Neutrophil-Percentage-to-Albumin Ratio as a Predictor of Coronary Artery Ectasia: A Comparative Analysis with Inflammatory Biomarkers
Abstract
1. Introduction
2. Methods
2.1. Patients and Methods
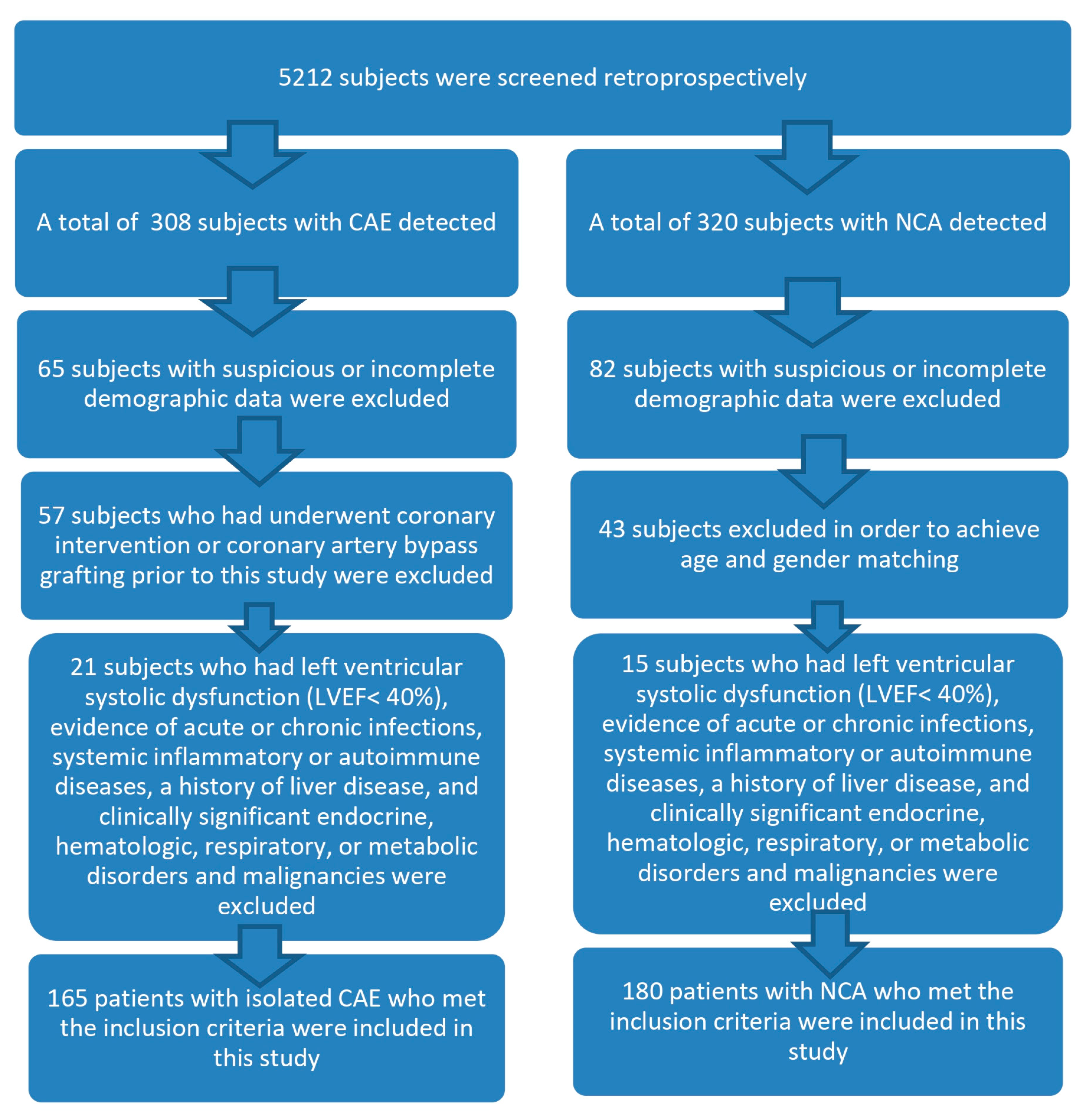
2.1.1. Study Design and Population
2.1.2. Data Collection
2.1.3. Angiography Evaluation
2.1.4. CAE Assessment
2.1.5. Laboratory Measurements
2.1.6. Left Ventricular Ejection Fraction (LVEF) Measurement
2.1.7. Statistical Analysis
3. Results
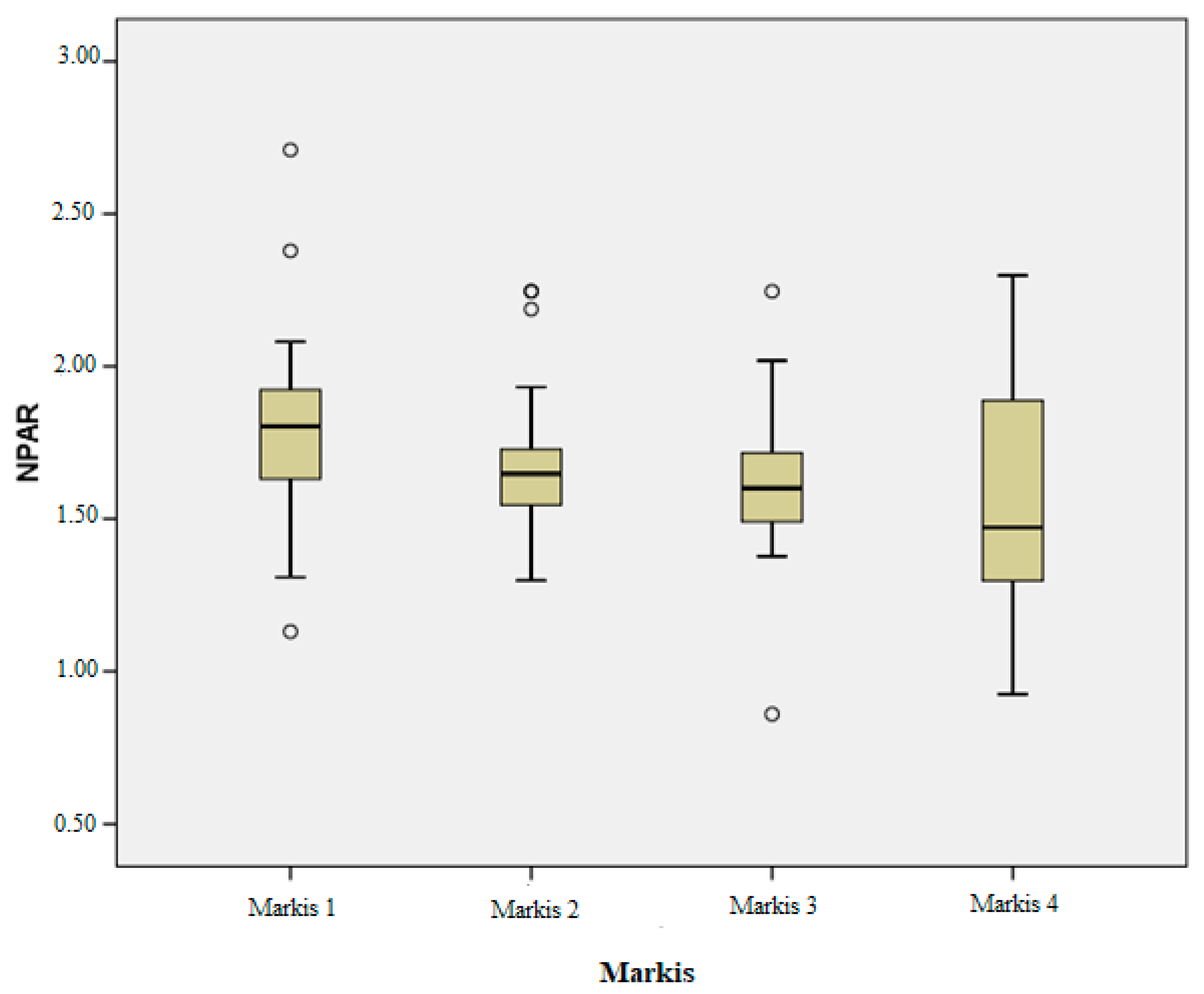
- A statistically significant difference was identified between the Markis 1 and Markis 4 groups for the NPAR, with a p-value of 0.002.
- For neutrophils, significant differences were noted between Markis 1 and Markis 4, as well as between Markis 3 and Markis 4, with p-values of 0.006 and 0.046, respectively.
- Regarding lymphocytes, significant differences were found between Markis 1 and Markis 4 and between Markis 2 and Markis 4, with p-values of 0.002 and 0.032, respectively.
- For the NLR, a significant difference was observed between Markis 1 and Markis 4, with a p-value of 0.001.
- Significant differences in the PLR were identified between Markis 1 and Markis 4, as well as between Markis 2 and Markis 4, with p-values of 0.003 and 0.025, respectively.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swaye, P.S.; Fisher, L.D.; Litwin, P.; Vignola, P.A.; Judkins, M.P.; Kemp, H.G.; Mudd, J.G.; Gosselin, A.J. Aneurysmal coronary artery disease. Circulation 1983, 67, 134–138. [Google Scholar] [CrossRef]
- Krüger, D.; Stierle, U.; Herrmann, G.; Simon, R.; Sheikhzadeh, A. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (“dilated coronopathy”). J. Am. Coll. Cardiol. 1999, 34, 1461–1470. [Google Scholar] [CrossRef]
- Markis, J.E.; Joffe, C.; Cohn, P.F.; Feen, D.J.; Herman, M.V.; Gorlin, R. Clinical significance of coronary arterial ectasia. Am. J. Cardiol. 1976, 37, 217–222. [Google Scholar] [CrossRef]
- Antoniadis, A.P.; Chatzizisis, Y.S.; Giannoglou, G.D. Pathogenetic mechanisms of coronary ectasia. Int. J. Cardiol. 2008, 130, 335–343. [Google Scholar] [CrossRef]
- Turhan, H.; Erbay, A.R.; Yasar, A.S.; Balci, M.; Bicer, A.; Yetkin, E. Comparison of C-reactive protein levels in patients with coronary artery ectasia versus patients with obstructive coronary artery disease. Am. J. Cardiol. 2004, 94, 1303–1306. [Google Scholar] [CrossRef]
- Kundi, H.; Gök, M.; Çetin, M.; Kızıltunç, E.; Çiçekcioğlu, H.; Çetin, Z.G.; Karayiğit, O.; Örnek, E. Relationship between platelet-to-lymphocyte ratio and the presence and severity of coronary artery ectasia. Anatol. J. Cardiol. 2016, 16, 857–862. [Google Scholar] [CrossRef]
- Sarli, B.; Baktir, A.O.; Saglam, H.; Arinc, H.; Kurtul, S.; Sivgin, S.; Akpek, M.; Kaya, M.G. Neutrophil-to-lymphocyte ratio is associated with severity of coronary artery ectasia. Angiology 2014, 65, 147–151. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Cheng, B.; Ying, B.; Wang, B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol. Infect. 2020, 148, e87. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, J.; Ma, Q.; Li, C.; Qiu, F. Prognostic value of inflammation biomarkers for 30-day mortality in critically ill patients with stroke. Front. Neurol. 2023, 14, 1110347. [Google Scholar] [CrossRef]
- Shin, K.W.; Choi, S.; Oh, H.; Hwang, S.Y.; Park, H.-P. A High Immediate Postoperative Neutrophil-to-Albumin Ratio is Associated with Unfavorable Clinical Outcomes at Hospital Discharge in Patients with Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. Anesthesiol. 2024, 36, 142–149. [Google Scholar] [CrossRef]
- Tawfik, B.; Mokdad, A.A.; Patel, P.M.; Li, H.C.; Huerta, S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anti-Cancer Drugs 2016, 27, 879–883. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, Y.; Liu, J.; Xu, X.; Liu, J.; Chen, M.; Shi, L. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with chronic heart failure. BMC Cardiovasc. Disord. 2023, 23, 568. [Google Scholar] [CrossRef]
- Sun, T.; Shen, H.; Guo, Q.; Yang, J.; Zhai, G.; Zhang, J.; Zhang, B.; Ding, Y.; Cai, C.; Zhou, Y.; et al. Association between Neutrophil Percentage-to-Albumin Ratio and All-Cause Mortality in Critically Ill Patients with Coronary Artery Disease. BioMed Res. Int. 2020, 2020, 8137576. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Zhu, C.; Song, D.; Wu, B.; Ji, K.; Li, J. The Neutrophil Percentage-to-Albumin Ratio is Associated with All-Cause Mortality in Patients with Atrial Fibrillation: A Retrospective Study. J. Inflamm. Res. 2023, 16, 691–700. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Yue, J.; Zou, Q. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2022, 22, 115. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Ling, X.; Huang, R.; Wang, S.; Min, J.; Xiao, J.; Zhang, Y.; Wang, Z.; Carrabba, N. The Neutrophil Percentage-to-Albumin Ratio as a New Predictor of All-Cause Mortality in Patients with Cardiogenic Shock. BioMed Res. Int. 2020, 2020, 7458451. [Google Scholar] [CrossRef]
- Cannata, A.; Segev, A.; Madaudo, C.; Bobbio, E.; Baggio, C.; Schütze, J.; Gentile, P.; Sanguineti, M.; Monzo, L.; Schettino, M.; et al. Elevated Neutrophil-to-Lymphocyte Ratio Predicts Prognosis in Acute Myocarditis. JACC Heart Fail. 2025, 13, 770–780. [Google Scholar] [CrossRef]
- Vrachatis, D.A.; Papathanasiou, K.A.; Kazantzis, D.; Sanz-Sánchez, J.; Giotaki, S.G.; Raisakis, K.; Kaoukis, A.; Kossyvakis, C.; Deftereos, G.; Reimers, B.; et al. Inflammatory Biomarkers in Coronary Artery Ectasia: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Cagirci, G.; Kucukseymen, S.; Yuksel, I.O.; Bayar, N.; Koklu, E.; Guven, R.; Arslan, S. The Relationship between Vitamin D and Coronary Artery Ectasia in Subjects with a Normal C-Reactive Protein Level. Korean Circ. J. 2017, 47, 231–237. [Google Scholar] [CrossRef]
- Cekici, Y.; Kilic, S.; Saracoglu, E.; Cetin, M.; Duzen, I.V.; Yilmaz, M. The Relationship between Blood Viscosity and Isolated Coronary Artery Ectasia. Acta Cardiol. Sin. 2019, 35, 20–26. [Google Scholar][Green Version]
- Demir, M.; Demir, C.; Keceoglu, S. The Relationship Between Blood Monocyte Count and Coronary Artery Ectasia. Cardiol. Res. 2014, 5, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.; Korkmaz, H.; Bilen, M.N.; Uku, Ö.; Kurtoğlu, E. Could neutrophil/lymphocyte ratio be an indicator of coronary artery disease, coronary artery ectasia and coronary slow flow? J. Int. Med. Res. 2016, 44, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-H.; Hao, Y.; Liu, Y.-H.; Li, X.-L.; Huang, Z.-H.; Luo, Y.; Li, R.-L. Anti-inflammatory effects of rosuvastatin treatment on coronary artery ectasia patients of different age groups. BMC Cardiovasc. Disord. 2020, 20, 330. [Google Scholar] [CrossRef]
- Tosu, A.R.; Çinar, T.; Güler, A.; Kahraman, S.; Gürbak, İ. Monosit/yüksek yoğunluklu lipoprotein oranının koroner arter ektaziyi öngörmedeki yararı Turk. J. Clin. Lab. 2019, 1, 68–73. [Google Scholar]
- Shereef, A.; Kandeel, N. Predictors for coronary artery ectasia. J. Indian Coll. Cardiol. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Yalcin, A.A.; Topuz, M.; Akturk, I.F.; Celik, O.; Erturk, M.; Uzun, F.; Duran, M.; Karadeniz, M.; Sarikamis, C.; Oner, E. Is there a correlation between coronary artery ectasia and neutrophil-lymphocyte ratio? Clin. Appl. Thromb./Hemost. 2015, 21, 229–234. [Google Scholar] [CrossRef]
- Liu, R.; Sheng, Q.; Liang, S.; Zhao, H. Peripheral blood soluble elastin and elastase as auxiliary diagnostic indicators for coronary artery ectasia. Turk. J. Med. Sci. 2021, 51, 1058–1064. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Joles, J.A.; Willekes-Koolschijn, N.; Koomans, H.A. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997, 52, 761–770. [Google Scholar] [CrossRef]
- Mikhailidis, D.P.; Ganotakis, E.S. Plasma albumin and platelet function: Relevance to atherogenesis and thrombosis. Platelets 1996, 7, 125–137. [Google Scholar] [CrossRef]
- Sercelik, A.; Tanrıverdi, O.; Askin, L.; Turkmen, S. Association of C-Reactive Protein to Albumin Ratio in Patients with Isolated Coronary Artery Ectasia. A Associação da Relação Proteína C-Reativa/Albumina em Pacientes com Ectasia da Artéria Coronária Isolada. Arq. Bras. Cardiol. 2021, 116, 48–54. [Google Scholar] [CrossRef]
- Onsrud, M.; Thorsby, E. Influence of in vivo hydrocortisone on some human blood lymphocyte subpopulations. I. Effect on natural killer cell activity. Scand. J. Immunol. 1981, 13, 573–579. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef]
- Dragu, R.; Huri, S.; Zukermann, R.; Suleiman, M.; Mutlak, D.; Agmon, Y.; Kapeliovich, M.; Beyar, R.; Markiewicz, W.; Hammerman, H.; et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis 2008, 196, 405–412, Erratum in Atherosclerosis 2012, 221, 605. [Google Scholar] [CrossRef]
| Overall (345) | CAE (165) | NCA (180) | p | |
|---|---|---|---|---|
| Mean ± Std. Deviation | Mean ± Std. Deviation | Mean ± Std. Deviation | ||
| Age, years | 59.99 ± 10.46 | 61.11 ± 10.16 | 58.97 ± 10.65 | 0.057 |
| NPAR | 1.28 ± 0.47 | 1.64 ± 0.31 | 1.42 ± 0.22 | <0.001 |
| Ejection fraction, % | 61.13 ± 3.05 | 60.90 ± 3.07 | 61.50 ± 2.84 | 0.061 |
| Plasma glucose | 136.67 ± 54.20 | 141.77 ± 48.76 | 131.99 ± 58.49 | 0.094 |
| Creatinine, mg/dL | 0.81 ± 0.17 | 0.82 ± 0.16 | 0.80 ± 0.18 | 0.410 |
| T cholesterol, mg/dL | 197.97 ± 48.71 | 194.04 ± 44.26 | 201.56 ± 52.33 | 0.149 |
| LDL-C, mg/dL | 118.47 ± 39.33 | 115.65 ± 37.02 | 121.04 ± 41.28 | 0.204 |
| HDL-C, mg/dL | 43.64 ± 9.66 | 43.22 ± 8.89 | 44.03 ± 10.32 | 0.438 |
| Triglyceride, mg/dL | 177.82 ± 91.36 | 176.95 ± 117.08 | 178.62 ± 58.98 | 0.866 |
| Albumin, g/dL | 39.55 ± 3.08 | 39.08 ± 3.55 | 39.98 ± 2.52 | 0.008 |
| hs-CRP, mg/L | 5.50 ± 2.78 | 6.02 ± 2.76 | 5.02 ± 2.73 | 0.001 |
| WBC count, ×10/μL | 8.04 ± 1.96 | 8.20 ± 1.95 | 7.88 ± 1.96 | 0.135 |
| Neutrophil count, ×10/μL | 4.84 ± 1.54 | 5.25 ± 1.67 | 4.47 ± 1.29 | <0.001 |
| Lymphocyte count, ×10/μL | 2.35 ± 0.94 | 2.12 ± 0.82 | 2.55 ± 0.99 | <0.001 |
| Monocyte count, ×10/μL | 0.68 ± 0.23 | 0.73 ± 0.34 | 0.63 ± 0.21 | 0.105 |
| Platelet count, ×10/μL | 271.67 ± 66.94 | 278.86 ± 67.19 | 265.08 ± 66.22 | 0.056 |
| Hemoglobin, g/dL | 13.97 ± 1.78 | 14.05 ± 1.77 | 13.89 ± 1.79 | 0.405 |
| NLR | 2.41 ± 1.46 | 2.93 ± 1.75 | 1.95 ± 0.90 | <0.001 |
| PLR | 133.51 ± 66.85 | 152.41 ± 77.20 | 116.19 ± 49.97 | <0.001 |
| n (%) | n (%) | n (%) | p | |
| Male, n (%) | 235 (68.1) | 116 (70.3) | 119 (66.1) | 0.404 |
| DM, n (%) | 136 (39.4) | 67 (40.6) | 69 (38.3) | 0.666 |
| Hypertension, n (%) | 197 (57.1) | 97 (58.8) | 100 (55.6) | 0.545 |
| Current smoker, n (%) | 130 (37.7) | 65 (39.4) | 65 (36.1) | 0.530 |
| B | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | |||
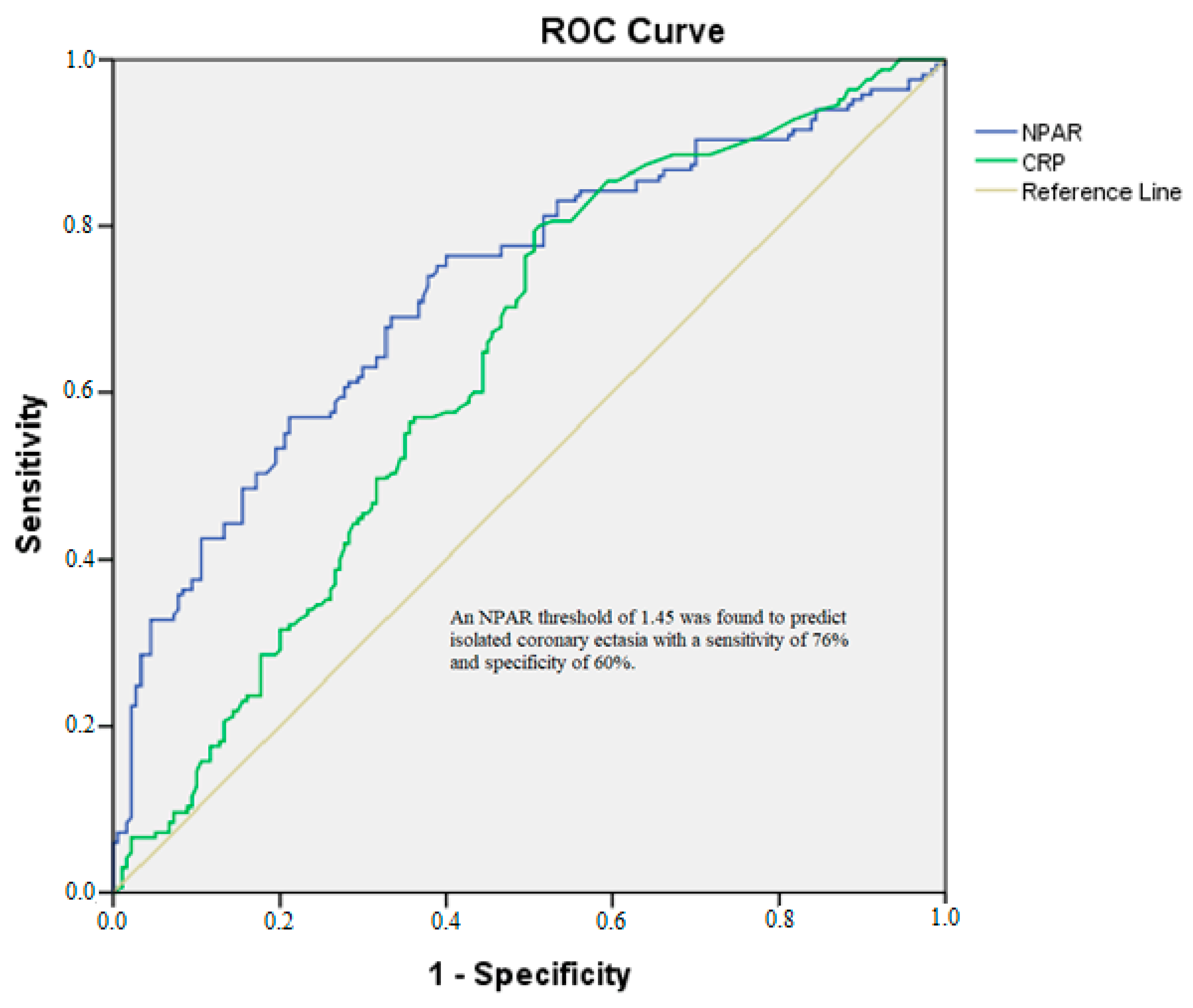
| NPAR | 6.829 | 0.016 | 1.422 | 32.803 |
| hsCRP | 1.129 | 0.007 | 1.034 | 1.231 |
| Neutrophil count | 1.131 | 0.454 | 0.819 | 1.563 |
| Lymphocyte count | 1.012 | 0.961 | 0.624 | 1.642 |
| NLR | 1.046 | 0.859 | 0.638 | 1.714 |
| PLR | 1.005 | 0.096 | 0.999 | 1.012 |
| Markıs 1(39) | Markıs 2(30) | Markıs 3(36) | Markıs 4(60) | p (Between Groups) | |
|---|---|---|---|---|---|
| Mean ± Std. Deviation | Mean ± Std. Deviation | Mean ± Std. Deviation | Mean ± Std. Deviation | ||
| NPAR | 1.78 ± 0.29 | 1.68 ± 0.24 | 1.62 ± 0.23 | 1.54 ± 0.35 | 0.001 |
| Albumin, g/dL | 38.64 ± 3.51 | 39.40 ± 3.93 | 39.28 ± 2.70 | 39.08 ± 3.87 | 0.816 |
| hsCRP | 5.44 ± 2.32 | 6.19 ± 2.64 | 6.40 ± 3.62 | 6.09 ± 2.48 | 0.471 |
| Neutrophil count | 5.77 ± 1.58 | 5.31 ± 1.50 | 5.63 ± 1.79 | 4.66 ± 1.59 | 0.004 |
| Lymphocyte count | 1.78 ± 0.73 | 1.94 ± 0.60 | 2.20 ± 0.86 | 2.38 ± 0.87 | 0.002 |
| NLR | 3.79 ± 1.94 | 3.01 ± 1.67 | 2.94 ± 1.69 | 2.31 ± 1.48 | 0.001 |
| PLR | 189.45 ± 104.56 | 172.05 ± 82.37 | 143.13 ± 59.69 | 124.07 ± 45.85 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karasu, M.; Şahin, Ş. Neutrophil-Percentage-to-Albumin Ratio as a Predictor of Coronary Artery Ectasia: A Comparative Analysis with Inflammatory Biomarkers. Diagnostics 2025, 15, 1638. https://doi.org/10.3390/diagnostics15131638
Karasu M, Şahin Ş. Neutrophil-Percentage-to-Albumin Ratio as a Predictor of Coronary Artery Ectasia: A Comparative Analysis with Inflammatory Biomarkers. Diagnostics. 2025; 15(13):1638. https://doi.org/10.3390/diagnostics15131638
Chicago/Turabian StyleKarasu, Mehdi, and Şeyda Şahin. 2025. "Neutrophil-Percentage-to-Albumin Ratio as a Predictor of Coronary Artery Ectasia: A Comparative Analysis with Inflammatory Biomarkers" Diagnostics 15, no. 13: 1638. https://doi.org/10.3390/diagnostics15131638
APA StyleKarasu, M., & Şahin, Ş. (2025). Neutrophil-Percentage-to-Albumin Ratio as a Predictor of Coronary Artery Ectasia: A Comparative Analysis with Inflammatory Biomarkers. Diagnostics, 15(13), 1638. https://doi.org/10.3390/diagnostics15131638






