A Rare Case of Exophiala Dermatitidis Isolation in a Patient with Non-Cystic Fibrosis Bronchiectasis: Colonization or True Infection?
Abstract
1. Introduction
2. Case Presentation
2.1. Physical Examination Findings
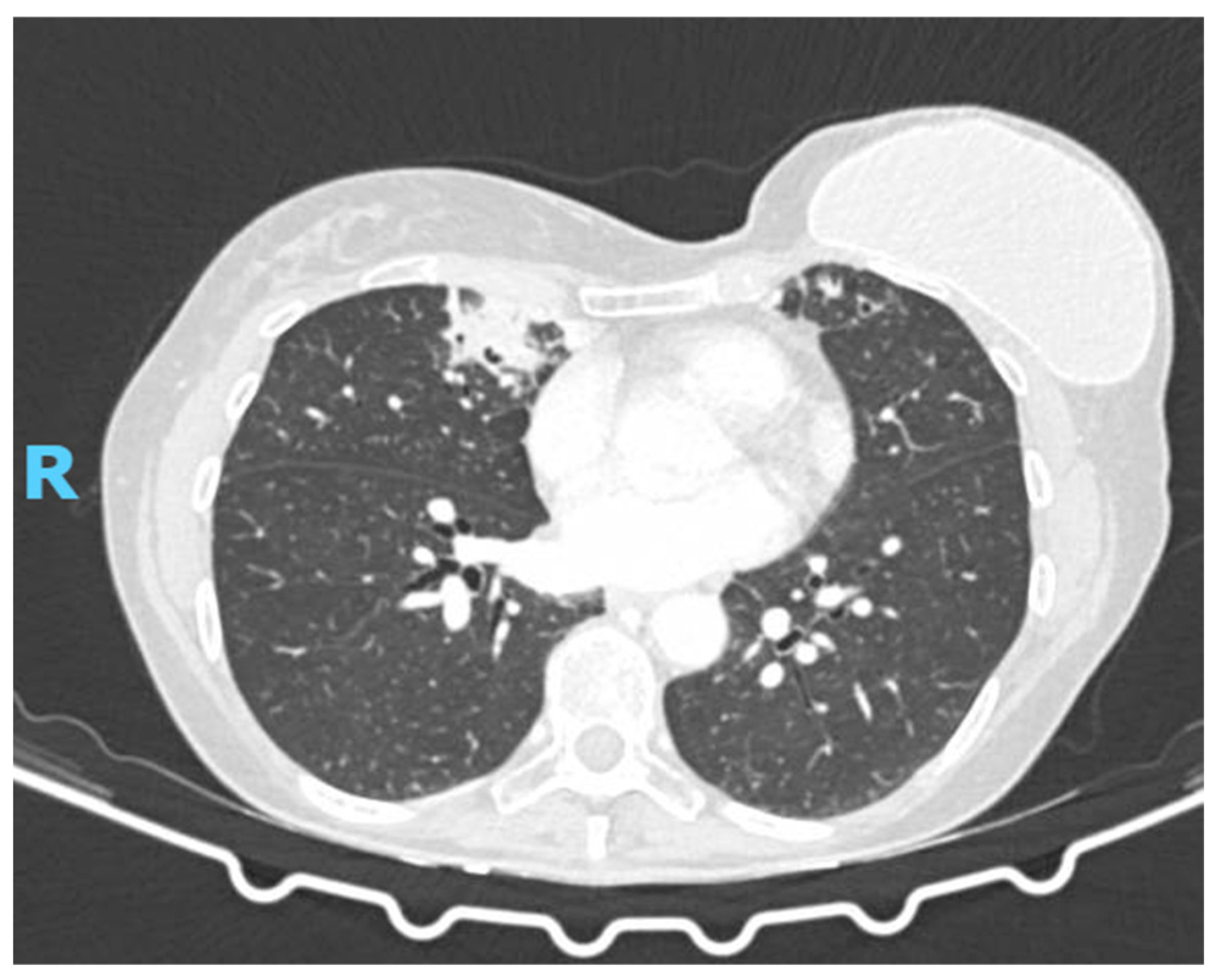
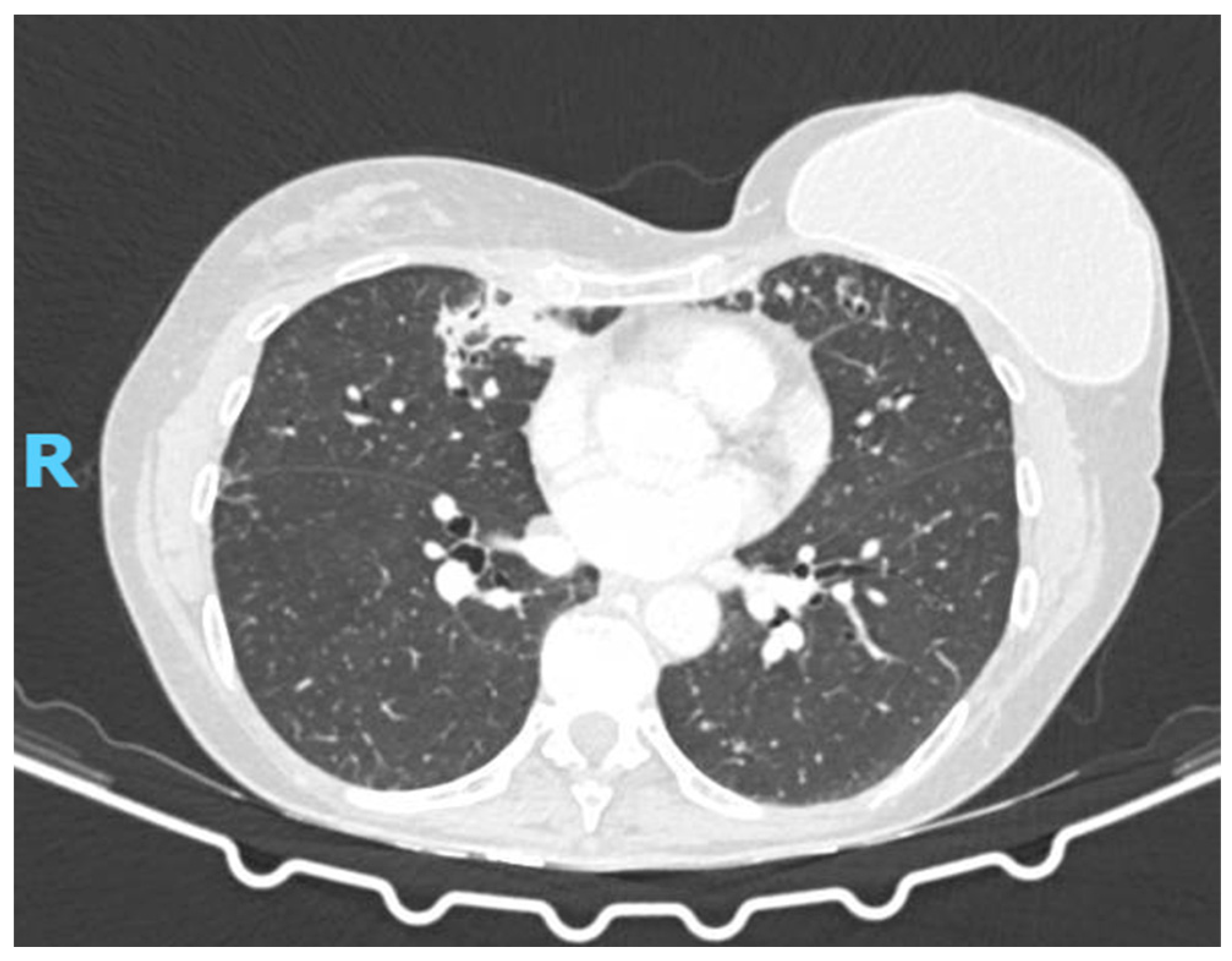
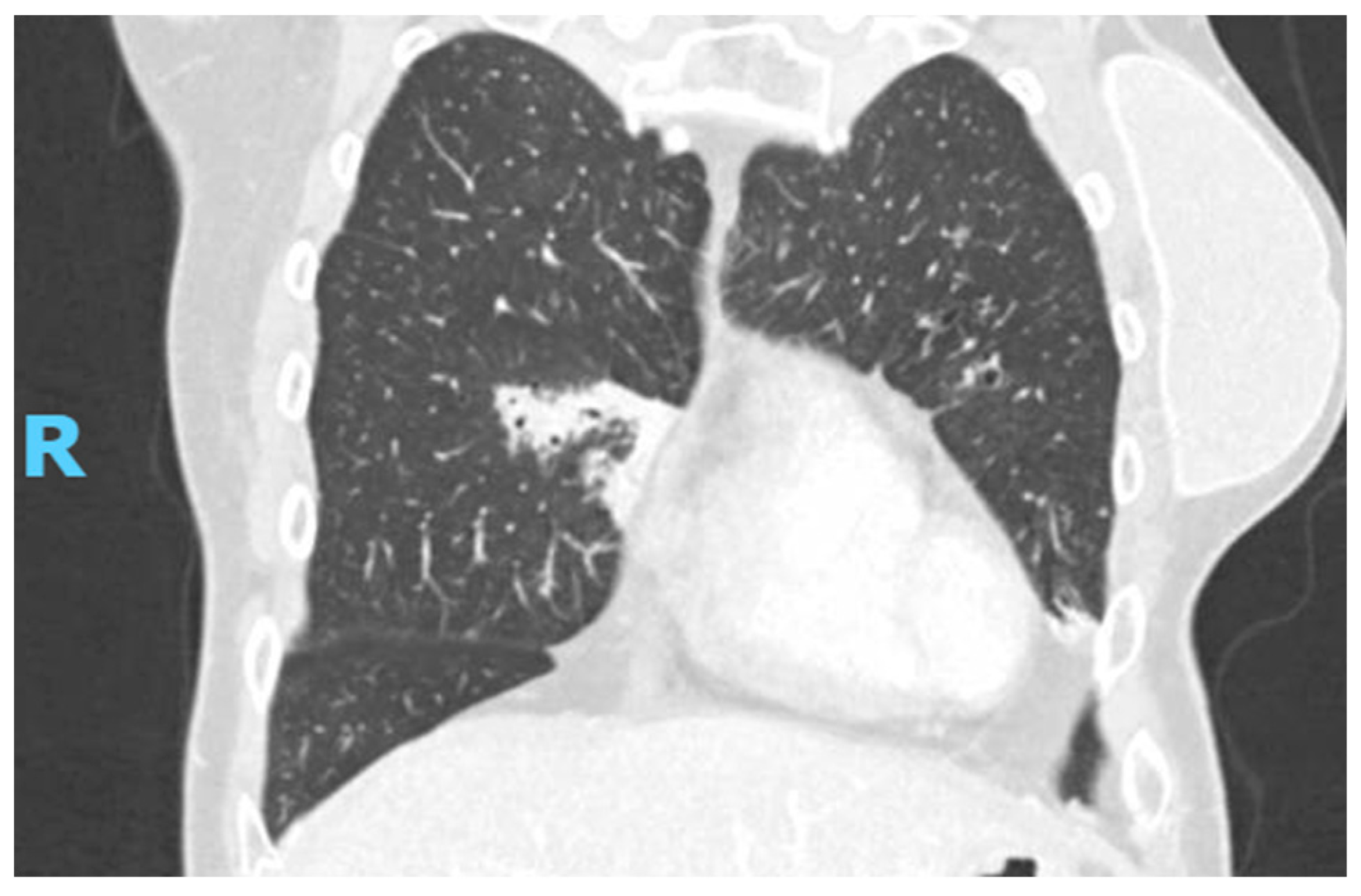
2.2. Diagnostic Studies
2.3. What Is the Diagnosis?
3. Discussion
Clinical Course
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahadori, T.; Didehdar, M.; Khansarinezhad, B.; Shokohi, T. Identification of Opportunistic and Nonopportunistic Exophiala Species Using High Resolution Melting Analysis. Med. Mycol. 2020, 58, 938–945. [Google Scholar] [CrossRef]
- Babič, M.N.; Zupančič, J.; Gunde-Cimerman, N.; de Hoog, S.; Zalar, P. Ecology of the Human Opportunistic Black Yeast Exophiala Dermatitidis Indicates Preference for Human-Made Habitats. Mycopathologia 2018, 183, 201–212. [Google Scholar] [CrossRef]
- de Jong, C.C.M.; Slabbers, L.; Engel, T.G.P.; Yntema, J.B.; van Westreenen, M.; Croughs, P.D.; Roeleveld, N.; Brimicombe, R.; Verweij, P.E.; Meis, J.F.; et al. Clinical Relevance of Scedosporium Spp. and Exophiala Dermatitidis in Patients with Cystic Fibrosis: A Nationwide Study. Med. Mycol. 2020, 58, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Chang, A.B.; Chotirmall, S.H.; Dhar, R.; McShane, P.J. Bronchiectasis. Nat. Rev. Dis. Prim. 2018, 4, 45. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society Guidelines for the Management of Adult Bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Richardson, H.; Dicker, A.J.; Barclay, H.; Chalmers, J.D. The Microbiome in Bronchiectasis. Eur. Respir. Rev. 2019, 28, 190048. [Google Scholar] [CrossRef] [PubMed]
- Usuda, D.; Higashikawa, T.; Hotchi, Y.; Usami, K.; Shimozawa, S.; Tokunaga, S.; Osugi, I.; Katou, R.; Ito, S.; Yoshizawa, T.; et al. Exophiala Dermatitidis. World J. Clin. Cases 2021, 9, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Revankar, S.G.; Sutton, D.A. Melanized Fungi in Human Disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef]
- Miyoshi, S.; Tanabe, M.; Semba, M.; Sato, C.; Aoyama, S.; Watanabe, A.; Ito, R.; Hamada, K.; Watanabe, A.; Abe, M. Exophiala Dermatitidis Coinfection with Nontuberculous Mycobacteria: A Case Report and Literature Review. Respirol. Case Rep. 2023, 11, e01221. [Google Scholar] [CrossRef]
- Horré, R.; Schaal, K.P.; Siekmeier, R.; Sterzik, B.; de Hoog, G.S.; Schnitzler, N. Isolation of Fungi, Especially Exophiala Dermatitidis, in Patients Suffering from Cystic Fibrosis. A Prospective Study. Respiration. 2004, 71, 360–366. [Google Scholar] [CrossRef]
- Haase, G.; Skopnik, H.; Groten, T.; Kusenbach, G.; Posselt, H.G. Long-Term Fungal Cultures from Sputum of Patients with Cystic Fibrosis. Mycoses 1991, 34, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Diemert, D.; Kunimoto, D.; Sand, C.; Rennie, R. Sputum Isolation of Wangiella Dermatitidis in Patients with Cystic Fibrosis. Scand. J. Infect. Dis. 2001, 33, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Matsuda, T.; McGinnis, M.R.; Ajello, L. Clinical and Mycological Spectra of Wangiella Dermatitidis Infections. Mycoses 1993, 36, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Ringshausen, F.C.; Harris, B.; Elborn, J.S.; Posthumus, A.; Haworth, C.S.; Pilkington, N.; Polverino, E.; Ruddy, T.; Aliberti, S.; et al. Cross-Infection Risk in Patients with Bronchiectasis: A Position Statement from the European Bronchiectasis Network (EMBARC), EMBARC/ELF Patient Advisory Group and European Reference Network (ERN-Lung) Bronchiectasis Network. Eur. Respir. J. 2018, 51, 1701937. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Mall, M.A.; McShane, P.J.; Nielsen, K.G.; Shteinberg, M.; Sullivan, S.D.; Chotirmall, S.H. A Systematic Literature Review of the Clinical and Socioeconomic Burden of Bronchiectasis. Eur. Respir. Rev. 2024, 33, 240049. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Olsowski, M.; Rath, P.-M.; Steinmann, J. Exophiala Dermatitidis: Key Issues of an Opportunistic Fungal Pathogen. Virulence 2019, 10, 984–998. [Google Scholar] [CrossRef]
- Boral, H.; Metin, B.; Döğen, A.; Seyedmousavi, S.; Ilkit, M. Overview of Selected Virulence Attributes in Aspergillus Fumigatus, Candida Albicans, Cryptococcus Neoformans, Trichophyton Rubrum, and Exophiala Dermatitidis. Fungal Genet. Biol. 2018, 111, 92–107. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, A.; Fujieda, A.; Nakase, K.; Katayama, N. Pulmonary Infection Caused by Exophiala Dermatitidis in a Patient with Multiple Myeloma: A Case Report and a Review of the Literature. Med. Mycol. Case Rep. 2012, 1, 95–98. [Google Scholar] [CrossRef]
- Myoken, Y.; Sugata, T.; Fujita, Y.; Kyo, T.; Fujihara, M.; Katsu, M.; Mikami, Y. Successful Treatment of Invasive Stomatitis Due to Exophiala Dermatitidis in a Patient with Acute Myeloid Leukemia. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2003, 32, 51–54. [Google Scholar] [CrossRef]
- Silva, W.C.; Gonçalves, S.S.; Santos, D.W.C.L.; Padovan, A.C.B.; Bizerra, F.C.; Melo, A.S.A. Species Diversity, Antifungal Susceptibility and Phenotypic and Genotypic Characterisation of Exophiala Spp. Infecting Patients in Different Medical Centres in Brazil. Mycoses 2017, 60, 328–337. [Google Scholar] [CrossRef]
- Yoshinouchi, T.; Yamamoto, K.; Migita, M.; Yokoyama, T.; Nakamura, T.; Matsuoka, M. Diagnosis and Clinical Management of Exophiala Dermatitidis Pneumonia in a Patient with Anorexia Nervosa: A Case Report. Med. Mycol. Case Rep. 2023, 42, 100617. [Google Scholar] [CrossRef]
- Setoguchi, D.; Iwanaga, N.; Ito, Y.; Ashizawa, N.; Hirayama, T.; Takeda, K.; Ide, S.; Takemoto, S.; Tashiro, M.; Hosogaya, N.; et al. Pulmonary Phaeohyphomycosis Due to Exophiala Dermatitidis in a Patient with Pulmonary Non-Tuberculous Mycobacterial Infection. J. Infect. Chemother. Off. J. Japan Soc. Chemother. 2023, 29, 615–619. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sano, H.; Konno, S.; Kamioka, Y.; Hariu, M.; Takano, K.; Yamada, M.; Seki, M. Sinobronchial Syndrome Patients with Suspected Non-Tuberculous Mycobacterium Infection Exacerbated by Exophiala Dermatitidis Infection. Infect. Drug Resist. 2022, 15, 1135–1141. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Zhu, J.; Xie, M.; Huang, S.; Li, S.; Zhan, Y.; Zeng, W.; Xu, T.; Ye, F. The Convoluted Process of Diagnosing Pulmonary Mycosis Caused by Exophiala Dermatitidis: A Case Report. BMC Infect. Dis. 2022, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, R.; Urabe, N.; Sakamoto, S.; Sasaki, M.; Homma, S.; Kishi, K. Exophiala Dermatitidis Pneumonia with Bronchiectasis Required Prolonged Voriconazole Treatment. Respirol. Case Rep. 2021, 9, e00783. [Google Scholar] [CrossRef]
- Mukai, Y.; Nureki, S.; Hata, M.; Shigenaga, T.; Tokimatsu, I.; Miyazaki, E.; Kadota, J.; Yarita, K.; Kamei, K. Exophiala Dermatitidis Pneumonia Successfully Treated with Long-Term Itraconazole Therapy. J. Infect. Chemother. Off. J. Japan Soc. Chemother. 2014, 20, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Bulloch, M.N. The Treatment of Pulmonary Wangiella Dermatitidis Infection with Oral Voriconazole. J. Clin. Pharm. Ther. 2011, 36, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Suda, T.; Kaida, Y.; Kato, M.; Hasegaw, H.; Fujii, M.; Ida, M.; Nogimura, H.; Nagayama, M.; Chida, K. A case of bronchial infection of Wangiella dermatitidis. Nihon Kokyuki Gakkai Zasshi 2007, 45, 907–911. [Google Scholar]
- Taj-Aldeen, S.J.; El Shafie, S.; Alsoub, H.; Eldeeb, Y.; de Hoog, G.S. Isolation of Exophiala Dermatitidis from Endotracheal Aspirate of a Cancer Patient. Mycoses 2006, 49, 504–509. [Google Scholar] [CrossRef]
- Mukaino, T.; Koga, T.; Oshita, Y.; Narita, Y.; Obata, S.; Aizawa, H. Exophiala Dermatitidis Infection in Non-Cystic Fibrosis Bronchiectasis. Respir. Med. 2006, 100, 2069–2071. [Google Scholar] [CrossRef]
- Barenfanger, J.; Ramirez, F.; Tewari, R.P.; Eagleton, L. Pulmonary Phaeohyphomycosis in a Patient with Hemoptysis. Chest 1989, 95, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Poyntner, C.; Mirastschijski, U.; Sterflinger, K.; Tafer, H. Transcriptome Study of an Exophiala Dermatitidis PKS1 Mutant on an Ex Vivo Skin Model: Is Melanin Important for Infection? Front. Microbiol. 2018, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Poyntner, C.; Blasi, B.; Arcalis, E.; Mirastschijski, U.; Sterflinger, K.; Tafer, H. The Transcriptome of Exophiala Dermatitidis during Ex-Vivo Skin Model Infection. Front. Cell. Infect. Microbiol. 2016, 6, 136. [Google Scholar] [CrossRef]
- Yazdanparast, S.A.; Mohseni, S.; De Hoog, G.S.; Aslani, N.; Sadeh, A.; Badali, H. Consistent High Prevalence of Exophiala Dermatitidis, a Neurotropic Opportunist, on Railway Sleepers. J. Mycol. Med. 2017, 27, 180–187. [Google Scholar] [CrossRef]
- Lang, R.; Minion, J.; Skinner, S.; Wong, A. Disseminated Exophiala Dermatitidis Causing Septic Arthritis and Osteomyelitis. BMC Infect. Dis. 2018, 18, 255. [Google Scholar] [CrossRef]
- Kenney, R.T.; Kwon-Chung, K.J.; Waytes, A.T.; Melnick, D.A.; Pass, H.I.; Merino, M.J.; Gallin, J.I. Successful Treatment of Systemic Exophiala dermatitidis Infection in a Patient with Chronic Granulomatous Disease. Clin. Infect. Dis. 1992, 14, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tanamachi, C.; Hashimoto, K.; Nakata, K.; Sagawa, K. A case of pulmonary chromomycosis caused by Exophiala dermatitidis. J. Jpn. Soc. Clin. Microbiol. 2008, 18, 25–30, (In Japanese, Abstract in English). [Google Scholar]
- Shintani, R.; Hagiwara, E.; Yamakawa, H.; Ikeda, S.; Kitamura, H.; Baba, T. Pulmonary phaeohyphomycosis caused by Exophiala dermatitidis. JJA Inf. 2017, 91, 785–789. [Google Scholar]
- Goto, Y.; Murakami, N.; Yamasaki, Y. Sequelae of pulmonary chromoblastomycosis caused by the viscous species Exophiala dermatitidis in a patient with nontuberculosis mycobacterial disease. Igakukensa 2020, 69, 451–456. [Google Scholar]
- Masuo, M.; Hanazawa, S.; Nukui, Y. Pulmonary chromomycosis caused by Exophiala dermatitidis in a patient with pulmonary non-tuberculous mycobacteriosis. J. Jap. Soc. Respir. Endos. 2021, 43, 619–623. [Google Scholar]
| Author (Reference) | Age | Sex | Country | Underlying Disease(s) | Symptoms | Treatment | Antifungal Therapy and Estimated Duration | Notes |
|---|---|---|---|---|---|---|---|---|
| Barenfanger et al. [31] | 79 | F | USA | Bronchiectasis | Hemoptysis, fever | Amphotericin B, 5-FC | Amphotericin B + 5-FC (IV), ~6–8 weeks | Likely IV start, step-down unknown |
| Kenney et al [36] | 21 | F | USA | Chronic granulomatous disease | Fever, chills, SOB | Surgery, Amphotericin B, 5-FC, Fluconazole | Combination therapy, ~6–12 weeks + oral Fluconazole | Immunocompromised host |
| Mukaino et al. [30] | 54 | F | Japan | Bronchiectasis | Cough, sputum | Miconazole, Amphotericin B | Likely topical/oral Miconazole + IV AmB, ~4–6 weeks | Non-severe |
| Taj-Aldeen et al. [29] | 54 | F | Quatar | Diabetes, cervical cancer | Cough, sputum, hemoptysis | Fluconazole, Itraconazole, Amphotericin B | Likely stepwise approach, total ~2–3 months | Fatal outcome, likely severe |
| Ozawa et al. [28] | 81 | F | Japan | None | Hemoptysis | Itraconazole | Itraconazole PO, ~3 months | Good response |
| Tanamachi et al. [37] | 53 | F | Japan | Bronchiectasis | Sputum, chest pain | Miconazole, 5-FC, Itraconazole | Combination (oral), likely ~3–6 months | Oral triple therapy |
| Bulloch et al. [27] | 86 | F | USA | Dementia | Asymptomatic | Voriconazole | VRCZ PO, likely short course (~4 weeks) | Incidental finding |
| Suzuki et al. [18] | 65 | M | Japan | Multiple myeloma | Asymptomatic | Voriconazole, Surgery | VRCZ PO, likely ~6 weeks post-surgery | Immunosuppressed |
| Mukai et al. [26] | 63 | F | Japan | None | Chest pain | Itraconazole | ITCZ PO, ~3 months | Standard regimen |
| Shintani et al. [38] | 56 | F | Japan | Bronchiectasis | Sputum, fever | Itraconazole | ITCZ PO, ~3–4 months | Bronchiectasis context |
| Goto et al. [39] | 70 | F | Japan | NTM | Sputum, chest pain | Voriconazole | VRCZ PO, ~4–6 months | NTM co-infection |
| Masuo et al. [40] | 58 | F | Japan | NTM | Cough | Voriconazole | VRCZ PO, ~4–6 months | NTM context |
| Sekiguchi et al. [25] | 65 | F | Japan | RA, bronchiectasis | Cough, sputum | Voriconazole | VRCZ PO, ~6 months | Autoimmune comorbidity |
| Li et al. [24] | 52 | M | China | None | Cough, sputum, hemoptysis | VRCZ, Amphotericin B, Posaconazole | Escalated therapy, total ~3–4 months | Likely initial failure |
| Watanabe et al. [23] | 65 | F | Japan | NTM, sinusitis | Cough, sputum | Voriconazole | VRCZ PO, ~3–4 months | Coinfection context |
| Watanabe et al. [23] | 47 | F | Japan | RA, NTM, sinusitis | Nasal discharge, anosmia | Amphotericin B, VRCZ, Itraconazole | Sequential therapy, total ~6 months | Upper + lower airway involvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccio, F.R.; Baio, N.; Montini, S.; Ferroni, V.; Chino, V.; Pisanu, L.; Russo, M.; Giana, I.; Gallo, E.; Arlando, L.; et al. A Rare Case of Exophiala Dermatitidis Isolation in a Patient with Non-Cystic Fibrosis Bronchiectasis: Colonization or True Infection? Diagnostics 2025, 15, 1661. https://doi.org/10.3390/diagnostics15131661
Bertuccio FR, Baio N, Montini S, Ferroni V, Chino V, Pisanu L, Russo M, Giana I, Gallo E, Arlando L, et al. A Rare Case of Exophiala Dermatitidis Isolation in a Patient with Non-Cystic Fibrosis Bronchiectasis: Colonization or True Infection? Diagnostics. 2025; 15(13):1661. https://doi.org/10.3390/diagnostics15131661
Chicago/Turabian StyleBertuccio, Francesco Rocco, Nicola Baio, Simone Montini, Valentina Ferroni, Vittorio Chino, Lucrezia Pisanu, Marianna Russo, Ilaria Giana, Elisabetta Gallo, Lorenzo Arlando, and et al. 2025. "A Rare Case of Exophiala Dermatitidis Isolation in a Patient with Non-Cystic Fibrosis Bronchiectasis: Colonization or True Infection?" Diagnostics 15, no. 13: 1661. https://doi.org/10.3390/diagnostics15131661
APA StyleBertuccio, F. R., Baio, N., Montini, S., Ferroni, V., Chino, V., Pisanu, L., Russo, M., Giana, I., Gallo, E., Arlando, L., Mucaj, K., Tafa, M., Arminio, M., Stefano, E. D., Cascina, A., Corsico, A. G., Stella, G. M., & Conio, V. (2025). A Rare Case of Exophiala Dermatitidis Isolation in a Patient with Non-Cystic Fibrosis Bronchiectasis: Colonization or True Infection? Diagnostics, 15(13), 1661. https://doi.org/10.3390/diagnostics15131661






