Stent Failure Management in Contemporary Clinical Practice
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dangas, G.D.; Serruys, P.W.; Kereiakes, D.J.; Hermiller, J.; Rizvi, A.; Newman, W.; Sudhir, K.; Smith, R.S., Jr.; Cao, S.; Theodoropoulos, K.; et al. Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: Final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc. Interv. 2013, 6, 914–922. [Google Scholar] [CrossRef]
- Toyota, T.; Shiomi, H.; Morimoto, T.; Kimura, T. Meta-analysis of long-term clinical outcomes of everolimus-eluting stents. Am. J. Cardiol. 2015, 116, 187–194. [Google Scholar] [CrossRef]
- Torrado, J.; Buckley, L.; Durán, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzmán, L.A. Restenosis, STSTosis, and Bleeding Complications: Navigating Between Scylla and Charybdis. J. Am. Coll. Cardiol. 2018, 71, 1676–1695. [Google Scholar] [CrossRef]
- Bangalore, S.; Toklu, B.; Patel, N.; Feit, F.; Stone, G.W. Newer-Generation Ultrathin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation 2018, 138, 2216–2226. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Redfors, B.; Ali, Z.A.; Prasad, M.; Shahim, B.; Smits, P.C.; von Birgelen, C.; Zhang, Z.; Mehran, R.; Serruys, P.W.; et al. Long-Term Outcomes After Revascularization for Stable Ischemic Heart Disease: An Individual Patient-Level Pooled Analysis of 19 Randomized Coronary Stent Trials. Circ. Cardiovasc. Interv. 2020, 13, e008565. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Kirtane, A.J.; Redfors, B.; Généreux, P.; Ben-Yehuda, O.; Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Smits, P.C.; von Birgelen, C.; et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2020, 75, 590–604. [Google Scholar] [CrossRef]
- Alfonso, F.; Coughlan, J.J.; Giacoppo, D.; Kastrati, A.; Byrne, R.A. Management of in-stent restenosis. EuroIntervention 2022, 18, e103–e123. [Google Scholar] [CrossRef]
- Piraino, D.; Cimino, G.; Buccheri, D.; Dendramis, G.; Andolina, G.; Cortese, B. Recurrent in-stent restenosis, certainty of its origin, uncertainty about treatment. Int. J. Cardiol. 2017, 230, 91–96. [Google Scholar] [CrossRef]
- Klein, L.W.; Nathan, S.; Maehara, A.; Messenger, J.; Mintz, G.S.; Ali, Z.A.; Rymer, J.; Sandoval, Y.; Al-Azizi, K.; Mehran, R.; et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100971. [Google Scholar] [CrossRef]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef]
- Alfonso, F.; Kastrati, A. Clinical burden and implications of coronary interventions for in-stent restenosis. EuroIntervention 2021, 17, e355–e357. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Joner, M.; Godschalk, T.C.; Malik, N.; Alfonso, F.; Xhepa, E.; De Cock, D.; Komukai, K.; Tada, T.; Cuesta, J.; et al. Optical Coherence Tomography Findings in Patients With Coronary Stent Thrombosis: A Report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation 2017, 136, 1007–1021. [Google Scholar] [CrossRef]
- Gori, T.; Polimeni, A.; Indolfi, C.; Räber, L.; Adriaenssens, T.; Münzel, T. Predictors of stent thrombosis and their implications for clinical practice. Nat. Rev. Cardiol. 2019, 16, 243–256. [Google Scholar] [CrossRef]
- Karamasis, G.V.; Varlamos, C.; Benetou, D.R.; Kalogeropoulos, A.S.; Keeble, T.R.; Tsigkas, G.; Xenogiannis, I. The Usefulness of Intracoronary Imaging in Patients with ST-Segment Elevation Myocardial Infarction. J. Clin. Med. 2023, 12, 5892. [Google Scholar] [CrossRef]
- Verheye, S.; Vrolix, M.; Kumsars, I.; Erglis, A.; Sondore, D.; Agostoni, P.; Cornelis, K.; Janssens, L.; Maeng, M.; Slagboom, T.; et al. The SABRE Trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): Angiographic Results and 1-Year Clinical Outcomes. JACC Cardiovasc. Interv. 2017, 10, 2029–2037. [Google Scholar] [CrossRef]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Tamez, H.; Secemsky, E.A.; Valsdottir, L.R.; Moussa, I.D.; Song, Y.; Simonton, C.A.; Gibson, C.M.; Popma, J.J.; Yeh, R.W. Long-term outcomes of percutaneous coronary intervention for in-stent restenosis among Medicare beneficiaries. EuroIntervention 2021, 17, e380–e387. [Google Scholar] [CrossRef]
- Waldo, S.W.; O’Donnell, C.I.; Prouse, A.; Plomondon, M.E.; Rao, S.V.; Maddox, T.M.; Ho, P.M.; Armstrong, E.J. Incidence, procedural management, and clinical outcomes of coronary in-stent restenosis: Insights from the National VA CART Program. Catheter. Cardiovasc. Interv. 2018, 91, 425–433. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, Y.K.; Kim, S.W.; Hong, Y.J.; Koo, B.K.; Bae, J.W.; Lee, S.H.; Yang, T.H.; Park, H.S.; Choi, S.W.; et al. Clinical Results of Drug-Coated Balloon Treatment in a Large-Scale Multicenter Korean Registry Study. Korean Circ. J. 2022, 52, 444–454. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Matteau, A.; Yeh, R.W.; Steg, P.G.; Camenzind, E.; Wijns, W.; McFadden, E.; Mauri, L. Comparison of Short- and Long-Term Cardiac Mortality in Early Versus Late Stent Thrombosis (from Pooled PROTECT Trials). Am. J. Cardiol. 2015, 115, 1678–1684. [Google Scholar] [CrossRef]
- Katsikis, A.; Keeble, T.R.; Davies, J.R.; Jagathesan, R.; Kabir, A.; Sayer, J.W.; Robinson, N.M.; Kalogeropoulos, A.S.; Aggarwal, R.K.; Gamma, R.A.; et al. Contemporary management of stent thrombosis: Predictors of mortality and the role of new-generation drug-eluting stents. Catheter. Cardiovasc. Interv. 2020, 96, E8–E16. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 145, E4–E17. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Byrne, R.A.; Neumann, F.J.; Mehilli, J.; Pinieck, S.; Wolff, B.; Tiroch, K.; Schulz, S.; Fusaro, M.; Ott, I.; Ibrahim, T.; et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet 2013, 381, 461–467. [Google Scholar] [CrossRef]
- Kastrati, A.; Mehilli, J.; von Beckerath, N.; Dibra, A.; Hausleiter, J.; Pache, J.; Schühlen, H.; Schmitt, C.; Dirschinger, J.; Schömig, A. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: A randomized controlled trial. JAMA 2005, 293, 165–171. [Google Scholar] [CrossRef]
- Kufner, S.; Byrne, R.A.; de Waha, A.; Schulz, S.; Joner, M.; Laugwitz, K.L.; Kastrati, A. Sirolimus-eluting versus paclitaxel-eluting stents in diabetic and non-diabetic patients within sirolimus-eluting stent restenosis: Results from the ISAR-DESIRE 2 trial. Cardiovasc. Revasc Med. 2014, 15, 69–75. [Google Scholar] [CrossRef]
- Gao, X.F.; Ge, Z.; Kong, X.Q.; Kan, J.; Han, L.; Lu, S.; Tian, N.L.; Lin, S.; Lu, Q.H.; Wang, X.Y.; et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 247–257. [Google Scholar] [CrossRef]
- Hong, S.J.; Mintz, G.S.; Ahn, C.M.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; Kang, T.S.; Kang, W.C.; Kim, Y.H.; Hur, S.H.; et al. Effect of Intravascular Ultrasound-Guided Drug-Eluting Stent Implantation: 5-Year Follow-Up of the IVUS-XPL Randomized Trial. JACC Cardiovasc. Interv. 2020, 13, 62–71. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography-Guided versus Angiography-Guided PCI. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef]
- Karamasis, G.V.; Katsikis, A.; Konstantinou, K.; Clesham, G.J.; Kelly, P.A.; Jagathesan, R.; Prati, F.; Bourantas, C.V.; Davies, J.R.; Keeble, T.R. Clinical Impact of Intracoronary Imaging in the Management of Stent Thrombosis. J. Clin. Med. 2024, 13, 4667. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Case, B.C.; Chen, Y.; Chezar-Azerrad, C.; Hashim, H.; Garcia-Garcia, H.M.; Mintz, G.S.; Waksman, R. Waksman In-Stent Restenosis Classification: A Mechanism-Based Approach to the Treatment of Restenosis. Cardiovasc. Revasc Med. 2021, 33, 62–67. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alvarez-Covarrubias, H.A.; Koch, T.; Cassese, S.; Xhepa, E.; Kessler, T.; Wiebe, J.; Joner, M.; Hochholzer, W.; Laugwitz, K.L.; et al. Coronary artery restenosis treatment with plain balloon, drug-coated balloon, or drug-eluting stent: 10-year outcomes of the ISAR-DESIRE 3 trial. Eur. Heart J. 2023, 44, 1343–1357. [Google Scholar] [CrossRef]
- Rittger, H.; Waliszewski, M.; Brachmann, J.; Hohenforst-Schmidt, W.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Schlundt, C.; et al. Long-Term Outcomes After Treatment With a Paclitaxel-Coated Balloon Versus Balloon Angioplasty: Insights From the PEPCAD-DES Study (Treatment of Drug-eluting Stent [DES] In-Stent Restenosis With SeQuent Please Paclitaxel-Coated Percutaneous Transluminal Coronary Angioplasty [PTCA] Catheter). JACC Cardiovasc. Interv. 2015, 8, 1695–1700. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.Y.; Degenhardt, R.; Pleva, L.; et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients With Coronary Stent Restenosis. J. Am. Coll. Cardiol. 2020, 75, 2664–2678. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahmoud, A.N.; Elgendy, A.Y.; Mojadidi, M.K.; Elbadawi, A.; Eshtehardi, P.; Pérez-Vizcayno, M.J.; Wayangankar, S.A.; Jneid, H.; David Anderson, R.; et al. Drug-Eluting Balloons Versus Everolimus-Eluting Stents for In-Stent Restenosis: A Meta-Analysis of Randomized Trials. Cardiovasc. Revascularization Med. 2019, 20, 612–618. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Kufner, S.; Joner, M.; Schneider, S.; Tölg, R.; Zrenner, B.; Repp, J.; Starkmann, A.; Xhepa, E.; Ibrahim, T.; Cassese, S.; et al. Neointimal Modification With Scoring Balloon and Efficacy of Drug-Coated Balloon Therapy in Patients With Restenosis in Drug-Eluting Coronary Stents: A Randomized Controlled Trial. JACC Cardiovasc. Interv. 2017, 10, 1332–1340. [Google Scholar] [CrossRef]
- Kleber, F.X.; Mathey, D.G.; Rittger, H.; Scheller, B. How to use the drug-eluting balloon: Recommendations by the German consensus group. EuroIntervention 2011, 7 (Suppl. K), K125–K128. [Google Scholar] [CrossRef]
- Leick, J.; Rheude, T.; Denne, M.; Tobias, K.; Cassese, S.; Kastrati, A.; Afzal, S.; Hug, K.P.; Saad, L.; Lauterbach, M.; et al. Comparison of long-term outcome in patients with in-stent restenosis treated with intravascular lithotripsy or with modified balloon angioplasty. Clin. Res. Cardiol. 2024, 113, 1030–1040. [Google Scholar] [CrossRef]
- van Werkum, J.W.; Heestermans, A.A.; de Korte, F.I.; Kelder, J.C.; Suttorp, M.J.; Rensing, B.J.; Zwart, B.; Brueren, B.R.; Koolen, J.J.; Dambrink, J.H.; et al. Long-term clinical outcome after a first angiographically confirmed coronary stent thrombosis: An analysis of 431 cases. Circulation 2009, 119, 828–834. [Google Scholar] [CrossRef]
- de la Torre-Hernández, J.M.; Alfonso, F.; Hernández, F.; Elizaga, J.; Sanmartin, M.; Pinar, E.; Lozano, I.; Vazquez, J.M.; Botas, J.; Perez de Prado, A.; et al. Drug-eluting stent thrombosis: Results from the multicenter Spanish registry ESTROFA (Estudio ESpañol sobre TROmbosis de stents FArmacoactivos). J. Am. Coll. Cardiol. 2008, 51, 986–990. [Google Scholar] [CrossRef]
- Burzotta, F.; Parma, A.; Pristipino, C.; Manzoli, A.; Belloni, F.; Sardella, G.; Rigattieri, S.; Danesi, A.; Mazzarotto, P.; Summaria, F.; et al. Angiographic and clinical outcome of invasively managed patients with thrombosed coronary bare metal or drug-eluting stents: The OPTIMIST study. Eur. Heart J. 2008, 29, 3011–3021. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Feldman, D.N.; Wang, T.Y.; Kaltenbach, L.A.; Yeo, K.K.; Wong, S.C.; Spertus, J.; Shaw, R.E.; Minutello, R.M.; Moussa, I.; et al. Clinical presentation, management, and outcomes of angiographically documented early, late, and very late stent thrombosis. JACC Cardiovasc. Interv. 2012, 5, 131–140. [Google Scholar] [CrossRef]
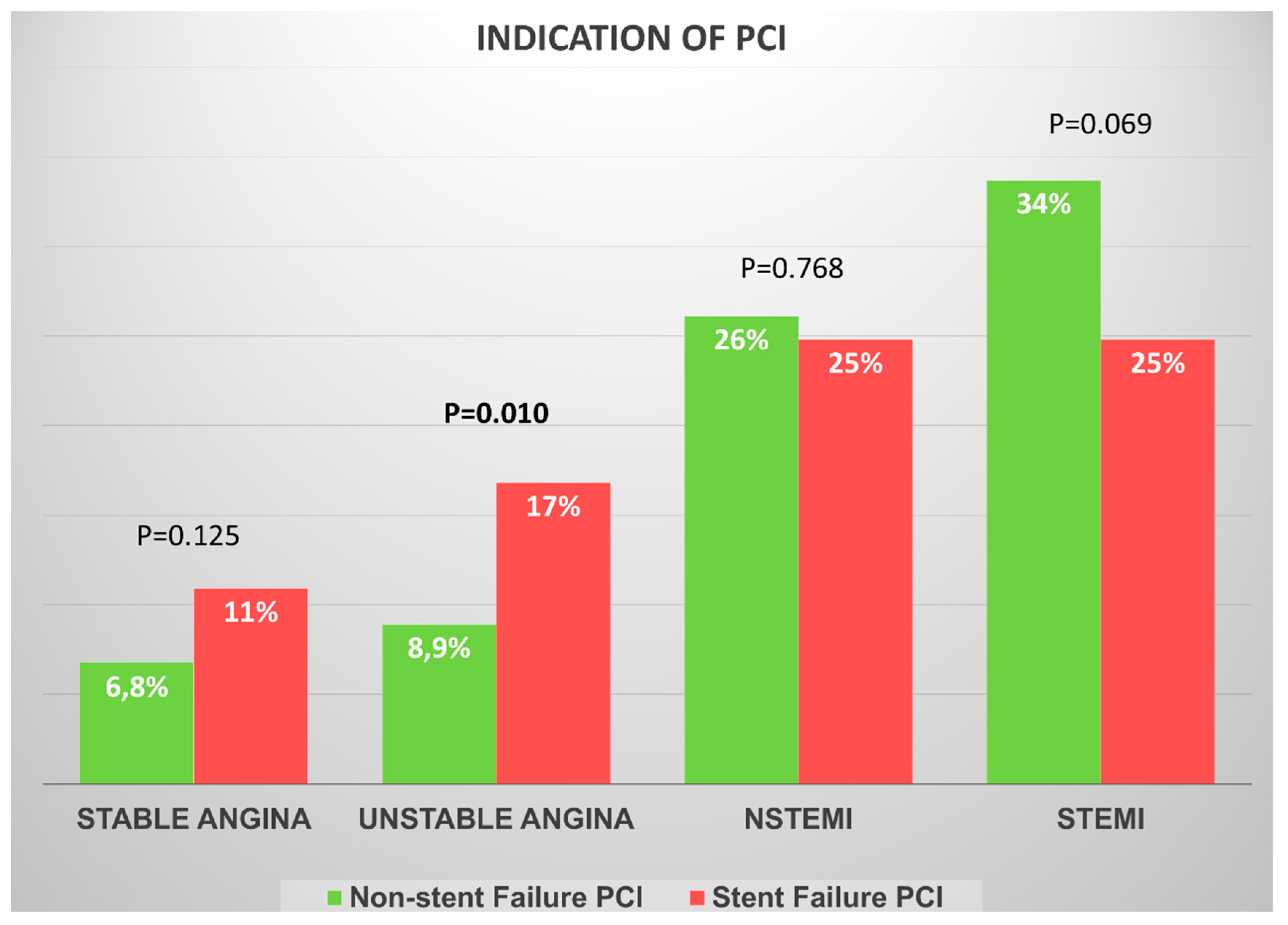
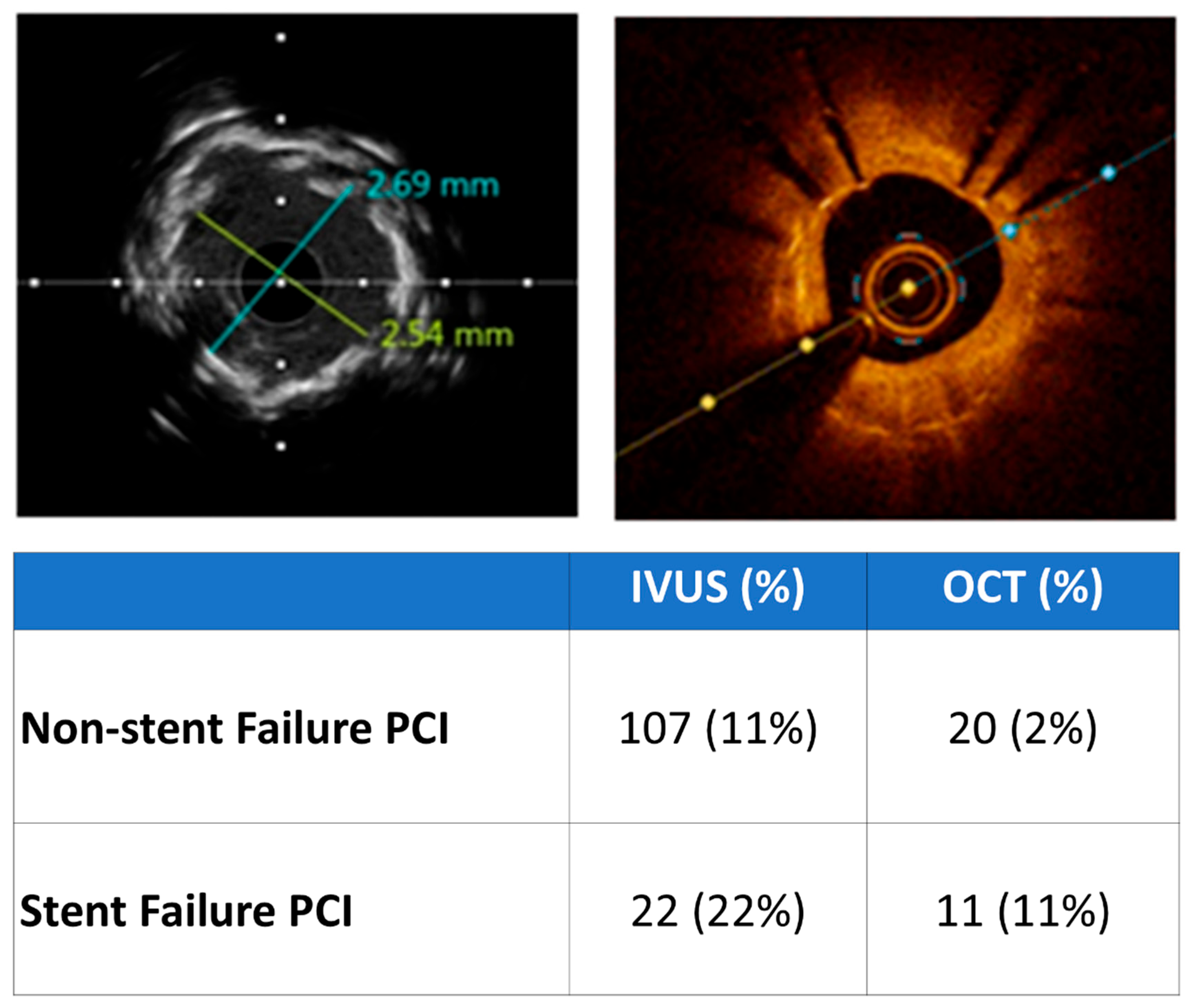
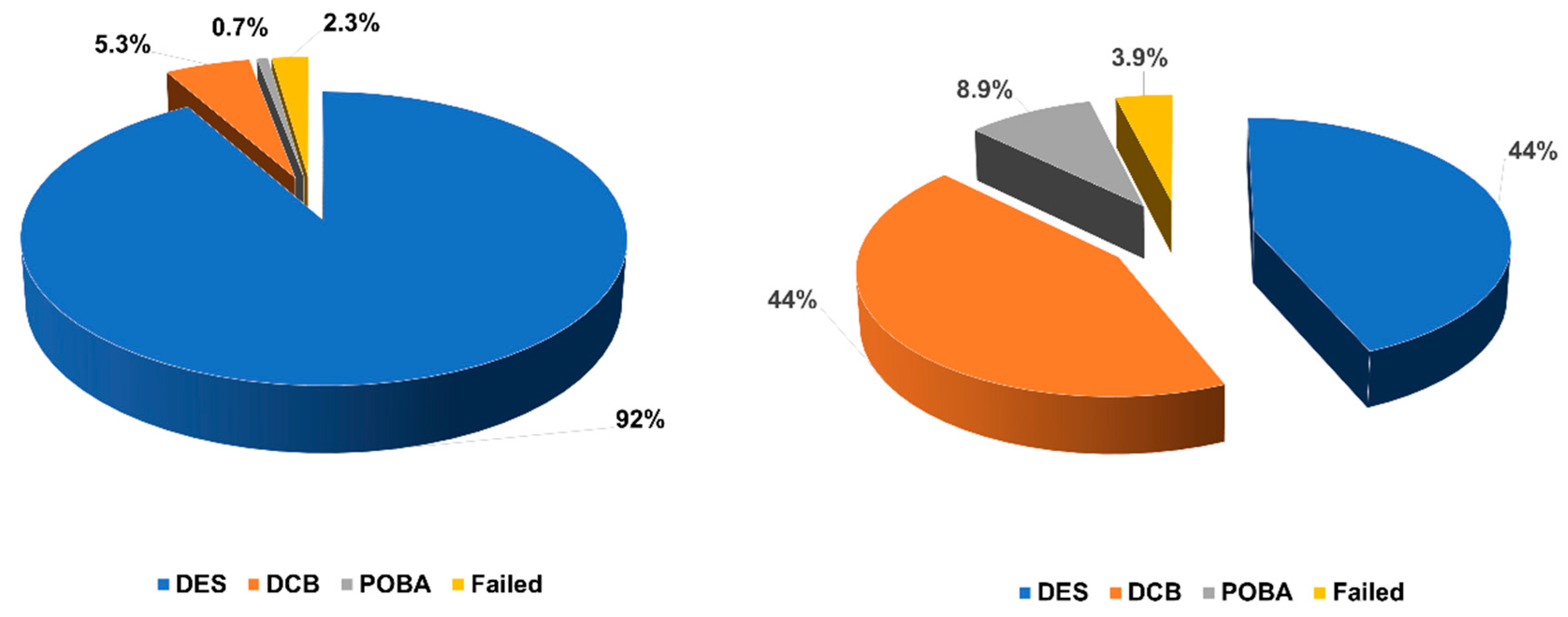
| Variables | Non-Stent Failure (n = 1019) | Stent Failure (n = 101) | In-Stent Restenosis (n = 76) | Stent Thrombosis (n = 25) | p-Value |
|---|---|---|---|---|---|
| Mean age (years) | 65 | 68 | 68 | 65 | 0.031 |
| Female gender (%) | 224 (22%) | 16 (16%) | 12 (16%) | 4 (16%) | 0.151 |
| Current smoker (%) | 532 (52%) | 33 (33%) | 21 (28%) | 12 (48%) | <0.001 |
| Diabetes mellitus (%) | 296 (29%) | 53 (53%) | 43 (57%) | 10 (40%) | 0.001 |
| Arterial hypertension (%) | 577(57%) | 64 (63%) | 51 (67%) | 13 (52%) | 0.191 |
| Dyslipidemia (%) | 511 (50%) | 89 (88%) | 67 (88%) | 22 (88%) | <0.001 |
| Family history of CAD (%) | 143 (14%) | 6 (5.9%) | 3 (3.9%) | 3 (12%) | 0.022 |
| Atrial fibrillation (%) | 72 (7.1%) | 11 (11%) | 9 (12%) | 2 (8%) | 0.162 |
| Prior CABG (%) | 38 (3.7%) | 8 (7.9%) | 8 (11%) | 0 (0.0%) | 0.043 |
| Characteristics | Non-Stent Failure (n = 1019) | Stent Failure (n = 101) | In-Stent Restenosis (n = 76) | Stent Thrombosis (n = 25) | p-Value |
|---|---|---|---|---|---|
| Radial access (%) | 967 (95%) | 90 (89%) | 67 (88%) | 23 (92%) | 0.016 |
| Vessel of PCI | |||||
| LM PCI (%) | 79 (7.8%) | 10 (9.9%) | 6 (7.9%) | 4 (16%) | 0.446 |
| LAD PCI (%) | 419 (41%) | 43 (43%) | 31 (41%) | 12 (48%) | 0.777 |
| LCX PCI (%) | 181 (18%) | 6 (5.9%) | 5 (6.6%) | 1 (4%) | 0.002 |
| RCA PCI (%) | 267 (26%) | 27 (27%) | 21 (28%) | 6 (24%) | 0.908 |
| Multivessel PCI (%) | 59 (5.8%) | 14 (14%) | 12 (16%) | 2 (8%) | 0.001 |
| Graft PCI (%) | 14 (1.4%) | 1 (1.0%) | 1 (1.3%) | 0 (0.0%) | 1.000 |
| Mean stent length per patients (mm) | 31 | 30.8 | 32.2 | 25.3 | 0.873 |
| Largest stent mean diameter (mm) | 3.21 | 3.24 | 3.23 | 3.31 | 0.639 |
| Utilization of balloons/adjunctive devices | |||||
| NC balloon (%) | 446 (44%) | 72 (71%) | 53 (70%) | 19 (76%) | <0.001 |
| Cutting/scoring balloon (%) | 4 (0.4%) | 9 (8.9%) | 8 (11%) | 1 (4%) | <0.001 |
| Lithotripsy (%) | 31 (3.0%) | 12 (12%) | 12 (16%) | 0 (0%) | <0.001 |
| Rotablation (%) | 12 (1.2%) | 1 (1%) | 1 (1.3%) | 0 (0%) | 1.000 |
| Technical success (%) | 996 (98%) | 97 (96%) | 72 (95%) | 25 (100%) | 0.296 |
| Malapposition | Raptured Neoathersoclerotic Plaque | Underexpansion | Stent Edge Dissection | |
|---|---|---|---|---|
| OCT (6) | 3 (50%) | 1 (17%) | 2 (33%) | 1 (17%) |
| IVUS (3) | 1 (33%) | 2 (66%) | 0 | 0 |
| Total (9) | 4 (44%) | 3 (33%) | 2 (22%) | 1 (11%) |
| Neoatherosclerosis | Underexpansion | Underexpansion and Neoatherosclerosis | |
|---|---|---|---|
| OCT (5) | 3 (60%) | 1 (20%) | 1 (20%) |
| IVUS (19) | 9 (47%) | 7 (37%) | 3 (16%) |
| Total (24) | 12 (50%) | 8 (33%) | 4 (17%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenogiannis, I.; Varlamos, C.; Benetou, D.-R.; Dragona, V.-M.; Vlachos, S.; Pappas, C.; Kolokathis, F.; Karamasis, G.V. Stent Failure Management in Contemporary Clinical Practice. Diagnostics 2025, 15, 1709. https://doi.org/10.3390/diagnostics15131709
Xenogiannis I, Varlamos C, Benetou D-R, Dragona V-M, Vlachos S, Pappas C, Kolokathis F, Karamasis GV. Stent Failure Management in Contemporary Clinical Practice. Diagnostics. 2025; 15(13):1709. https://doi.org/10.3390/diagnostics15131709
Chicago/Turabian StyleXenogiannis, Iosif, Charalampos Varlamos, Despoina-Rafailia Benetou, Vassiliki-Maria Dragona, Stefanos Vlachos, Christos Pappas, Fotios Kolokathis, and Grigoris V. Karamasis. 2025. "Stent Failure Management in Contemporary Clinical Practice" Diagnostics 15, no. 13: 1709. https://doi.org/10.3390/diagnostics15131709
APA StyleXenogiannis, I., Varlamos, C., Benetou, D.-R., Dragona, V.-M., Vlachos, S., Pappas, C., Kolokathis, F., & Karamasis, G. V. (2025). Stent Failure Management in Contemporary Clinical Practice. Diagnostics, 15(13), 1709. https://doi.org/10.3390/diagnostics15131709






