| 4 | Posterior peritoneum & USL dissection | Uterosacral ligament transection with optical rotation | Enhances exposure to deep pelvis; switch of dominant instrument hand improves ergonomics |
| 5 | Cardinal ligament dissection | Transection of cardinal ligament and distal uterine artery | Performed under direct traction of manipulator for safety |
| 6 | Colpotomy | Circumferential incision around cervix | Posterior to anterior; monopolar cutting for minimal thermal damage |
| 7 | Uterus extraction | Removal via vagina or morcellation as needed | Depends on size; vaginal preferred if feasible |
| 8 | Vaginal cuff closure | Laparoscopic figure-of-eight intracorporeal suturing | Includes mucosa, fascia, and uterosacral ligaments for support |
| Patients (n = 109) | Mean ± SD or Count |
|---|---|
| Age (years) | 51.1 ± 10.3 |
| BMI | 26.8 ± 5.3 |
| BMI >30 (n,%) | 19 (17.4) |
| Parity: 1–4 (n,%) | 99 (90.8) |
| Postmenopausal status (n,%) | 36 (33) |
| History of vaginal delivery (n,%) | 88 (80.7) |
| Previous laparoscopic surgery (n,%) | 19 (17.4) |
| Previous open surgery (n,%) | 38 (34.9) |
| Patients (n = 109) | Value (±SD) |
|---|---|
| Indication: Fibroids n, (%) | 35 (32.1) |
| Indication: Abnormal uterine bleeding n, (%) | 43 (39.5) |
| Indication: Other n, (%) | 31 (28.4) |
| Uterine weight (g) | 211.9 (±95.3) |
| Total operative time (min) | 67.2 (±18.3) |
| Preoperative hemoglobin (g/dL) | 12.5 (±1.5) |
| Hemoglobin drop on POD-1 (g/dL) | 1.2 (±0.9) |
| Hospital stay (days) | 2.49 (±1.14) |
| Prolonged hospital stay >4 days, n (%) | 10 (9.2) |
| Complication Type | n (%) |
|---|---|
| Bladder injury | 2 (1.8) |
| Small bowel injury | 2 (1.8) |
| Vaginal cuff dehiscence | 2 (1.8) |
| Vaginal evisceration | 0 (0) |
| Vaginal cuff hematoma/abscess | 1 (0.9) |
| Postoperative vaginal bleeding | 4 (3.7) |
| Postoperative infection (non-surgical) | 0 (0) |
| Reoperation | 1 (0.9) |
| Laparoconversion | 0 (0) |
| Blood transfusion | 2 (1.8) |
| Paralytic ileus | 2 (1.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampé, R.; Margitai, N.; Török, P.; Lukács, L.; Orosz, M. A Stepwise Anatomy-Based Protocol for Total Laparoscopic Hysterectomy: Educational Tool with Broad Clinical Utility. Diagnostics 2025, 15, 1736. https://doi.org/10.3390/diagnostics15141736
Lampé R, Margitai N, Török P, Lukács L, Orosz M. A Stepwise Anatomy-Based Protocol for Total Laparoscopic Hysterectomy: Educational Tool with Broad Clinical Utility. Diagnostics. 2025; 15(14):1736. https://doi.org/10.3390/diagnostics15141736
Chicago/Turabian StyleLampé, Rudolf, Nóra Margitai, Péter Török, Luca Lukács, and Mónika Orosz. 2025. "A Stepwise Anatomy-Based Protocol for Total Laparoscopic Hysterectomy: Educational Tool with Broad Clinical Utility" Diagnostics 15, no. 14: 1736. https://doi.org/10.3390/diagnostics15141736
APA StyleLampé, R., Margitai, N., Török, P., Lukács, L., & Orosz, M. (2025). A Stepwise Anatomy-Based Protocol for Total Laparoscopic Hysterectomy: Educational Tool with Broad Clinical Utility. Diagnostics, 15(14), 1736. https://doi.org/10.3390/diagnostics15141736







