Pressure Gradient-Driven Embolization b-TACE for HCC: Technical and Diagnostic Step-by-Step Procedural Guide and Literature Review
Abstract
1. Introduction
2. Microballoon Interventions (Balloon-Occluded TACE -bTACE- and TARE -bTARE-): History and Rationale
3. bTACE and bTARE: Safety and Oncological Results
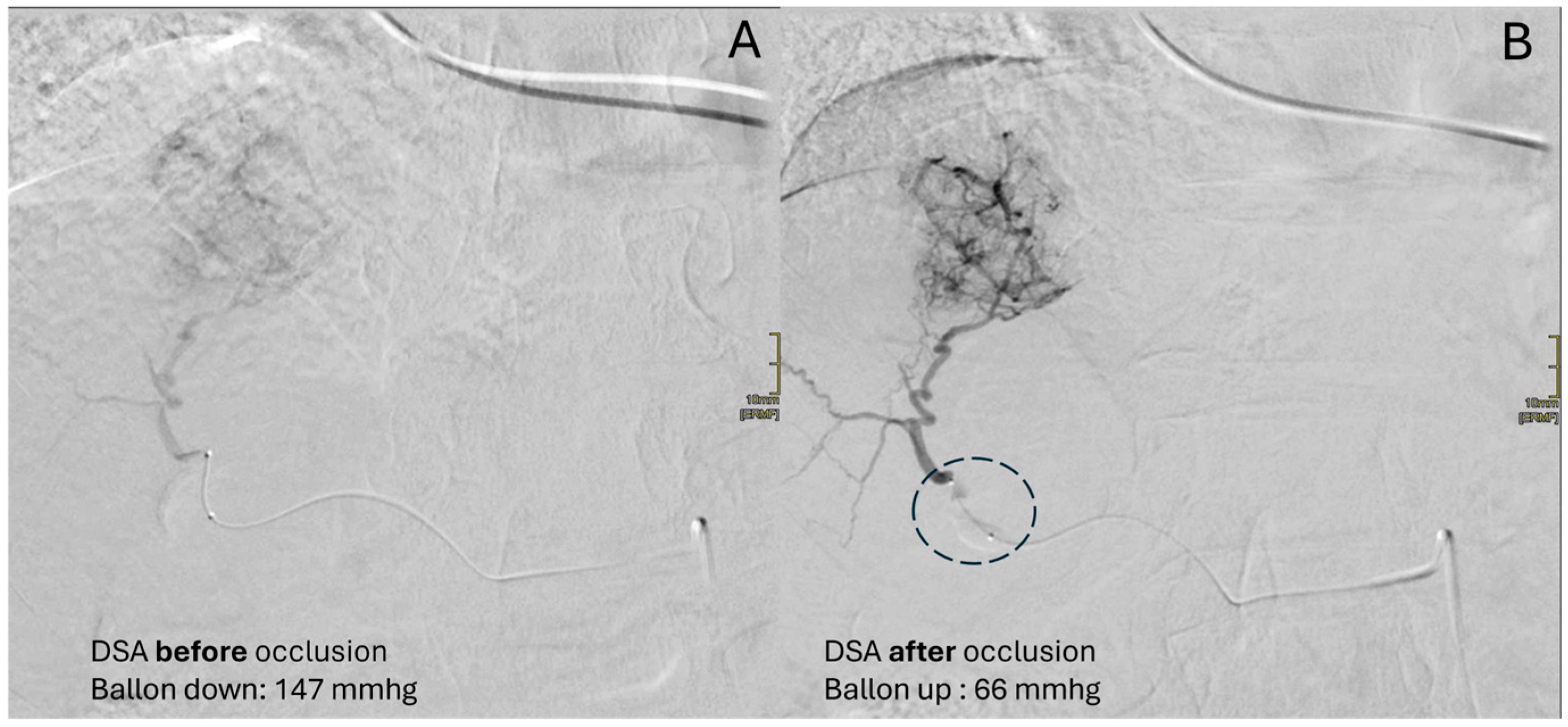
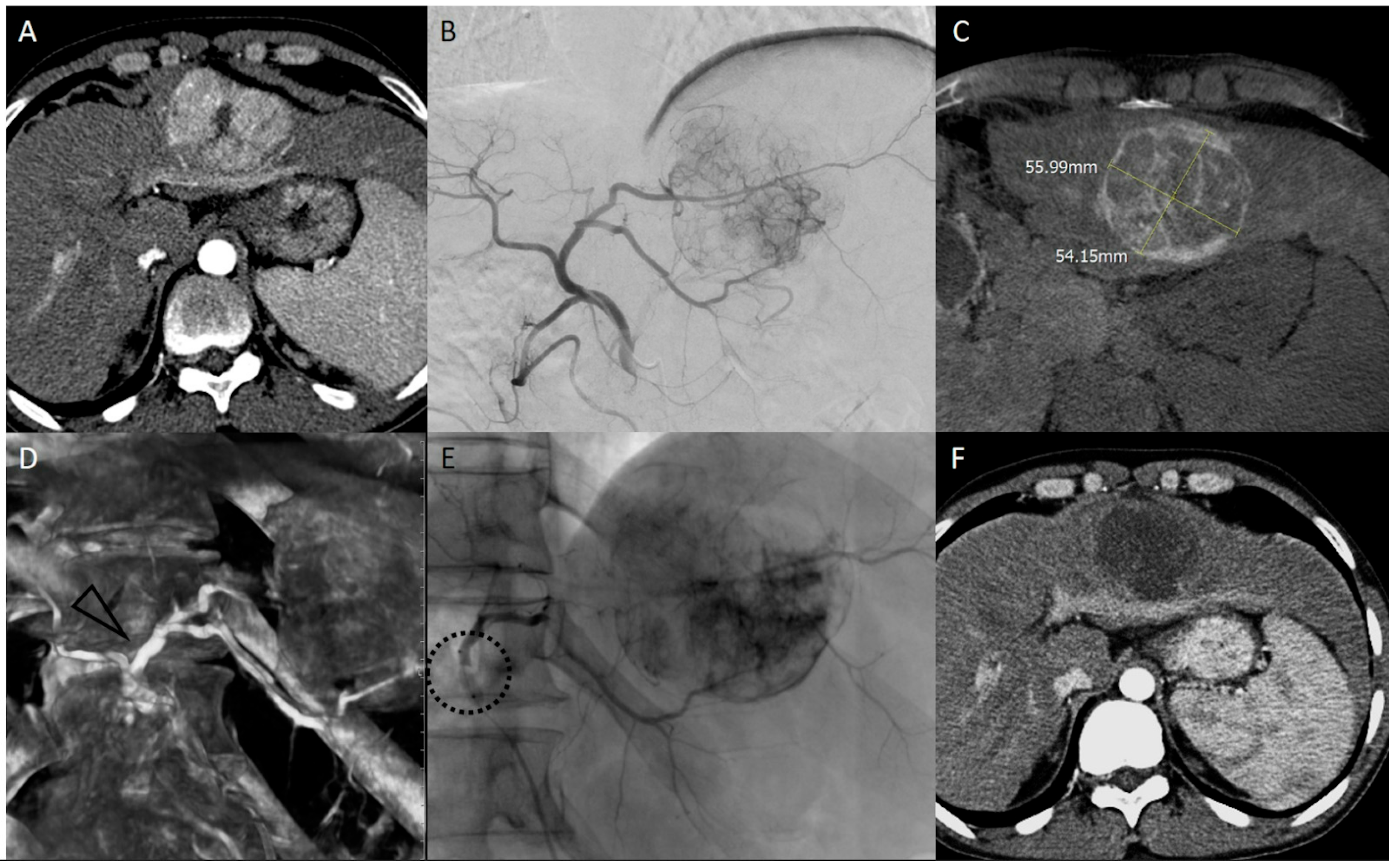
4. bTACE Technical Notes: How We Do It
- Perception of resistance: Even through forced manual injection, no more embolic agent can be injected.
- Reflux despite the presence of the inflated microballoon: Forced injection could determine the overdilation of the vessel wall at the level of the balloon causing reflux.
- Inversion of flow into collaterals: To understand this concept, take a step back to the mechanism of action of the device. Once the balloon is inflated, the restoration of flow beyond the balloon is performed due to the intersegmental collateral opening. This permits the embolic agent to reach the lesion despite the “absence” of the vis a tergo. During the embolization, when the target lesion has been filled with embolics and the pressure within it rises, these collaterals that opened could reverse their flow, pushing our embolics further towards healthy liver segments. Thus, if hepatofugal collateral became appreciable during the embolization, then the embolization should be stopped. Continuous fluoroscopic guidance is mandatory throughout the entire embolization procedure.
- Maximum threshold of drug: The added value of the employment of microballoon catheter has clinical relevance since, as mentioned above, the main factor influencing overall survival is achieving a complete response, preferably sustained for at least six months, a result that is more difficult to obtain in nodules larger than 3 cm.
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular Carcinoma; |
| TACE | Transarterial Chemoembolization; |
| bTACE | Balloon-occluded TACE; |
| BCLC | Barcelona Clinic Liver Cancer; |
| TARE | Transarterial Radio Embolization; |
| bTARE | Balloon-occluded TARE; |
| DEM-TACE | Drug-eluting Microsphere TACE; |
| c-TACE | Conventional TACE; |
| DSA | Digital Subtracted Angiography; |
| BOASP | Balloon-occluded Arterial Stump Pressure. |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanneet, M.; Soerjomataramal, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–337. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; Sangro, B.; Singal, A.G.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prince, D.; Liu, K.; Xu, W.; Chen, M.; Sun, J.; Lu, X.; Ji, J. Management of patients with intermediate stage hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Chung-Mau, L.; Henry, N.; Wai-Kuen, T.; Chi-Leung, L.; Chi-Ming, L.; Tung-Ping, P.R.; Sheung-Tat, F.; John, W. Randomized controlled trial of transarterial Lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, J.E.; Kim, D.Y. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J. Hepatol. 2017, 9, 808–814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, J.H.; Zhang, L.; Ren, Z.G.; Ye, S.L. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: A meta-analysis. J. Dig. Dis. 2016, 17, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R. New data supporting modified RECIST (mRECIST) for hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 1312–1314. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.-J.; Park, M.-S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J. Hepatol. 2015, 62, 1304–1310. [Google Scholar] [CrossRef]
- Peng, C.-W.; Teng, W.; Lui, K.-W.; Hung, C.F.; Jeng, W.J.; Huang, C.H.; Chen, W.T.; Lin, C.C.; Lin, C.Y.; Lin, S.M.; et al. Complete response at first transarterial chemoembolization predicts favorable outcome in hepatocellular carcinoma. Am. J. Cancer Res. 2021, 11, 4956–4965. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34765303 (accessed on 3 July 2025).
- Zhang, Y.; Zhang, M.; Chen, M.; Mei, J.; Xu, L.; Guo, R.; Lin, X.; Li, J.; Peng, Z. Association of Sustained Response Duration with Survival after Conventional Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. JAMA Netw. Open 2018, 1, e183213. [Google Scholar] [CrossRef]
- Jeong, S.O.; Kim, E.B.; Jeong, S.W.; Jang, Y.J.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Predictive factors for complete response and recurrence after transarterial chemoembolization in hepatocellular carcinoma. Gut Liver 2017, 11, 409–416. [Google Scholar] [CrossRef]
- Vesselle, G.; Quirier-Leleu, C.; Velasco, S.; Charier, F.; Silvain, C.; Boucebci, S.; Ingrand, P.; Tasu, J.-P. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur. Radiol. 2016, 26, 1640–1648. [Google Scholar] [CrossRef]
- Bryant, M.K.; Dorn, D.P.; Zarzour, J.; Smith, J.K.; Redden, R.T.; Saddekni, S.; Aal, A.K.A.; Gray, S.H.; Eckhoff, D.E.; DuBay, D.A. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB 2014, 16, 327–335. [Google Scholar] [CrossRef]
- Asano, K.; Kageyama, K.; Yamamoto, A.; Jogo, A.; Uchida-Kobayashi, S.; Sohgawa, E.; Murai, K.; Kawada, N.; Miki, Y. Transcatheter Arterial Chemoembolization for Treatment-Naive Hepatocellular Carcinoma Has Different Treatment Effects Depending on Central or Peripheral Tumor Location. Liver Cancer 2023, 12, 576–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, H.; Tanaka, M.; Oi, H. Hepatic embolization for the common hepatic artery using balloon occlusion technique. Am. J. Roentgenol. 1985, 145, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Kuramochi, M.; Takahashi, N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: Measurement of balloon-occluded arterial stump pressure. Cardiovasc. Interv. Radiol. 2013, 36, 706–713. [Google Scholar] [CrossRef]
- Lucatelli, P.; Corradini, L.G.; Rubeis, G.D.; Rocco, B.; Basilico, F.; Cannavale, A.; Nardis, P.G.; Corona, M.; Saba, L.; Catalano, C.; et al. Balloon-Occluded Transcatheter Arterial Chemoembolization (b-TACE) for Hepatocellular Carcinoma Performed with Polyethylene-Glycol Epirubicin-Loaded Drug-Eluting Embolics: Safety and Preliminary Results. Cardiovasc. Interv. Radiol. 2019, 42, 853–862. [Google Scholar] [CrossRef]
- Saltarelli, A.; Pelle, G.; Notarianni, E.; Pasqualini, V.; Cianni, R. Y90 Radioembolization with Occlusafe Catheter Infusion System in Patients with Unresectable Hepatic Metastasis. J. Vasc. Interv. Radiol. 2017, 28, S213–S214. [Google Scholar] [CrossRef]
- Lucatelli, P.; Rubeis, G.D.; Trobiani, C.; Ungania, S.; Rocco, B.; Gyurgyokai, S.Z.D.; Masi, M.; Pecorella, I.; Cappelli, F.; Lai, A.; et al. In Vivo Comparison of Microballoon Interventions (MBI) Advantage: A Retrospective Cohort Study of DEB-TACE Versus b-TACE and of SIRT Versus b-SIRT. Cardiovasc Intervent Radiol. 2022, 45, 306–314. [Google Scholar] [CrossRef]
- Rose, S.C.; Narsinh, K.H.; Isaacson, A.J.; Fischman, A.M.; Golzarian, J. The beauty and bane of pressure-directed embolotherapy: Hemodynamic principles and preliminary clinical evidence. Am. J. Roentgenol. 2019, 212, 686–695. [Google Scholar] [CrossRef]
- Aramburu, J.; Antón, R.; Rivas, A.; Ramos, J.C.; Larraona, G.S.; Sangro, G. A methodology for numerically analysing the hepatic artery haemodynamics during B-TACE: A proof of concept. Comput. Methods Biomech Biomed. Engin. 2019, 22, 518–532. [Google Scholar] [CrossRef]
- Aramburu, J.; Antón, R.; Fukamizu, J.; Nozawa, D.; Takahashi, M.; Ozaki, K.; Ramos, J.C.; Sangro, B.; Bilbao, J.I.; Tomita, K.; et al. In vitro model for simulating drug delivery during balloon-occluded transarterial chemoembolization. Biology 2021, 10, 1341. [Google Scholar] [CrossRef]
- Aramburu, J.; Antón, R.; Rodríguez-Fraile, M.; Sangro, B.; Bilbao, J.I. Computational fluid dynamics modeling of liver radioembolization: A review. Cardiovasc. Interv. Radiol. 2022, 45, 12–20. [Google Scholar] [CrossRef]
- Lucatelli, P.; Ciaglia, S.; Rocco, B.; Rubeis, G.D.; Bolognesi, G.; Damato, E.; Corona, M.; Nardis, P.G.; Cannavale, A.; Ricci, P.; et al. Two-dimensional perfusion angiography permits direct visualization of redistribution of flow in hepatocellular carcinoma during b-TACE. Radiol. Med. 2024, 129, 823–833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucatelli, P.; Rocco, B.; Ciaglia, S.; Damato, E.; Mosconi, C.; Argirò, R.; Catalano, C. Microballoon Interventions for Liver Tumors: Review of Literature and Future Perspectives. J. Clin. Med. 2022, 11, 5334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maruyama, M.; Yoshizako, T.; Nakamura, T.; Nakamura, M.; Yoshida, R.; Kitagaki, H. Initial Experience with Balloon-Occluded Trans-catheter Arterial Chemoembolization (B-TACE) for Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2016, 39, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Rubeis, G.D.; Rocco, B.; Basilico, F.; Cannavale, C.A.; Abbatecola, A.; Nardis, P.G.; Corona, M.; Brozzetti, S.; Catalano, C.; et al. Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: A single center retrospective case control study. BMC Gastroenterol. 2021, 21, 51. [Google Scholar] [CrossRef]
- Golfieri, R.; Bezzi, M.; Verset, G.; Fucilli, F.; Mosconi, C.; Cappelli, A.; Paccapelo, A.; Lucatelli, P.; Magand, N.; Rode, A.; et al. Retrospective European Multicentric Evaluation of Selective Transarterial Chemoembolisation with and without Balloon-Occlusion in Patients with Hepatocellular Carcinoma: A Propensity Score Matched Analysis. Cardiovasc. Interv. Radiol. 2021, 44, 1048–1059. [Google Scholar] [CrossRef]
- Golfieri, R.; Bezzi, M.; Verset, G.; Fucilli, F.; Mosconi, C.; Cappelli, A.; Paccapelo, A.; Lucatelli, P.; Magand, A.; Rode, A.; et al. Balloon-occluded transarterial chemoembolization: In which size range does it perform best? A comparison of its efficacy versus conventional transarterial chemoembolization, using propensity score matching. Liver Cancer 2021, 10, 522–534. [Google Scholar] [CrossRef]
- Lucatelli, P.; Rocco, B.; Beare, T.D.; Verset, G.; Fucilli, F.; Damato, E.; Paccapelo, A.; Braccischi, L.; Tomassoni, M.T.; Bucalau, A.M.; et al. Long-Term Outcomes of Balloon TACE for HCC: An European Multicentre Single-Arm Retrospective Study. Cardiovasc. Interv. Radiol. 2024, 47, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumoto, T.; Endo, J.; Hashida, K.; Ichikawa, H.; Kojima, S.; Takashimizu, S.; Watanabe, N.; Yamagami, T.; Hasebe, T. Balloon-Occluded Transarterial Chemoembolization Using A 1.8-French Tip Coaxial Microballoon Catheter for Hepatocellular Carcinoma: Technical and Safety Considerations. Minim. Invasive Ther. Allied Technol. 2014, 24, 94–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, B.; Madoff, D.C.; Basilico, F.; Damato, E.; Vetri, P.; Panebianco, V.; Catalano, C.; Lucatelli, P. Pressure Gradient-Driven Embolization b-TACE for HCC: Technical and Diagnostic Step-by-Step Procedural Guide and Literature Review. Diagnostics 2025, 15, 1726. https://doi.org/10.3390/diagnostics15131726
Rocco B, Madoff DC, Basilico F, Damato E, Vetri P, Panebianco V, Catalano C, Lucatelli P. Pressure Gradient-Driven Embolization b-TACE for HCC: Technical and Diagnostic Step-by-Step Procedural Guide and Literature Review. Diagnostics. 2025; 15(13):1726. https://doi.org/10.3390/diagnostics15131726
Chicago/Turabian StyleRocco, Bianca, David C. Madoff, Fabrizio Basilico, Elio Damato, Paolo Vetri, Valeria Panebianco, Carlo Catalano, and Pierleone Lucatelli. 2025. "Pressure Gradient-Driven Embolization b-TACE for HCC: Technical and Diagnostic Step-by-Step Procedural Guide and Literature Review" Diagnostics 15, no. 13: 1726. https://doi.org/10.3390/diagnostics15131726
APA StyleRocco, B., Madoff, D. C., Basilico, F., Damato, E., Vetri, P., Panebianco, V., Catalano, C., & Lucatelli, P. (2025). Pressure Gradient-Driven Embolization b-TACE for HCC: Technical and Diagnostic Step-by-Step Procedural Guide and Literature Review. Diagnostics, 15(13), 1726. https://doi.org/10.3390/diagnostics15131726






