Bisphosphonate Use and Cardiovascular Outcomes According to Kidney Function Status in Post-Menopausal Women: An Emulated Target Trial from the Multi-Ethnic Study of Atherosclerosis
Abstract
1. Introduction
2. Methods
3. Target Trial Emulation
3.1. Study Population, Eligibility, and Exclusion Criteria
3.2. Propensity Score Matching
3.3. Treatment Assignment
3.4. Outcome
4. Statistical Analysis
4.1. Analysis of Association Between NCB Use and CAC
4.2. Association Between NCB Use and CVD/CHD Events
4.3. Sensitivity Analyses
5. Results
6. Discussion
7. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golub, I.S.; Termeie, O.G.; Kristo, S.; Schroeder, L.P.; Lakshmanan, S.; Shafter, A.M.; Hussein, L.; Verghese, D.; Aldana-Bitar, J.; Manubolu, V.S.; et al. Major Global Coronary Artery Calcium Guidelines. Cardiovasc. Imaging 2023, 16, 98–117. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rogers, J.R.; Fulchino, L.A.; Kim, C.A.; Solomon, D.H.; Kim, S.C.; Pizzi, C. Bisphosphonates and Risk of Cardiovascular Events: A Meta-Analysis. PLoS ONE 2015, 10, e0122646. [Google Scholar] [CrossRef]
- Caffarelli, C.; Montagnani, A.; Nuti, R.; Gonnelli, S. Bisphosphonates, atherosclerosis and vascular calcification: Update and systematic review of clinical studies. Clin. Interv. Aging 2017, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arter. Thromb. Vasc. Biol. 2001, 21, 817–824. [Google Scholar] [CrossRef]
- Nitta, K.; Akiba, T.; Suzuki, K.; Uchida, K.; Watanabe, R.-I.; Majima, K.; Aoki, T.; Nihei, H. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am. J. Kidney Dis. 2004, 44, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Russell, R.G.G.; Rogers, M.J.D. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Min. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, N.-C.; Wu, F.-Z.; Wu, M.-T. Impact of denosumab on cardiovascular calcification in patients with secondary hyperparathyroidism undergoing dialysis: A pilot study. Osteoporos. Int. 2020, 31, 1507–1516. [Google Scholar] [CrossRef]
- Ariyoshi, T.; Eishi, K.; Sakamoto, I.; Matsukuma, S.; Odate, T. Effect of etidronic acid on arterial calcification in dialysis patients. Clin. Drug Investig. 2006, 26, 215–222. [Google Scholar] [CrossRef]
- Hill, J.A.; Goldin, J.G.; Gjertson, D.; Emerick, A.M.; Greaser, L.D.; Yoon, H.-C.; Khorrami, S.; Aziz, D.; Adams, J.S. Progression of coronary artery calcification in patients taking alendronate for osteoporosis. Acad. Radiol. 2002, 9, 1148–1152. [Google Scholar] [CrossRef]
- Pawade, T.A.; Doris, M.K.; Bing, R.; White, A.C.; Forsyth, L.; Evans, E.; Graham, C.; Williams, M.C.; van Beek, E.J.; Fletcher, A.; et al. Effect of Denosumab or Alendronic Acid on the Progression of Aortic Stenosis: A Double-Blind Randomized Controlled Trial. Circulation 2021, 143, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.L.; Chaudhri, K.; McOrist, N.S.; Gladysz, K.; Gnanenthiran, S.R.; Shalaby, G. Do bisphosphonates and RANKL inhibitors alter the progression of coronary artery calcification? A systematic review. BMJ Open 2024, 14, e084516. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamanaka, S.; Yoshimoto, W.; Shigematsu, T. Alendronate as an effective treatment for bone loss and vascular calcification in kidney transplant recipients. J. Transpl. 2014, 2014, 269613. [Google Scholar] [CrossRef] [PubMed]
- Atieh, K.; Miles, B.; Mackey, J.; Mehta, A. The potential impact of bisphosphonates on major adverse cardiovascular events: A study of 1,097,406 patients. Eur. Heart J. 2024, 45, 1555. [Google Scholar] [CrossRef]
- Wu, S.T.; Chen, J.F.; Tsai, C.J. The impact of bisphosphonates on mortality and cardiovascular risk among osteoporosis patients after cardiovascular disease. J. Formos. Med. Assoc. 2021, 120, 1957–1966. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.Y.; Eastell, R.; Allen, I.E. Association Between Drug Treatments for Patients With Osteoporosis and Overall Mortality Rates: A Meta-analysis. JAMA Intern. Med. 2019, 179, 1491–1500. [Google Scholar] [CrossRef]
- Händel, M.N.; Cardoso, I.; von Bülow, C.; Rohde, J.F.; Ussing, A.; Nielsen, S.M.; Christensen, R.; Body, J.-J.; Brandi, M.L.; Diez-Perez, A.; et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: Systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ 2023, 381, e068033. [Google Scholar] [CrossRef]
- Elmariah, S.; Delaney, J.A.; O’BRien, K.D.; Budoff, M.J.; Vogel-Claussen, J.; Fuster, V.; Kronmal, R.A.; Halperin, J.L. Bisphosphonate Use and Prevalence of Valvular and Vascular Calcification in Women MESA (The Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010, 56, 1752–1759. [Google Scholar] [CrossRef]
- MESA Overview and Protocol. Available online: https://www.mesa-nhlbi.org/aboutMESAOverviewProtocol.aspx (accessed on 31 August 2024).
- MESA Measurements. Available online: https://www.mesa-nhlbi.org/measurements.aspx (accessed on 13 October 2024).
- Bluemke, D.A.; Kronmal, R.A.; Lima, J.A.; Liu, K.; Olson, J.; Burke, G.L.; Folsom, A.R. The Relationship of Left Ventricular Mass and Geometry to Incident Cardiovascular Events: The MESA Study. J. Am. Coll. Cardiol. 2008, 52, 2148. [Google Scholar] [CrossRef]
- Blaha, M.J.; Budoff, M.J.; Tota-Maharaj, R.; Dardari, Z.A.; Wong, N.D.; Kronmal, R.A.; Eng, J.; Post, W.S.; Blumenthal, R.S.; Nasir, K. Improving the CAC Score by Addition of Regional Measures of Calcium Distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging 2016, 9, 1407–1416. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Kronmal, R.; Eng, J.; Folsom, A.; Burke, G.; Carr, J.J.; Shea, S.; Lima, J.A.C.; Bluemke, D.A. Left Ventricular Mass at MRI and Long-term Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2019, 293, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A. Methods of Public Health Research—Strengthening Causal Inference from Observational Data. N. Engl. J. Med. 2021, 385, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am. J. Epidemiol. 2010, 172, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Contr 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Stone, M. Comments on Model Selection Criteria of Akaike and Schwarz. J. R. Stat. Soc. Ser. B Stat. Methodol. 1979, 41, 276–278. [Google Scholar] [CrossRef]
- Hartle, J.E.; Tang, X.; Kirchner, H.L.; Bucaloiu, I.D.; Sartorius, J.A.; Pogrebnaya, Z.V.; Akers, G.A.; Carnero, G.E.; Perkins, R.M. Bisphosphonate therapy, death, and cardiovascular events among female patients with CKD: A retrospective cohort study. Am. J. Kidney Dis. 2012, 59, 636–644. [Google Scholar] [CrossRef]
- Kranenburg, G.; Bartstra, J.W.; Weijmans, M.; de Jong, P.A.; Mali, W.P.; Verhaar, H.J.; Visseren, F.L.; Spiering, W. Bisphosphonates for cardiovascular risk reduction: A systematic review and meta-analysis. Atherosclerosis 2016, 252, 106–115. [Google Scholar] [CrossRef]

| Protocol Components | Ideal Randomized Trial | Emulated Target Trial |
|---|---|---|
| Eligibility criteria |
|
|
| Exclusion criteria |
|
|
| Treatment strategy |
|
|
| Assignment |
|
|
| Follow-up |
|
|
| Outcome |
|
|
| Causal contrast of interest |
|
|
| Data analysis plan |
|
|
| Characteristic | Non-User, N = 473 1 | NCB 2 Initiator, N = 165 1 | SMD 3 |
|---|---|---|---|
| Age (years) | 68 (60, 75) | 68 (63, 74) | 0.03 |
| Race/ethnicity | |||
| White | 160 (34%) | 51 (31%) | 0.01 |
| Chinese | 113 (24%) | 44 (27%) | 0.006 |
| Black | 91 (19%) | 34 (21%) | 0.01 |
| Hispanic/Latino | 109 (23%) | 36 (22%) | 0.04 |
| BMI (kg/m2) | 26.2 (23.5, 29.5) | 25.9 (23.1, 29.4) | 0.02 |
| LDL (mg/dL) | 117 (99, 136) | 114 (92, 139) | 0.07 |
| HDL (mg/dL) | 53 (45, 63) | 52 (46, 64) | |
| Exam 1 diabetes mellitus by 2003 ADA fasting criteria algorithm | |||
| Normal | 348 (74%) | 132 (80%) | |
| IFG | 62 (13%) | 20 (12%) | |
| Untreated diabetes | 19 (3.8%) | 1 (0.6%) | |
| Treated diabetes | 44 (9.1%) | 12 (7.3%) | |
| Hypertension medication | |||
| Yes | 174 (37%) | 63 (38%) | |
| Hypertension status | |||
| Yes | 250 (53%) | 80 (48%) | |
| Baseline CAC Agatston score phantom-adjusted | 0 (0, 62) | 3 (0, 73) | 0.04 |
| Seated SBP (mmHg) | 133 (114, 152) | 128 (114, 142) | |
| Consumed alcoholic beverages | |||
| Yes | 282 (60%) | 101 (61%) | 0.02 |
| Smoking status | |||
| Never | 322 (69%) | 116 (70%) | 0.02 |
| Former | 106 (23%) | 40 (24%) | 0.08 |
| Current | 41 (8.7%) | 9 (5.5%) | 0.02 |
| Steroid use | 13 (2.7%) | 3 (1.8%) | 0.002 |
| Initiators 1 | Non-Users | Estimated Difference Per Year (95% CI) 2,3 | |||
|---|---|---|---|---|---|
| (n) | Delta CAC Agatston Score per Year (Median (IQR)) | (n) | Delta CAC Agatston Score per Year (Median (IQR)) | ||
| All participants | 165 | 473 | −0.01 (−0.05, 0.01) | ||
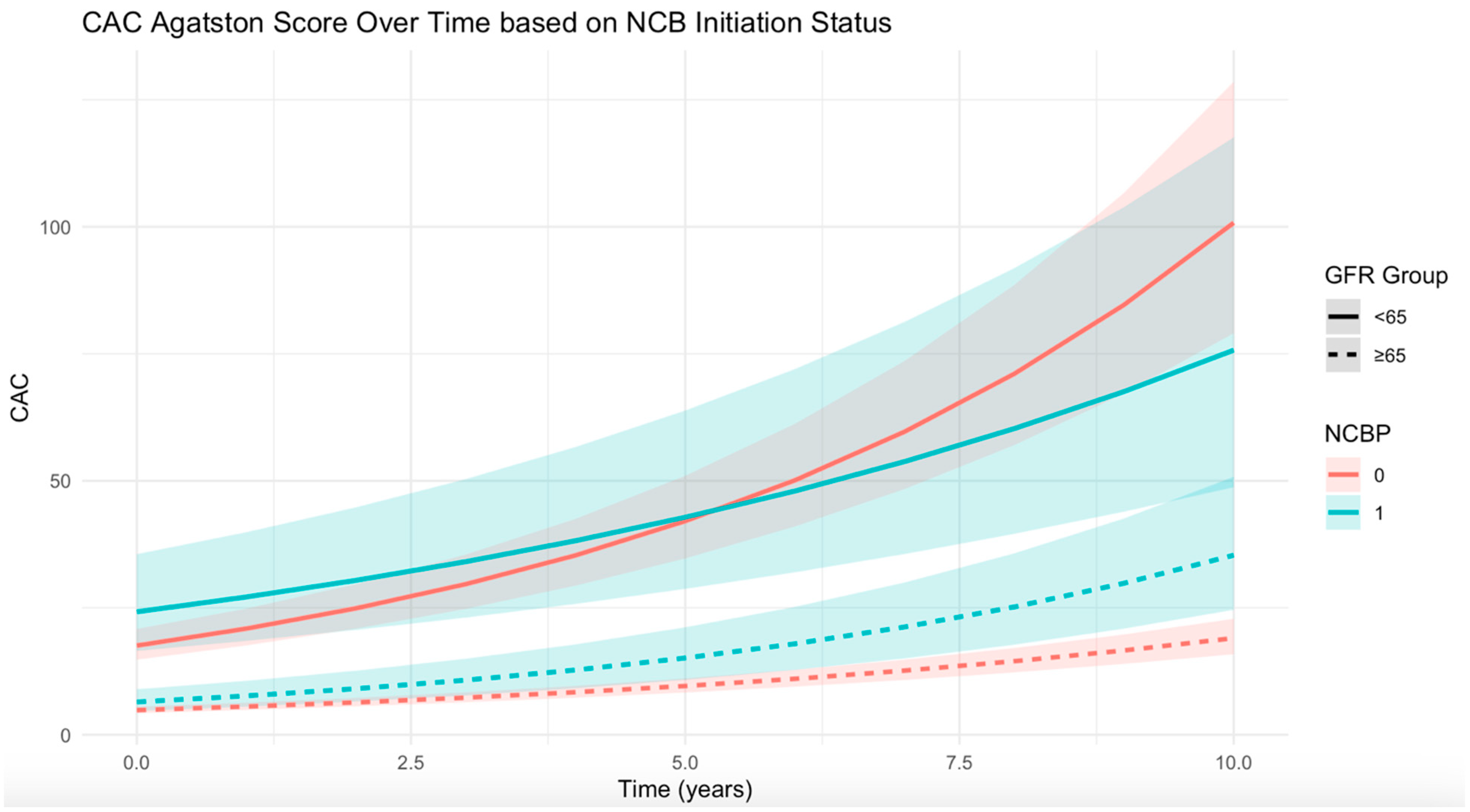
| eGFR < 65 | 40 | 8 (0, 40.5) | 146 | 11.5 (1.0, 55.0) | −0.06 (−0.12,−0.007) |
| eGFR ≥ 65 | 125 | 3 (0, 12.5) | 327 | 2.5 (0, 15) | 0.016 (−0.02, 0.05) |
| CVD 1,2,3 | CHD 1,2,4 | |||
|---|---|---|---|---|
| (n Events) | HR (95% CI) | (n Events) | HR (95% CI) | |
| All participants | 121 | 0.90 (0.60, 1.36) | 67 | 0.92 (0.53, 1.59) |
| eGFR < 65 | 23 | 0.91 (0.30, 2.68) | 15 | 1.06 (0.29, 3.78) |
| eGFR ≥ 65 | 98 | 0.94 (0.61, 1.48) | 52 | 0.94 (0.51, 1.73) |
| Restricted to age > 65 at baseline, NCB initiators vs. PS-matched non-users | |||
|---|---|---|---|
| Initiators 1 (n) | Non-users (n) | Estimated difference per year (95% CI) 2,3 | |
| All | 88 | 246 | −0.03 (−0.08, 0.008) |
| eGFR < 65 | 35 | 102 | −0.07 (−0.13,−0.01) |
| eGFR ≥ 65 | 53 | 144 | −0.007 (−0.06, 0.05) |
| Restricted to age > 75 at baseline, NCB initiators vs. PS-matched non-users | |||
| Initiators 1 (n) | Non-users (n) | Estimated difference per year (95% CI) 2,3 | |
| All | 21 | 61 | −0.06 (−0.14, 0.01) |
| eGFR < 65 | 12 | 28 | −0.1 (−0.2, −0.004) |
| eGFR ≥ 65 | 9 | 33 | −0.01 (−0.12, 0.10) |
| NCB initiators vs. non-users, adjusted for PSs 4 | |||
| Initiators 1 (n) | Non-users (n) | Estimated difference per year (95% CI) 2,3 | |
| All | 166 | 1571 | 0.004 (−0.02, 0.03) |
| eGFR < 65 | 40 | 405 | −0.04 (−0.08, −0.003) |
| eGFR ≥ 65 | 126 | 1166 | 0.02 (−0.01, 0.05) |
| NCB initiators vs. non-users, outcome: calcium volume | |||
| Initiators 1 (n) | Non-users (n) | Estimated difference per year (95% CI) 2,3 | |
| All | 165 | 473 | −0.005 (−0.04, 0.29) |
| eGFR < 65 | 40 | 146 | −0.06 (−0.12, −0.007) |
| eGFR ≥ 65 | 125 | 327 | 0.015 (−0.03, 0.06) |
| NCB users vs. PS-matched non-users | |||
| Users 1,5 (n) | Non-users (n) | Estimated difference per year (95% CI) 2,3 | |
| All | 297 | 748 | −0.003 (−0.03, 0.02) |
| eGFR < 65 | 97 | 230 | −0.02 (−0.06, 0.01) |
| eGFR ≥ 65 | 200 | 518 | 0.01 (−0.01, 0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghotbi, E.; Subhas, N.; Bancks, M.P.; Elmariah, S.; Halperin, J.L.; Bluemke, D.A.; Kestenbaum, B.R.; Barr, R.G.; Post, W.S.; Budoff, M.; et al. Bisphosphonate Use and Cardiovascular Outcomes According to Kidney Function Status in Post-Menopausal Women: An Emulated Target Trial from the Multi-Ethnic Study of Atherosclerosis. Diagnostics 2025, 15, 1727. https://doi.org/10.3390/diagnostics15131727
Ghotbi E, Subhas N, Bancks MP, Elmariah S, Halperin JL, Bluemke DA, Kestenbaum BR, Barr RG, Post WS, Budoff M, et al. Bisphosphonate Use and Cardiovascular Outcomes According to Kidney Function Status in Post-Menopausal Women: An Emulated Target Trial from the Multi-Ethnic Study of Atherosclerosis. Diagnostics. 2025; 15(13):1727. https://doi.org/10.3390/diagnostics15131727
Chicago/Turabian StyleGhotbi, Elena, Nikhil Subhas, Michael P. Bancks, Sammy Elmariah, Jonathan L. Halperin, David A. Bluemke, Bryan R Kestenbaum, R. Graham Barr, Wendy S. Post, Matthew Budoff, and et al. 2025. "Bisphosphonate Use and Cardiovascular Outcomes According to Kidney Function Status in Post-Menopausal Women: An Emulated Target Trial from the Multi-Ethnic Study of Atherosclerosis" Diagnostics 15, no. 13: 1727. https://doi.org/10.3390/diagnostics15131727
APA StyleGhotbi, E., Subhas, N., Bancks, M. P., Elmariah, S., Halperin, J. L., Bluemke, D. A., Kestenbaum, B. R., Barr, R. G., Post, W. S., Budoff, M., Lima, J. A. C., & Demehri, S. (2025). Bisphosphonate Use and Cardiovascular Outcomes According to Kidney Function Status in Post-Menopausal Women: An Emulated Target Trial from the Multi-Ethnic Study of Atherosclerosis. Diagnostics, 15(13), 1727. https://doi.org/10.3390/diagnostics15131727







